Abstract
Objective
To investigate the effect of reconstruction slice thickness on image quality at CT virtual cystoscopy (VC).
Methods
Pelvic CT examinations in bladder cancer patients were reconstructed at different slice thicknesses (0.6–5 mm) and intervals, and resulting VC images assessed. Quality indicators were ridging, holes, floaters and dimpling artefacts, tumour definition, and an overall score, ranked 1 (best) to 7 (worst). CT number and standard deviation (SD) for bladder contents and bladder wall were recorded. The mean SD was used as a measure of noise, and the contrast-to-noise ratio (CNR) was calculated as the CT number difference between them divided by the average image noise. The mean CNR across the three levels was used for analysis. Each qualitative image quality measure was compared with CT number, noise and CNR measurements.
Results
Dimpling artefacts increased with thinner slice reconstruction and correlated with increased noise, often resulting in poor tumour definition. The best overall image quality score was seen for VC images reconstructed at 1.2 mm slice thickness, probably because of the competing effects of spatial resolution and CNR.
Conclusion
A slice thickness reconstruction <1.2 mm does not provide for better image quality at VC owing to the presence of increased noise.
Flexible and rigid cystoscopy are invasive techniques employed in the diagnosis and follow-up of patients with suspected and treated bladder malignancy. Areas of mucosal abnormality can be directly visualised and resected or biopsied. However, the procedure is associated with a complication rate of 4–6%, depending on expertise [1-3], and visualisation of the entire mucosal surface of the bladder is not always possible, particularly in the presence of clot and debris. Other anatomical abnormalities within the bladder (such as diverticula) cannot be adequately accessed. Alternative diagnostic tools and imaging techniques have until now been unable to provide sufficient accuracy to replace this direct visualisation of the bladder.
Recently, the availability of rapid image acquisition, and software permitting three-dimensional computer-rendered images, has led to interest in virtual reality imaging. Volume rendering processing techniques have developed such that it is now possible to simulate intraluminal navigation through any hollow viscus, as in conventional endoscopic procedures. The majority of virtual endoscopy development has been in CT virtual colonoscopy, which has developed into an accepted tool for the diagnosis and screening of colonic lesions [4]. The bladder also provides a suitable organ for virtual imaging: it is a hollow fluid-filled organ into which additional positive or negative contrast material can be instilled via urethral catheter or intravenously. CT virtual cystoscopy (VC) has been reported for the investigation of haematuria and diagnosis of bladder tumours [5-7].
VC as an imaging modality is well described in the literature, but the optimum scanning technique remains unclear. Initial studies of VC used single-detector CT scanners and a collimation of 3–5 mm. These early reports demonstrate a low sensitivity for the detection of smaller tumours using a shaded surface technique of virtual imaging [8,9]. Most of these studies have used a collimation of 3 mm and reconstruction interval of 1–2 mm [6,10-15]. Dectection rates of >90% are reported with a lower size limit of 5 mm.
Multidetector CT (MDCT) has the ability to image faster than single-detector CT and acquire multiple thin sections with near-isotropic voxels. Virtual reconstructions of these thin sections using volume rendering software could result in improved spatial resolution and the possibility to detect smaller abnormalities. As the technological capability to reconstruct with thinner slice thickness has improved, there has been a reported improvement in tumour detection rates without impairment of image quality for CT virtual colonoscopy; for every 1 mm increase in slice thickness, there is a decrease in sensitivity of 5% [16] and in specificity [17]. A slice thickness of 3 mm or below is currently recommended for CT virtual colonoscopy [18]. Although reports of VC using MDCT suggest an improvement in bladder tumour detection rates with reducing slice thickness [19-22], the optimal image acquisition variables for VC remain unknown.
This study was performed to investigate the effects of reducing slice thickness on virtual CT reconstructions with the following objectives:
document artefacts associated with CT VC and image quality
define the effect of slice thickness on image quality variables
define the optimum image acquisition for CT VC.
Method
10 CT examinations were performed in patients with known bladder tumours as part of a larger study investigating VC at a single institution. Scientific and ethics approval was granted, and the study was conducted in line with European Union guidelines for good clinical practice following signed informed consent from all patients.
