Abstract
Objective
The aim of the study was to evaluate the potential role of fludeoxyglucose (FDG)-positron emission tomography (PET)/CT in the detection of bone/bone marrow disease in patients with Hodgkin's lymphoma (HL).
Methods
We retrospectively reviewed (18F)-FDG-PET/CT scans of 122 newly diagnosed, biopsy-proven cases of HL performed between November 2009 and June 2010. All the patients were staged before treatment by both PET/CT and bone marrow biopsy (BMB). Patients were subdivided into three groups based on the findings of FDG-PET/CT. Group A consisted of patients showing diffuse FDG uptake, Group B consisted of patients showing unifocal FDG uptake and Group C patients showed multifocal FDG-avid foci on PET/CT scans. Bone marrow results were also reviewed and considered positive if lymphomatous involvement was detected on bone marrow trephine biopsy. BMB results were correlated with FDG-PET/CT findings.
Results
There were 122 patients in total—81 (66.4%) were male and 41 (33.6%) were female. The age range was from 6 years to 78 years (mean 35.70 years). PET/CT was reported as negative for bone/bone marrow involvement in 85 (69.7%) patients, while the remaining 37 showed abnormal FDG uptake. The sensitivity of FDG-PET/CT was calculated to be 100%, the specificity was 76.57%, the negative predictive value was 76.57%, the positive predictive value was 29.72% and the diagnostic accuracy was 78.62%.
Conclusion
18F-FDG-PET/CT and BMB are complementary in the evaluation of bone marrow disease.
Fluorine-18 (18F)-fludeoxyglucose (FDG) has found widespread use in the diagnosis and staging work-up of lymphomas. One of the most promising applications is in the determination of clinical stage of disease at presentation or recurrence [1]. Accurate staging is essential for planning an effective treatment regimen and minimising side effects and toxicity [2]. Bone marrow infiltration is of prime importance not only in staging the disease but also in the tailoring of treatment protocols [3]. Bone involvement can result from haematogenous spread or by extension from adjacent soft tissues [4,5]. Bone marrow involvement in patients with lymphoma is considered as a sign of generalised disease and with less favourable prognosis. Bone marrow biopsy (BMB) is the established method for the detection of bone marrow infiltration. BMB is generally safe but should not be considered as a risk-free procedure; adverse events (haemorrhage, infection etc) have been reported in about 0.12% of cases [6]. It is an invasive and painful experience for the patients and it sometimes results in only a small sample which may turn out to be inconclusive. Bone marrow involvement is diagnosed in 50–80% of patients with low-grade non-Hodgkin's lymphoma (NHL), 25–40% of those with high-grade NHL and 5–14% of those with Hodgkin's lymphoma (HL) [6,7]. Lymphoma staging is based on Ann Arbor classification with Cotswolds modifications [8], which includes CT and BMB. Radiologically, CT may depict cortical bone changes but has low sensitivity for early bone marrow involvement [8,9]. Unilateral or bilateral BMB of the dorsal iliac crest is considered as the standard method for detecting bone marrow involvement complemented by MRI when needed [2,10-12]. The potential role of FDG-positron emission tomography (PET)/CT is yet to be determined for the assessment of bone marrow involvement, as very few systematic studies have been carried out in this regard. Since the advent of FDG-PET/CT, functional imaging has emerged as an important imaging tool in differentiating viable tumour tissue from necrotic and therapy-induced fibrosis [13,14]. The aim of the current study was to correlate BMB and PET/CT results as part of baseline staging work-up and to assess the clinical utility of FDG-PET/CT in the detection of bone/bone marrow disease.
Methods and materials
Patients
A retrospective study was conducted, reviewing 122 biopsy-proven and untreated patients with HL who underwent 18F-FDG-PET/CT scanning between November 2009 and June 2010. All the patients were staged before treatment by both PET/CT and BMB. The routine lymphoma staging at our institution involves whole-body contrast-enhanced PET/CT along with iliac crest marrow aspirate (bilateral) and trephine biopsy. In all patients, PET/CT was performed within 2 weeks of the marrow biopsy as a baseline scan followed by a follow-up PET/CT (either mid treatment or end of therapy as per oncologist's protocol). The repeat or follow-up PET/CT scan was carried out to assess the response to therapy.
