Abstract
Objectives
: Haemophilic pseudotumour (HP) is an extremely rare lesion. The purpose of this study was to describe the CT and MRI features of maxillary bone HPs and introduce the key points to differentiate HP from the mimicking entities in the region.
Methods
: We retrospectively reviewed three paediatric patients with histology-proven HPs arising from the maxillary bone. All three patients underwent CT and/or MRI. Combined with six previously reported cases in the literature, the imaging features were comprehensively analysed.
Results
: All HPs showed a well-demarcated, multilobulated expansile osteolytic lesion in the maxillary bone. On non-enhanced CT, HPs appeared of mixed density relative to grey matter. The lesions appeared to have markedly heterogeneous signal intensity on both T1 and T2 weighted images, with septa-like enhancement following the administration of contrast material, which corresponded to blood products in various stages of evolution. The lesions caused cortical thinning and even focal disappearance and multiple bone septa were identified within the involved maxillary bone. Some HPs were associated with radiated periosteal proliferation, which can easily be misdiagnosed as a malignant bone tumour.
Conclusion
: A high index of suspicion for HP and a familiarity with imaging findings may help to accurately diagnose this rare entity.
Haemophilic pseudotumour (HP) is a rare and serious complication that occurs in 1–2% of patients with severe haemophilia, and most frequently develops in the femur, tibia, pelvic bones, small bones of the hand and, rarely, in the craniofacial bones [1-6]. It is essentially an encapsulated haematoma resulting from repetitive bleeding and is surrounded by a thick, fibrous capsule. To the best of our knowledge, a total of six cases of maxillary bone HPs have been reported in the literature to date [1,2,7-10]; unfortunately, the imaging findings were not described effectively. During the past 10 years, HPs in the maxillary bone have been confirmed by histopathology in three children at our hospital. The imaging findings of these three HPs were retrospectively reviewed and, combined with the literature, the value of using CT and MR imaging to diagnose, treat and follow up patients with HP in the maxillary bone was also discussed.
Methods and materials
This study was approved by the institutional review board. We retrospectively reviewed the CT and MRI findings of three patients with histology-proven HPs over a 10-year period (March 2000–July 2010) who were selected by review of clinical records. All three patients underwent surgical removal of HPs by endoscopic sinus surgery (ESS). The clinical presentations, surgical findings and histological diagnosis were extracted from the medical records.
All three patients initially presented to the otolaryngology clinic and underwent paranasal sinus CT. Images were acquired in both the axial and coronal planes using Siemens Somatom Plus 4 (Siemens Company, Berlin, Germany) or GE HiSpeed NX/i CT system (GE Healthcare, Waukesha, WI). The imaging parameters were as follows: voltage 120 KV, current 200 mA, matrix 512×512, section thickness 2 mm. Examinations were performed from the anterior wall of the frontal sinus to the posterior wall of the sphenoid sinus. Images were reconstructed using both bone algorithm (window width 1500 or 2000 HU at a window level of 150 or 200 HU) and soft-tissue algorithm (window width 400 HU, window level 40 HU).
Cases 1 and 2 underwent paranasal sinus MRI prior to surgery. The MR examinations were performed on a 1.5 T unit MR system (Signa; GE Healthcare) with a head coil. Fast spin-echo pulse sequences were used in these two patients. They underwent pre-enhanced T1 and T2 weighted images and Case 2 had also post-enhanced T1 weighted images in the axial, coronal and sagittal planes. The imaging parameters were as follows: T1 weighted images: repetition time (TR) 500–600 ms, echo time (TE) 10–15 ms; T2 weighted images: TR 3000–3500 ms, TE 120–130 ms; excitations 2–4; echo train length 11–27; matrix 256×256; field of view 20×20 cm; and section thickness 4–5 mm, intersection gap 0–0.5 mm. Post-contrast T1 weighted images with frequency-selective fat saturation were acquired in the optimal plane. Rapid manual bolus intravenous injection (2 ml s−1) of 0.1 mmol of gadopentetate dimeglumine (Magnevist®; Schering AG, Berlin, Germany) per kilogram of body weight was administered, followed by a 10-ml flush of normal saline solution.