Data acquisition
Following administration of intravenous contrast, patients were scanned with a time delay of 30–60 min when they expressed desire to void. Limited pelvic CT scans were acquired in the supine position (LightSpeed® 16; GE Healthcare, Little Chalfont, UK; 120 kV; 16×0.625 mm collimation; rotation 0.8 seconds; pitch 0.938; auto mA, noise index 12). Each CT examination was reconstructed with different slice thickness (5, 2.5, 1.2 and 0.6 mm). Data sets were sent to an independent workstation (Advantage® 4.1, GE Healthcare) and viewed after surface rendered processing with NavigatorTM software (GE Healthcare). This application uses a three-dimensional (3D) image set to generate a conical 3D view inside an anatomical structure that can be manipulated to examine luminal surfaces, mimicking the view seen through an endoscopic camera.
Each reconstruction was assessed qualitatively by viewing the VC and quantitatively by using the axial cross-sectional images.
Virtual cystoscopy assessment
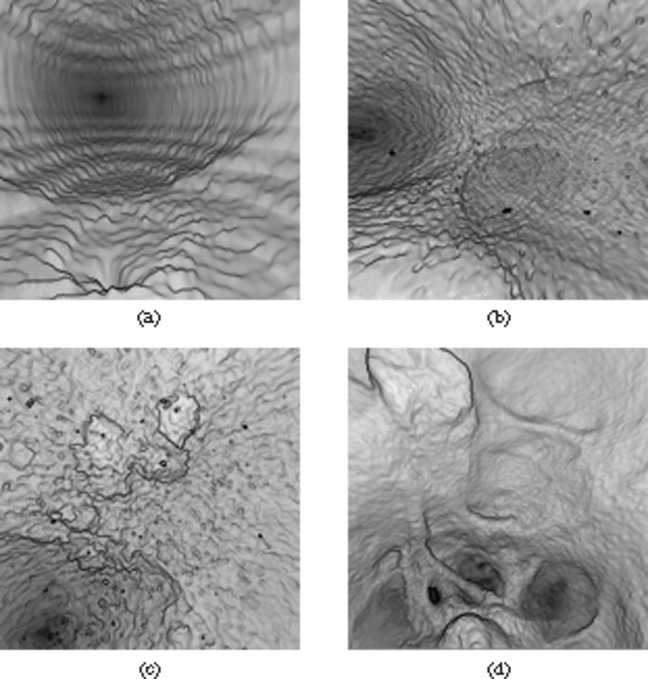
The threshold function of the Navigator software, which is used to set the voxel values of the structures displayed as hollow and solid, was manually adjusted by one observer (SL) to obtain the optimum VC view for each CT reconstruction set. The resulting quality of each virtual reconstruction was determined by scoring different image quality variables. The parameters used to assess image quality were chosen based on the different artefacts observed when performing VC. Examples of each parameter are shown in Figure 1. The presence of horizontal lines or steps was described as “ridging”. The appearance of pits and troughs in the mucosal surface was termed “dimples”. Extremes of threshold values generated the presence of “holes” and “floaters”, visible as gaps in the mucosal surface and black spots in the lumen, respectively. Tumour definition was assessed by the ability to determine the extent of tumour from normal surrounding mucosa. Image quality was assessed by assigning a rank from 1 to 7 (best to worst) for each parameter across the range of reconstructions for each scan. Finally, an overall rank (1–7) was given to each reconstruction. Each reconstruction was assessed jointly by two observers (SL and SAS).
Figure 1.
Virtual cystoscopy artefacts and parameters assessed: (a) ridging, (b) dimpling, (c) holes and floaters, and (d) tumour definition.
CT quantitative parameters
In order to provide an explanation for the observed differences in image variables, a number of quantitative parameters were examined. CT number and standard deviation (SD) for bladder contents and bladder wall were measured on axial images. Regions of interest (ROIs) for these measurements were defined at three levels through the craniocaudal dimension of the bladder corresponding to approximately the upper third, centre and lower third of the organ (Figure 2). The area and position of the ROI for each level was kept constant. Noise was expressed as the mean SD across the three levels for each reconstruction. Using the formula
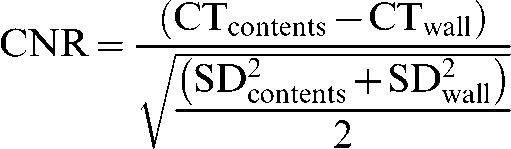 |
where CTcontents and CTwall are the CT numbers for bladder contents and bladder wall, respectively, and SDcontents and SDwall are the SD for bladder contents and bladder wall, respectively; the contrast-to-noise ratio (CNR) for each level was calculated. The mean CNR across the three levels for each reconstruction was then used for analysis. Subsequently, each image quality measure score for each reconstruction was compared with CT number, noise and CNR, expressed by linear regression r2, to determine any possible relationship.