Marrow histology
Trephine biopsy samples were decalcified and stained with haematoxyline and eosine (Gordon and Sweet's reticulin method) [15]. Accompanied marrow aspirates were stained with the May–Grunwald/Giemsa stain. All trephine biopsies were phenotyped with CD20 (B cell marker), CD3 (T cell marker), CD15 and CD30 (markers for Reed–Sternberg cells) and leukocyte common antigens (CD45). The marrow infiltration for Hodgkin's lymphoma was interpreted by two pathologists who were blinded to the PET/CT results.
18F-FDG-PET/CT scanning
PET/CT studies were obtained using a Philips Gemini TF (Philips Healthcare, Eindhoven, Netherlands), combining a germaniumoxyorthosilicate-based PET scanner and a 16-slice CT scanner. After fasting for 4–6 h and with a blood glucose level of <150 mg dl–1 at the time of injection, patients were injected with 5 MBq kg–1 of 18F-FDG (maximum 400 MBq). A 18F-FDG-PET/CT scan was acquired 60 min post injection from head up to mid-thigh level. A CT scan was performed first with intravenous contrast using a current of 100–150 mA and was taken as a diagnostic scan. On completion of the CT scan, PET study was acquired by advancing the patient couch into the field of view and acquiring multibed (8–10 positions) over the same range as the CT scan. CT images were used for attenuation correction of the 18F-FDG-PET emission data as well for exact anatomical localisation. PET images were reconstructed using a three-dimensional row action maximum likelihood algorithm.
Analysis of bone/bone marrow FDG uptake
In this study, the intensity and distribution of FDG activity within the bone/bone marrow was visually scored independently by two experienced nuclear medicine physicians and two radiologists. The marrow was assumed to be abnormal when the uptake was equal to or greater than the liver uptake, taking into account that the liver uptake was more than the background. The PET/CT scan was reported as positive (having focal or diffuse tracer uptake in the bone/bone marrow that could not be explained by benign findings on underlying CT or clinical history). The number and location of FDG-avid lesions in the bone/bone marrow were also noted as well as the underlying sclerotic or osteolytic abnormality found on the CT bone windows. The negative PET/CT scan did not show any abnormal FDG uptake in the bone/bone marrow. The BMB was taken as the gold standard in calculating the sensitivity of 18F-FDG in detecting bone/bone marrow involvement by lymphoma.
Patients were broadly divided into three groups based on the pattern and number of FDG avid focal lesions:
Group A: intense homogeneous FDG uptake in the axial and proximal appendicular skeletons.
Group B: unifocal (one or two sites) of increased FDG uptake in the bone/bone marrow.
Group C: multifocal (at least three sites) of increased FDG uptake in the bone/bone marrow.
True-positives were patients with a positive BMB and PET/CT scan. True-negatives were patients with a negative BMB and PET/CT scan. False-positive cases were those with positive PET/CT and negative BMB. False-negative cases were those with negative PET/CT and positive BMB.
Ethical considerations
BMB and whole-body contrast-enhanced PET/CT is part of the routine clinical work-up for the established diagnosis of HL at Shaukat Khanum Memorial Cancer Hospital & Research Center. All the patients were informed and written consent was obtained, explaining the need for this investigation and treatment. All the data were reviewed retrospectively, which complies with the institutional laws. None of the authors had any conflict of interest.
Results
There were 122 patients in total—81 (66.4%) were male and 41 (33.6%) were female. The age range was from 6 to 78 years (mean 35.70 years). There were 114 patients with classic HL subtypes (92 mixed cellularity and 22 nodular sclerosis), while 8 patients had lymphocyte predominant HL.
PET/CT was reported as negative for bone/bone marrow involvement in 85 (69.7%) patients as no abnormal FDG uptake was noted; all of these patients also had negative BMB results and were regarded as true negatives. The remaining 37 (30.3%) patients showed abnormal FDG uptake, independent of the BMB results.
Group A
11/37 (29.7%) patients with diffuse bone/bone marrow FDG uptake were included in this group. No radiological correlate was identified on the CT component in any of these patients. Only 2 out of 11 patients showed BMB evidence of disease involvement (Figure 1). Follow-up PET/CT performed in all Group A patients showed normal FDG uptake in the bone/bone marrow.