The CT and MR images were evaluated by three experienced radiologists and findings were reached by consensus.
In addition, the clinical data, radiological features and treatment information of six HPs of the maxillary bone previously reported in the literature were reviewed.
Results
Case 1
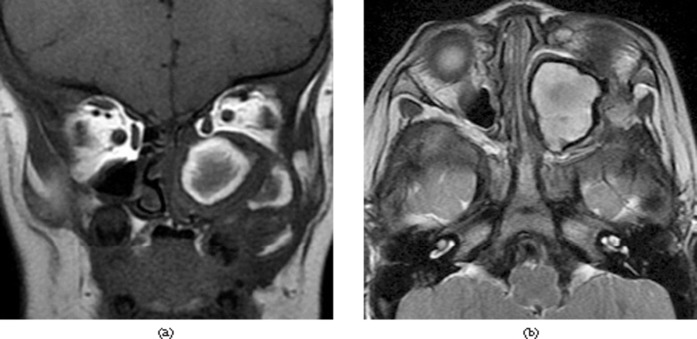
A 1-year-old male with moderate haemophilia B (2% factor IX) presented with a 1-month history of painless swelling of the left maxillary region and proptosis. There was no history of trauma to this region. Endoscopic examination showed a non-tender soft-tissue mass overlying alveolar process of the left maxillary bone. CT demonstrated a well-defined, multilobulated expansile osteolytic lesion occupying the left maxillary sinus with a mixed attenuation soft-tissue mass. Cortical thinning with peripheral sclerosis was also noted. The mass displayed mixed signal, with peripheral capsule appearing as low signal intensity on both MR T1 weighted and T2 weighted images (Figure 1). Prior to ESS, the patient was given adequate Factor IX replacement. The histopathological findings were consistent with HP. The patient was monitored every 6 months for 3 years, without evidence of recurrence.
Figure 1.
Case 1. (a) Coronal T1 weighted image shows mixed-signal soft tissue with multiple hyperintense signal areas. (b) Axial T2 weighted image demonstrates mainly hyperintense signal with hypointense signal rim of the lesion.
Case 2
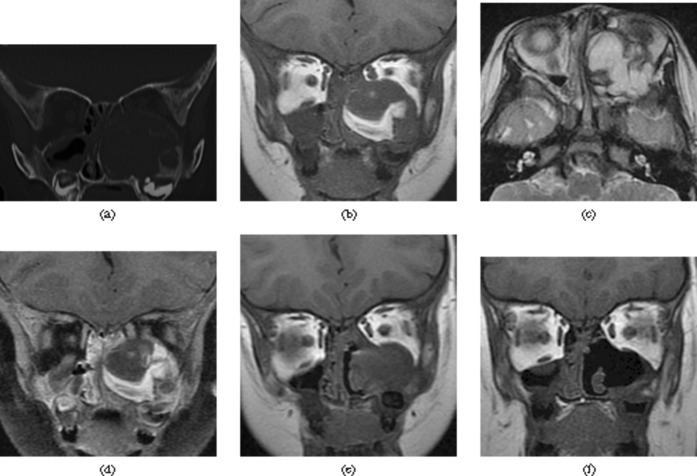
A 2-year-old male was admitted owing to left facial swelling and proptosis of 20 days' duration. The patient had left nasal obstruction, rhinorrhoea and intermittent facial pain. No association with a traumatic event was recollected. Clinical examination revealed left nasal cavity narrowing. Until just preparing for surgery, the patient was diagnosed with moderate haemophilia A (2% Factor VIII), based on the laboratory results. CT revealed a well-defined, multilobulated, expansile osteolytic lesion in the left maxillary bone with a heterogeneous attenuation soft-tissue mass. Diffuse cortical thinning with focal sclerosis, some septa-like structures crossing osteolytic zone and scattered flecks of calcification were also detected (Figure 2a). On MRI, the mass showed markedly heterogeneous signal intensity with fluid–fluid levels and peripherally surrounded by low-signal rim. The lesion displayed moderate nodular and linear contrast enhancement after contrast administration (Figure 2b–d). After administering adequate Factor VIII for 7 days, ESS was performed under general anaesthetic. The histopathological features were consistent with HP. 2 months later, the left maxillary sinus was found to have recurrent lesion on MRI (Figure 2e); thus, a second ESS was undertaken. Follow-up MRI 6 months after the second surgery revealed no evidence of recurrence (Figure 2f).