Figure 2.
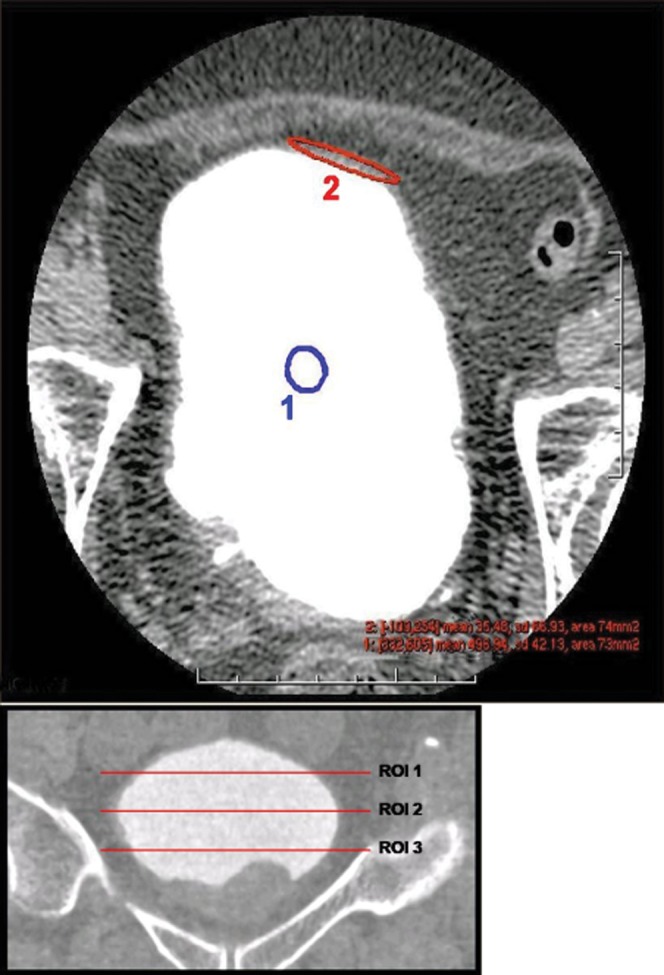
Assessment of CT quantitative parameters, CT number and standard deviation recorded for contents (1) and bladder wall (2) at three levels through the bladder [region of interest (ROI) 1, ROI 2, ROI 3].
Results
Image quality assessment
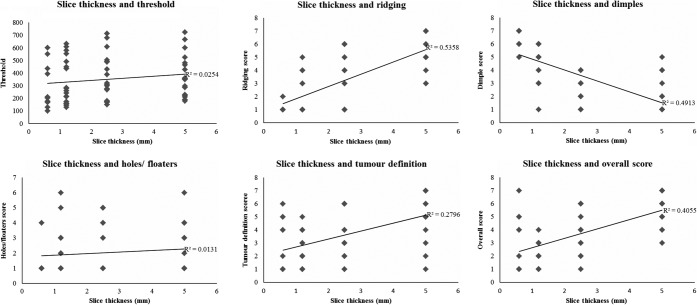
The effect of slice thickness on each of the qualitative variables is demonstrated in Figure 3. As slice thickness increases, an increase in the presence of ridges was noted. This may be explained by poor sampling along the axis of rotation of the scanner. Conversely, dimpling artefact increased as slice thickness reduced, and in some series the presence of dimpling on the thinnest slices resulted in very poor quality images and loss of tumour definition (Figure 4). The presence of holes and floaters did not correlate with slice thickness. Generally, as indicated by the correlation coefficients displayed in Figure 3, thinner slice thickness gave better tumour definition and overall score. The thinnest slice thickness of 0.6 mm gave the best tumour definition and overall score in some series where dimpling artefact did not appear in the virtual reconstructions; however, this was not the case for most series. The best slice thickness for tumour definition was 1.2 or 2.5 mm (mean tumour definition score 2.3 and 2.8, respectively). As seen with tumour definition, the best overall virtual reconstructions were obtained with a slice thickness of 1.2 mm (Figure 5).
Figure 3.
Effect of slice thickness on image quality variables.
Figure 4.
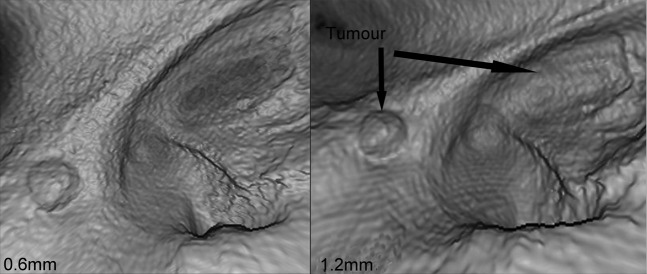
Dimpling artefact on thinner slices results in loss of tumour definition.