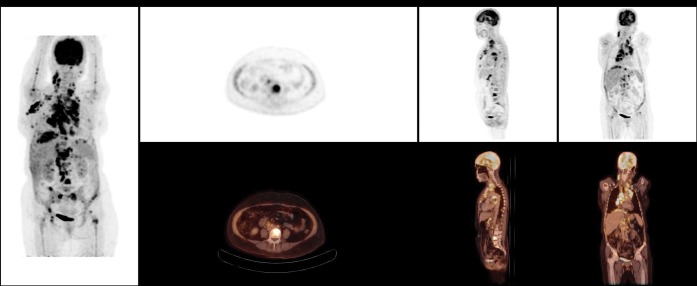
Figure 1.
Diffuse homogenous fludeoxyglucose (FDG) uptake in the axial and proximal appendicular skeletons in the maximal intensity projection image. FDG-avid disease is identified in bilateral cervical regions, the right axilla and the spleen. Bone marrow biopsy was negative in this patient.
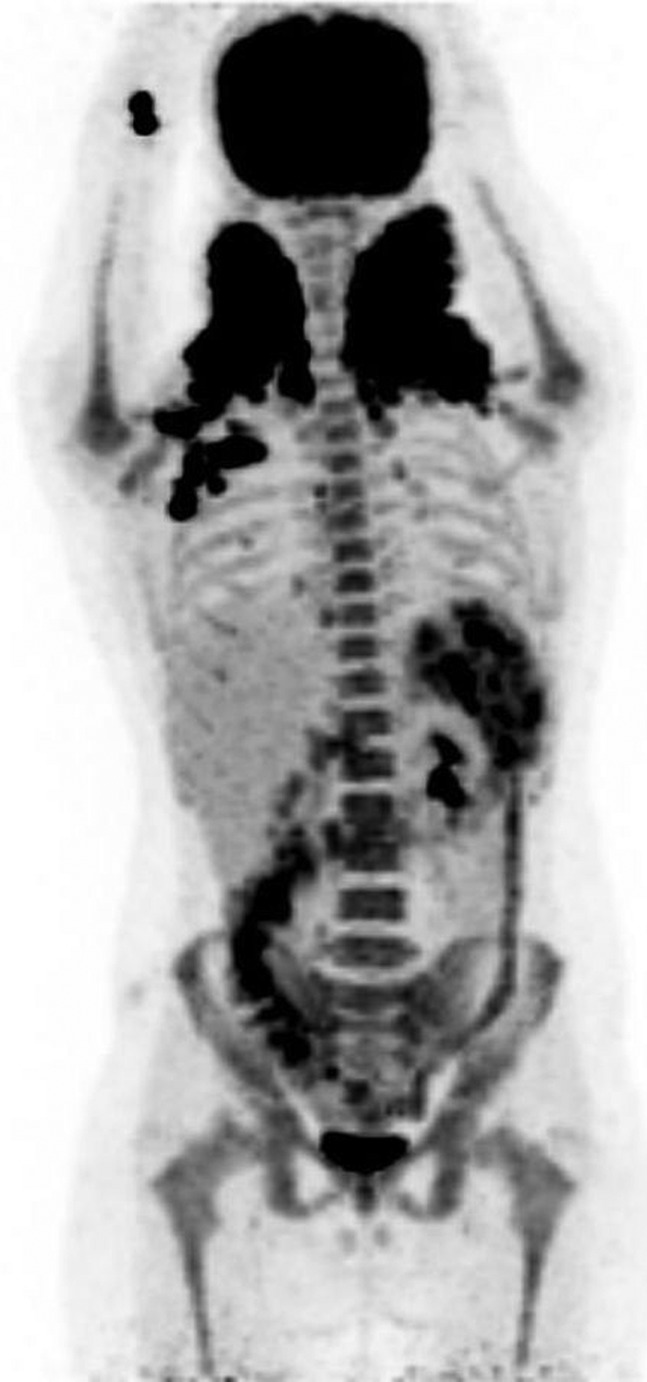
Group B
6/37 (16.2%) patients showed unifocal FDG abnormality in the bone/bone marrow. Only one of these six (16.6%) patients had positive BMB. A follow-up PET/CT scan of this patient showed normal FDG uptake. Another patient from this group, who had a negative BMB at baseline, later developed FDG-avid disease progression. CT correlate of FDG-avid unifocal bony uptake was found in three patients. Figure 2 shows an example of discordance between CT and PET (right ischium) while Figure 3 shows a concordant lesion in thoracic vertebra.
Figure 2.
Fluorine-18-fludeoxyglucose (18F-FDG)-positron emission tomography/CT shows an FDG-avid focus in the right ischium in the maximal intensity projection and transaxial fusion images. The corresponding CT image shows no matching abnormality. FDG-avid disease identified in the left supraclavicular region, the left axilla and the spleen. Bone marrow biopsy was negative in this patient.