Figure 2.
Case 2. (a) Coronal CT image shows a well-defined, multilobulated soft-tissue mass with diffuse cortical thinning with focal sclerosis. (b) Coronal T1 weighted image shows a mixed-signal lesion with hyperintense signal areas. (c) The lesion has mainly hyperintense signal with hypointense signal rim on the axial T2 weighted image. (d) Coronal contrast-enhanced T1 weighted image with fat saturation shows moderate septa-like contrast enhancement of the lesion. (e) Coronal T1 weighted image shows a recurrent lesion in the primary site. (f) Coronal T1 weighted image shows no evidence of recurrence.
Case 3
A 2-year-old male was referred with progressive left nasal swelling for 2 months without recalling trauma. The patient suffered from left nasal obstruction, rhinorrhoea and intermittent epistaxis. Clinical examination showed left nasal bulging. CT showed bone destruction of the frontal and alveolar processes in the left maxillary bone, with a well-defined, lobulated soft-tissue mass. Cortex showed thinning with focal radiating periosteal proliferation. Fluid–fluid levels within the mass were also found (Figure 3). Before surgery, the laboratory results confirmed the patient as having moderate haemophilia A (4% Factor VIII). The lesion was totally excised by an extranasal approach under general anaesthesia. The surgical and histopathological findings suggested HP. Clinical and imaging follow-up every 6 months for 7 years showed no evidence of recurrence.
Figure 3.
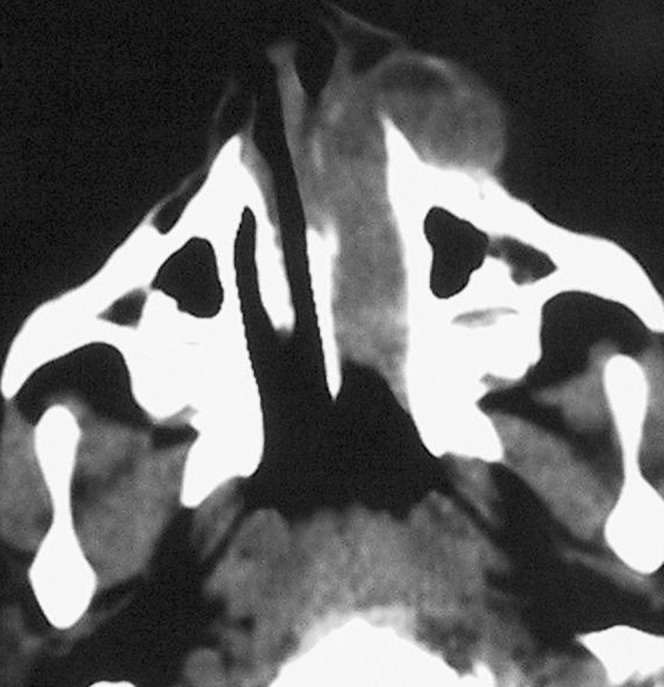
Case 3. Axial CT image shows a well-defined, lobulated soft-tissue mass with fluid–fluid levels in the left maxillary bone.
The six previously published cases of HP of the maxillary bone in the literature are summarised in Table 1.