Figure 5.
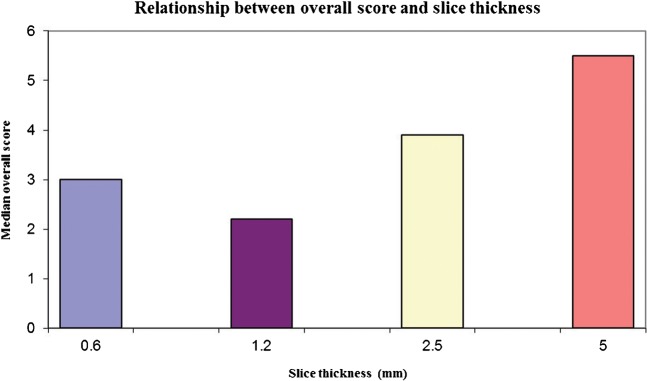
Slice thickness and overall score (a minimum value is seen for 1.2 mm slice thickness, indicating the optimum value).
Quantitative variables
No correlation was found between CT number of either bladder contents or bladder wall and slice thickness, tumour definition or overall score.
As slice thickness decreases, mean noise measured for bladder contents was observed to increase (Figure 6). This effect was not seen for noise ROI measured on the bladder wall. Increased noise also correlated with worsening dimple score (Figure 6). A trend for increasing noise (SDcontents) and reduced tumour definition (r2=0.13) and overall score (r2=0.17) was observed across all slice thicknesses. However, examining this relationship further for each slice thickness did not provide correlation between increased noise on the thinnest slices and reduced tumour definition score or overall score.
Figure 6.
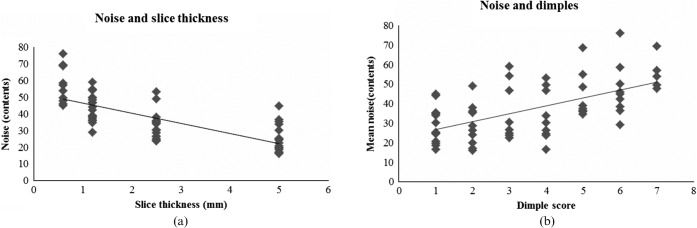
The relationship of image quality and noise: (a) noise increases with reducing slice thickness; (b) dimple score increases (worsens) as noise increases.
The mean CNR increased with increasing slice thickness. As with noise, a worsening dimple score was observed for higher CNR. For the thinnest slice thickness (0.6 mm) only, increased CNR was associated with better tumour definition and overall score.
Therefore, reducing the slice collimation provides a better tumour definition and an enhanced overall virtual cystoscopic image. However, at slice thickness <1.2 mm this effect is lost owing to the presence of increased noise. Noise is visualised on the virtual reconstructions as a dimpling artefact.
Discussion
We have identified several artefacts associated with CT VC and correlated the presence of these artefacts with image acquisition variables in order to determine the optimum scanning parameters for VC reconstruction. A slice thickness of 1.2 mm most commonly results in the best image quality. At thinner slice thickness (0.6 mm), increased noise may result in the appearance of dimpling artefact and the loss of tumour definition. Thus, although the thinner slice thickness of MDCT may improve tumour detection below 5 mm, there exists a noise-limited minimum thickness below which spatial resolution is not further enhanced.
This finding has not been widely reported in the virtual imaging literature. For virtual colonoscopy, where virtual imaging has been most widely investigated, the effect of thinner slice thickness has been studied predominantly in colonic phantoms [23-25]. Laghi et al [23] found the best image quality and sensitivity of lesion detection with 1.0 mm slice thickness, having studied a range of collimation (1.0–3.0 mm) and slice thickness (1.0–5.0 mm). Wessling et al [24] found that reduced tube current and thin section (1.25–2 mm) resulted in increased noise but that this did not impair colonic polyp detection. In a further study [26], they compared 4-section and 16-section CT with slice/reconstruction intervals of 1.25/0.8 mm and 0.75/0.7 mm, respectively. A non-significant difference between the 4-section (67% sensitivity) and 16-section (82.5% sensitivity) scans in detection of polyps <2 mm was seen. For lesions >3 mm there was no difference in detection rates between the scan protocols. An increase in detection of false-positive lesions was seen with 16-section CT (average 0.9 per reader versus average 0.5 per reader for 4-section CT). This is explained by increased noise on the thinnest sections resulting in image degradation. Their findings indicate that, as in our study, the best virtual images are not created by the thinnest slice thickness owing to the appearance of artefacts associated with increased noise. A clinical consensus statement on virtual colonoscopy recommends a collimation of <3 mm [18].