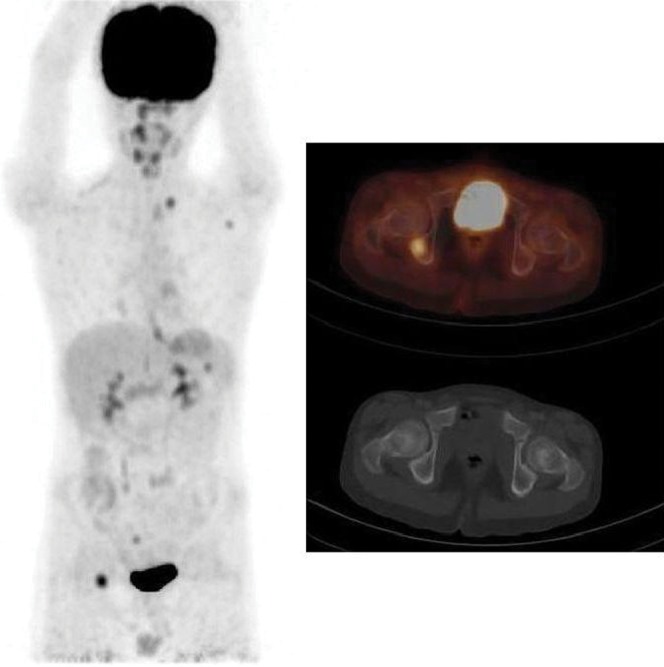
Figure 3.
Sclerosed thoracic vertebra shows abnormal increased fludeoxyglucose uptake, consistent with lymphomatous involvement. Bone marrow biopsy was positive for lymphomatous involvement in this patient.
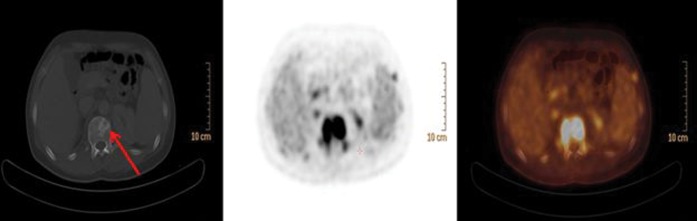
Group C
20/37 (54.1%) patients showed multifocal FDG abnormalities. 8 (40.0%) out of these 20 had positive BMB. CT correlate of FDG-avid bone/bone marrow uptake was found in 12/20 (60.0%) patients (Figure 4). Follow-up PET/CT scans showed progressive disease in 2/20 patients, 1 of these had a positive BMB at baseline. The remaining 18 patients showed normal FDG uptake in the bone/bone marrow on follow-up PET/CT scanning.
Figure 4.
Fludeoxyglucose-positron emission tomography/CT shows multiple hypermetabolic bone lesions involving mainly the axial skeleton, the ribs, the proximal humeri and both femurs. Bone marrow biopsy was negative in this patient.
Table 1 gives an elaborate overview of the three groups. All the patients were clinically followed, the median follow-up was 7 months (range 3–12 months). The sensitivity of FDG-PET/CT was calculated to be 100%, the specificity was 76.57%, the negative predictive value was 76.57%, the positive predictive value was 29.72% and the diagnostic accuracy in detecting bone/bone marrow disease in HL patients was 78.62%.
Table 1. Positron emission tomography (PET)/CT positive groups.
| Group | PET positive | BMB positive | CT correlate |
| A | 11/37 | 2/11 | 0/11 |
| B | 6/37 | 1/6 | 3/6 |
| C | 20/37 | 8/20 | 12/20 |
BMB, bone marrow biopsy.
The results of PET/CT vs BMB are summarised in a 2×2 table (Table 2). Table 3 gives detailed data of all 37 patients with positive bone/bone marrow findings on PET/CT, independent of BMB results.
Table 2. Summary of bone marrow biopsy (BMB) and positron emission tomography (PET)/CT results in 122 patients with Hodgkin's lymphoma.
| BMB status |
||
| PET/CT status | BMB positive (n=11) | BMB negative (n=111) |
| Positive (n=37) | 11 (TP) | 26 (FP) |
| Negative (n=85) | 0 (FN) | 85 (TN) |
FN, false-negative; FP, false-positive; TN, true-negative; TP, true-positive.