Table 1. Six haemophilic pseudotumours of the maxillary bone reported in the literature.
| Author | Sex (M/F)/age (years) | History | Symptoms | Haemophilia | Laterality | Imaging findings | Treatment | Follow-up |
| de Sousa et al (1995) [1] | M/1 | Tibia HP, nasal trauma 6 years previously | None (incidental finding) | A (<1% Factor VIII) | Left | Radiolucent lesion (CT) | Curettage | Healing (2 years) |
| Zheng K (1997) [2] | M/26 | Tibia HP | None (incidental finding) | A (11% Factor VIII) | Left | Radiolucent lesion (radiograph) | Curettage | Not specified |
| Stevenson and Keast (2002) [8] | M/75 | COAD, chronic atrial fibrillation, aortic valve replacement surgery | Spontaneous epistaxis | No haemophilia | Right | Soft-tissue mass associated bone expansion and destruction (CT) | Curettage, radiotherapy | No recurrence (18 months) |
| Steele et al (2004) [7] | M/0.5 | None | Cheek swelling, eye tearing for 3 weeks | A (<1% Factor VIII) | Left | Hypodense mass with peripheral nodular enhancement (CT), hypointense lesion with hyperintense rim on T1 weighted image (MRI) | Curettage, Factor VIII therapy | Not specified |
| Lima et al (2008) [9] | M/12 | Bone cyst of the radius | Spontaneous gingival bleeding for 2 months | A (14% Factor VIII) | Left | Osteolytic lesion with bony cortical preservation, (panoramic radiography and CT) | Curettage, Factor VIII therapy | No recurrence (9 months) |
| Xue et al (2011) [10] | M/24 | None | Pain, dysfunction for 1 month | A (<5% Factor VIII) | Right | Soft-tissue lump with erosion bony cortex | Replacement therapy | Resolution |
COAD, chronic obstructive airway disease; F, female; HP, haemophilic pseudotumours; M, male.
Discussion
HP is generally considered to be associated with severe Factor VIII and IX deficiency. However, our three cases had moderate-factor deficiency. The pathogenesis of HP is speculated to be pressure necrosis secondary to recurrent bleeding into a closed subperiosteal or intramedullary space [11,12]. Trauma acts as an initiating factor in most cases, whereas our three cases and the five cases in the literature [2,7-10] had no such history, which indicates that maxillary HPs may occur even without trauma.
HPs are found almost exclusively in men between 20 and 70 years of age [3], whereas our three male patients were aged under 3 years. HP may cause no symptoms and remain unchanged for decades. The onset of symptoms takes between months and years [6]. However, in the case of maxillary HPs, the most common clinical manifestations are superior jaw painless swelling and intermittent epistaxis. In this series, the symptoms lasted less than 2 months at the time of diagnosis, which indicates that these lesions may progress rapidly. Interestingly, our three cases and four of the six cases reported in the literature occurred in the left maxillary bone.
According to the literature [1-4,7-10,12-15] and the present study, HPs of the maxillary bone tend to be well-defined, multilobulated, expansile osteolytic lesions, demonstrate mixed attenuation on non-enhanced CT and heterogeneous signal intensity on both T1 and T2 weighted MR images, with septa-like enhancement after contrast administration. The lesions usually result in cortical thinning and even focal disappearance with peripheral reactive sclerosis and multiple bone septa formation, accompanying radiated periosteal proliferation. The lesions may become extensive and lead to facial deformity. The lesions may occasionally show internally curvilinear or punctate calcifications or ossifications on CT, which may be dystrophic or sequestral in nature, as in Case 2. The characteristic MRI appearance of the maxillary HPs is a cystic lesion containing fluid components, which have complex signal intensities, representing blood products in various stages of evolution, with peripheral rim of dark signal intensity on all sequences, which is consistent with the fibrous capsule containing haemosiderin. As a valuable imaging feature, fluid–fluid levels have been described in aneurysmal bone cysts, giant cell tumour and so on. The sign occurring in Cases 2 and 3 may represent different layers secondary to repeated bleeding into the cystic cavity [15]. CT and MRI are also useful in the follow-up for patients after surgery, as well as in monitoring the treatment response.