MDCT use for VC is also reported. Russell et al [20] investigated the effect of slice thickness using a four-channel MDCT on tumour detection (2–4 mm) in a bladder phantom. The thinnest slice thickness investigated of 2.5 mm showed the greatest sensitivity (93%) for detection of tumours 2 mm in size. Slice thicknesses of 3.75 and 5 mm were still able to detect lesions 3 and 4 mm in size. Using a 2.5 mm slice thickness in a clinical study, Scardapane et al [21] detected 100% of papillary lesions >5 mm and 60% of those <5 mm. A thinner slice thickness of 1.25 mm was used by Kim et al [19] to detect 56 of 59 lesions identified by conventional cystoscopy; 88% (15/17) of lesions <5 mm were correctly identified by VC. The thinnest slice thickness of 1 mm used in a clinical study was reported by Tsampoulas et al [22] with a detection rate of 96%, of which 18/55 lesions were <5 mm in size. These studies make no comment on any artefacts or problems encountered with virtual reconstructions at thinner slice thickness. They do, however, suggest that, as with virtual colonoscopy, with reducing slice thickness there is an improvement in detection rates of smaller lesions. Of note, none of these studies have used the thinnest collimation used in our study of 0.6 mm.
As well as CT scanning parameters, other factors play a role in the generation of an optimal VC image. The ability to generate an endoluminal view relies on achieving a large difference in density between the organ of interest and surrounding tissues. The bladder is therefore filled with air or contrast material via a catheter or delayed filling following oral or intravenous administration. For this study, CT images were taken following delayed intravenous injection of contrast when the patient reported a full bladder. A pitfall of this technique is that suboptimal bladder filling and sedimentation of contrast within the urine resulting in layering will generate suboptimal VC images. This effect was not observed in the cohort studied owing to attention to patient preparation by ensuring mixing of urine and contrast by patient motion, but it may explain why some series appear noisier at thinner collimation than others. The differences in image quality at thinner collimation could be due to the reduced difference in density at the mucosa–contrast interface.
Conclusion
A CT slice thickness of 1.2 mm appears optimal for tumour detection using VC, as thinner reconstructions may be noise limited, resulting in artefact.
Acknowledgments
Conflict of interest
This work was undertaken in The Royal Marsden NHS Foundation Trust, which received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive.
References
- 1.Collado A, Chechile GE, Salvador J, Vicente J. Early complications of endoscopic treatment for superficial bladder tumors. J Urol 2000;164:1529–32 [PubMed] [Google Scholar]
- 2.Hollenbeck BK, Miller DC, Taub D, Dunn RL, Khuri SF, Henderson WG, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006;106:1527–35 [DOI] [PubMed] [Google Scholar]
- 3.Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol 2005;174:2307–9 [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60 [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Park SY, Kim HS, Kim SH, Cho KS. Comparison of virtual cystoscopy, multiplanar reformation, and source CT images with contrast material-filled bladder for detecting lesions. AJR Am J Roentgenol 2005;185:689–96 [DOI] [PubMed] [Google Scholar]
- 6.Browne RF, Murphy SM, Grainger R, Hamilton S. CT cystography and virtual cystoscopy in the assessment of new and recurrent bladder neoplasms. Eur J Radiol 2005;53:147–53 [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt TM, Schmidl H, Philipp C, Allhoff EP, Rapp-Bernhardt U. Diagnostic potential of virtual cystoscopy of the bladder: MRI vs CT. Preliminary report. Eur Radiol 2003;13:305–12 [DOI] [PubMed] [Google Scholar]