Table 3. Patients with positive bone/bone marrow findings on fluorine-18 (18F)-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT.
| Number | Sex | Age (years) | H/P | Stage | BMB | FDG-avid foci | CT: bone findingsa | Response PET | Follow-up (months) | Status |
| 1 | M | 21 | MC | IV-B/LS | + | Diffuse | +ve CT | CMR | 6 | CR |
| 2 | M | 48 | MC | IV-BLS | – | Multifocal | +ve CT | CMR | 8 | CR |
| 3 | M | 59 | MC | IV-BS | – | Multifocal | +ve CT | PD | 3 | Died |
| 4 | M | 18 | MC | III-S | – | Multifocal | +ve CT | CMR | 10 | CR |
| 5 | M | 66 | NS | II-A | – | Unifocal femur | +ve CT | CMR | 4 | CR |
| 6 | M | 10 | MC | IV-S | + | Multifocal | +ve CT | PD | 9 | Che |
| 7 | F | 21 | MC | IV-BLS | – | Unifocal D-12 | –ve CT | CMR | 12 | CR |
| 8 | M | 53 | MC | II-B | – | Diffuse | –ve CT | CMR | 7 | CR |
| 9 | M | 40 | MC | IV-BSX | – | Multifocal | –ve CT | CMR | 6 | CR |
| 10 | F | 28 | NS | III-AS | – | Diffuse | –ve CT | CMR | 10 | CR |
| 11 | F | 35 | NS | III-BS | – | Unifocal L-1 | –ve CT | CMR | 8 | CR |
| 12 | M | 28 | NS | III-B | – | Multifocal | +ve CT | CMR | 6 | CR |
| 13 | M | 23 | NS | III-B | – | Diffuse | –ve CT | CMR | 6 | CR |
| 14 | M | 45 | MC | III-AS | – | Unifocal | –ve CT | CMR | 6 | CR |
| 15 | M | 40 | MC | III-S | – | Diffuse | –ve CT | CMR | 7 | CR |
| 16 | M | 43 | MC | IV-BLS | – | Multifocal | –ve CT | CMR | 6 | PR |
| 17 | M | 06 | MC | III-S | – | Multifocal | –ve CT | CMR | 9 | CR |
| 18 | M | 12 | MC | IV-LS | + | Multifocal | +ve CT | CMR | 8 | CR |
| 19 | M | 28 | MC | II-B | – | Diffuse | –ve CT | CMR | 10 | CR |
| 20 | F | 50 | MC | IV-S | – | Multifocal | –ve CT | CMR | 9 | CR |
| 21 | M | 15 | MC | III-S | – | Diffuse | –ve CT | CMR | 6 | CR |
| 22 | M | 21 | LP | IV | + | Unifocal ischium | +ve CT | CMR | 9 | CR |
| 23 | F | 24 | NS | II-AX | – | Diffuse | –ve CT | CMR | 6 | CR |
| 24 | M | 17 | MC | II-A | – | Diffuse | –ve CT | CMR | 10 | CR |
| 25 | F | 14 | MC | IV-L | + | Multifocal | –ve CT | CMR | 6 | CR |
| 26 | F | 17 | MC | IV-L | – | Unifocal ilium | +ve CT | PD | 6 | Che |
| 27 | M | 19 | MC | IV-L | – | Multifocal | –ve CT | CMR | 7 | CR |
| 28 | M | 22 | NS | IV-BS | – | Multifocal | +ve CT | CMR | 9 | CR |
| 29 | M | 23 | NS | IV-LS | + | Multifocal | –ve CT | CMR | 12 | CR |
| 30 | M | 11 | MC | III-S | – | Multifocal | +ve CT | CMR | 6 | CR |
| 31 | F | 24 | NS | IV-BL | + | Multifocal | –ve CT | CMR | 8 | CR |
| 32 | F | 29 | MC | III-A | – | Multifocal | +ve CT | CMR | 7 | CR |
| 33 | M | 49 | NS | IV-BS | + | Multifocal | +ve CT | CMR | 6 | CR |
| 34 | M | 30 | MC | IV-BS | + | Multifocal | +ve CT | CMR | 6 | CR |
| 35 | M | 26 | NS | II-BX | – | Diffuse | +ve CT | CMR | 6 | CR |
| 36 | M | 15 | MC | IV-BS | + | Multifocal | +ve CT | CMR | 8 | CR |
| 37 | M | 64 | MC | IV-BS | + | Diffuse | –ve CT | CMR | 12 | CR |
A, without B symptoms; B, B symptoms (fever, night sweats, weight loss); Che, second-line chemotherapy; CMR, complete metabolic response; CR, complete remission of Hodgkin's lymphoma; F, female; L, liver involvement; LP, lymphocyte predominant; M, male; MC, mixed cellularity; NS, nodular sclerosis; PD, progressive disease; PR, partial response; S, spleen involvement; X, bulky disease (>10 cm); −ve, negative CT of PET/CT; +ve, positive CT of PET/CT.