Although some imaging features may suggest the diagnosis, they are not specific for HP in the region and may be misdiagnosed as bone tumour or infectious lesion, especially in those who have not established haemophilia diagnosis. The differential diagnoses primarily include osteosarcoma, Ewing's sarcoma, aneurysmal bone cyst, bony cyst, giant cell tumour and haemangioma. The above-mentioned key points may be helpful in differentiating from those lesions. Nevertheless, a specific diagnosis of HP in this region is usually only made with knowledge of the underlying disease.
In conclusion, although HP is extremely rare in the craniofacial bones, it can occasionally occur in the maxillary bone. Accurate recognition of the imaging features of HP combined with history and clinical manifestations can help to support the diagnosis. In the correct clinical setting, this condition should be considered in the differential diagnosis for a rapidly expanding soft-tissue mass in children.
References
- 1.de Sousa SO, de Piratininga J, Pinto Júnior DS, de Araújo N. Hemophilic pseudotumor of the jaws: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;79:216–19 [DOI] [PubMed] [Google Scholar]
- 2.Zheng K, Zheng P. Hemophilic pseudotumor involving maxilla and tibia. Chin Med J (Engl) 1997;110:233–5 [PubMed] [Google Scholar]
- 3.Stafford JM, James TT, Allen AM, Dixon LR. Hemophilic pseudotumor: radiologic-pathologic correlation. Radiographics 2003;23:852–6 [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Ryu KN. Hemophilic pseudotumor involving the musculoskeletal system: spectrum of radiologic findings. AJR Am J Roentgenol 2004;183:55–61 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Merchan EC. Haemophilic cysts(pseudotumours). Haemophilia 2002;8:393–401 [DOI] [PubMed] [Google Scholar]
- 6.Rey EA, Puia S, Bianco RP, Pinto MT. Haemophilic pseudotumour of the mandible: report of three cases. Int J Oral Maxillofac Surg 2007;36:552–5 [DOI] [PubMed] [Google Scholar]
- 7.Steele NP, Myssiorek D, Zahtz GD, Diamond A. Pediatric hemophilic pseudotumor of the paranasal sinus. Laryngoscope 2004;114:1761–3 [DOI] [PubMed] [Google Scholar]
- 8.Stevenson DS, Keast AT. An unusual cause of epistaxis: a haemophilic pseudotumor in a non-haemophilic, arising in a paranasal sinus. J Laryngol Otol 2002;116:294–5 [DOI] [PubMed] [Google Scholar]
- 9.Lima GS, Robaina TF, de QueirozChavesLourenço S, Dias EP. Maxillary hemophilic pseudotumor in a patient with mild hemophilia A. J Pediatr Hematol Oncol 2008;30:605–7 [DOI] [PubMed] [Google Scholar]
- 10.Xue F, Sun C, Sui T, Zhang L, Jiang L, Yang R. Hemophilic pseudotumor in Chinese patients: a retrospectively single-centered analysis of 14 cases. Clin Appl Thromb Hemost 2011;17:279–82 [DOI] [PubMed] [Google Scholar]
- 11.Liu SS, White WL, Johnson PC, Gauntt C. Hemophilic pseudotumor of the spinal canal. Case report. J Neurosurg 1988;69:624–7 [DOI] [PubMed] [Google Scholar]
- 12.Wilson DA, Prince JR. MR imaging of hemophilic pseudotumors. AJR Am J Roentgenol 1988;150:349–50 [DOI] [PubMed] [Google Scholar]
- 13.Hermann G, Gilbert MS, Abdelwahab F. Hemophilia: evaluation of musculoskeletal involvement with CT, sonography, and MR imaging. AJR Am J Roentgenol 1992;158:119–23 [DOI] [PubMed] [Google Scholar]
- 14.Geyskens W, Vanhoenacker FM, Van derZijden T, Peerlinck K. MR imaging of intra-osseous hemophilic pseudotumor: case report and review of the literature. JBR-BTR 2004;87:289–93 [PubMed] [Google Scholar]
- 15.Kale HA, Rathod KR, Prasad SR, Madiwale CM, Sheth RJ. Mandibular haemophilic pseudotumour containing a fluid-fluid level. Br J Radiol 2001;74:186–8 [DOI] [PubMed] [Google Scholar]