- 8.Narumi Y, Kumatani T, Sawai Y, Kuriyama K, Kuroda C, Takahashi S, et al. The bladder and bladder tumors: imaging with three-dimensional display of helical CT data. AJR Am J Roentgenol 1996;167:1134–5 [DOI] [PubMed] [Google Scholar]
- 9.Song JH, Francis IR, Platt JF, Cohan RH, Mohsin J, Kielb SJ, et al. Bladder tumor detection at virtual cystoscopy. Radiology 2001;218:95–100 [DOI] [PubMed] [Google Scholar]
- 10.Fenlon HM, Bell TV, Ahari HK, Hussain S. Virtual cystoscopy: early clinical experience. Radiology 1997;205:272–5 [DOI] [PubMed] [Google Scholar]
- 11.Hussain S, Loeffler JA, Babayan RK, Fenlon HM. Thin-section helical computed tomography of the bladder: initial clinical experience with virtual reality imaging. Urology 1997;50:685–8 discussion: 689). [DOI] [PubMed] [Google Scholar]
- 12.Nambirajan T, Sohaib SA, Muller-Pollard C, Reznek R, Chinegwundoh FI. Virtual cystoscopy from computed tomography: a pilot study. BJU Int 2004;94:828–31 [DOI] [PubMed] [Google Scholar]
- 13.Prando A. CT-virtual endoscopy of the urinary tract. Int Braz J Urol 2002;28:317–22 [PubMed] [Google Scholar]
- 14.Tsili A, Tsampoulas C, Chatziparaskevas N, Silakos A, Kalef-Ezra J, Sofikitis N, et al. Computed tomographic virtual cystoscopy for the detection of urinary bladder neoplasms. Eur Urol 2004;46:579–85 [DOI] [PubMed] [Google Scholar]
- 15.Yazgan C, Fitoz S, Atasoy C, Turkolmez K, Yagci C, Akyar S. Virtual cystoscopy in the evaluation of bladder tumors. Clin Imaging 2004;28:138–42 [DOI] [PubMed] [Google Scholar]
- 16.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med 2005;142:635–50 [DOI] [PubMed] [Google Scholar]
- 17.Lui YW, Macari M, Israel G, Bini EJ, Wang H, Babb J. CT colonography data interpretation: effect of different section thicknesses – preliminary observations. Radiology 2003;229:791–7 [DOI] [PubMed] [Google Scholar]
- 18.Barish MA, Soto JA, Ferrucci JT. Consensus on current clinical practice of virtual colonoscopy. AJR Am J Roentgenol 2005;184:786–92 [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Ahn JH, Park T, Ahn HJ, Kim CS, Cho KS. Virtual cystoscopy of the contrast material-filled bladder in patients with gross hematuria. AJR Am J Roentgenol 2002;179:763–8 [DOI] [PubMed] [Google Scholar]
- 20.Russell ST, Kawashima A, Vrtiska TJ, LeRoy AJ, Bruesewitz MR, Hartman RP, et al. Three-dimensional CT virtual endoscopy in the detection of simulated tumors in a novel phantom bladder and ureter model. J Endourol 2005;19:188–92 [DOI] [PubMed] [Google Scholar]
- 21.Scardapane A, Pagliarulo V, Ianora AA, Pagliarulo A, Angelelli G. Contrast-enhanced multislice pneumo-CT-cystography in the evaluation of urinary bladder neoplasms. Eur J Radiol 2007;66:246–52 [DOI] [PubMed] [Google Scholar]
- 22.Tsampoulas C, Tsili AC, Giannakis D, Alamanos Y, Sofikitis N, Efremidis SC. 16-MDCT cystoscopy in the evaluation of neoplasms of the urinary bladder. AJR Am J Roentgenol 2008;190:729–35 [DOI] [PubMed] [Google Scholar]
- 23.Laghi A, Iannaccone R, Mangiapane F, Piacentini F, Iori S, Passariello R. Experimental colonic phantom for the evaluation of the optimal scanning technique for CT colonography using a multidetector spiral CT equipment. Eur Radiol 2003;13:459–66 [DOI] [PubMed] [Google Scholar]
- 24.Wessling J, Fischbach R, Meier N, Allkemper T, Klusmeier J, Ludwig K, et al. CT colonography: Protocol optimization with multi-detector row CT – study in an anthropomorphic colon phantom. Radiology 2003;228:753–9 [DOI] [PubMed] [Google Scholar]
- 25.Embleton KV, Nicholson DA, Hufton AP, Jackson A. Optimization of scanning parameters for multi-slice CT colonography: experiments with synthetic and animal phantoms. Clin Radiol 2003;58:955–63 [DOI] [PubMed] [Google Scholar]
- 26.Wessling J, Fischbach R, Borchert A, Kugel H, Allkemper T, Osada N, et al. Detection of colorectal polyps: comparison of multi-detector row CT and MR colonography in a colon phantom. Radiology 2006;241:125–31 [DOI] [PubMed] [Google Scholar]