Discussion
PET/CT is being exclusively used in lymphomas for baseline staging work-up and response evaluation. PET has a well-established role in the staging as well as restaging of patients with HL and aggressive NHL [16-18]. It has been found that FDG-PET exhibits higher sensitivity than CT in the evaluation of lymph node involvement as disease involvement is picked up in lymph nodes considered normal by CT criteria [3,19,20]. The prevalence of bone marrow involvement is <1% among patients with early-stage disease, and hence BMB is not routinely recommended in such patients in the absence of adverse prognostic factors [21-24]. The role of PET/CT in the evaluation of bone/bone marrow disease is still emerging as very few studies have evaluated this [1,2]. BMB is considered the gold standard for bone marrow involvement; however, the approach of unilateral vs bilateral BMB has limitations that need to be taken into account. Brunning et al have shown that in patients with a positive biopsy for HL, only one side is involved in about 40% of cases and a unilateral biopsy would miss 20% of cases compared with a bilateral biopsy [25]. In the present study, all the patients underwent bilateral BMB and 26 patients showed bone/bone marrow uptake on 18F-FDG-PET/CT scan with negative BMB. In every case, the iliac crest had no FDG-avid foci. There have been very few studies that examine the sensitivity of 18F-FDG-PET/CT for bone marrow involvement in a pure population of HL [9]. The meta-analysis by Pakos et al [1] summarising the results of 13 studies comprising a total of 587 patients showed that compared with BMB, the weighted sensitivity and specificity of FDG-PET were 51% and 91%, respectively. In the sub-group analysis, BMB showed better sensitivity in HL (76%) and in aggressive NHL, while FDG-PET gave false negative results in two-thirds of patients with bone marrow involvement in more indolent histological forms of NHL, leading to the conclusion that the role of PET/CT would be complementary [26-33]. Fuster et al [34] investigated the role of FDG-PET vs bone marrow disease in detecting bone marrow disease in patients with HL and NHL and found out that FDG-PET was more sensitive than BMB in HL and NHL with the exception of Grade 1 and 2 follicular lymphomas. Their findings were consistent with those of Pakos et al [1] regarding the overall accuracy and high sensitivity of PET compared with BMB. In our retrospective study, we found that the sensitivity of FDG-PET/CT is 100% in picking up bone/bone marrow disease while the specificity was found to be 76.57%. The negative predictive value was 76.57%, the positive predictive value was 29.72% and the diagnostic accuracy was 78.62%. The high sensitivity in this study was due to the fact that all the patients with a positive BMB had a positive PET/CT scan as well. It has been cited in literature that diffuse homogeneous FDG uptake in the axial and appendicular skeletons often reflects benign enhancement owing to inflammation or cytokine release; however, bone marrow involvement cannot be entirely excluded [9]. In our study we found that only 2/11 patients with diffuse skeletal uptake had a positive BMB. All 11 patients showing diffuse FDG uptake showed normal bone/bone marrow uptake on the follow-up PET/CT scan. These findings highlight that homogeneous increased FDG uptake is a non-specific finding that could be due to resolution of cytokine-induced reactive marrow. With negative BMB which is considered the gold standard, diffuse bone/bone marrow FDG uptake should not be interpreted as disease involvement. In the present study, we found that 85/122 patients had a negative PET/CT scan for bone/bone marrow involvement along with negative BMB, which suggests that routine BMB might be unnecessary when 18F-FDG-PET/CT is negative. It has been reported that focal mono or polyostotic bone marrow disease may produce abnormal FDG-PET/CT scans, but if the disease does not extend to the dorsal iliac crests then bone marrow sampling would be negative [2]. In our study, 26 patients with negative bilateral BMB had uni- or multifocal abnormal bone/bone marrow FDG uptake. When divided into subgroups, 12/20 patients with multifocal abnormal FDG uptake and 5/6 patients with unifocal abnormal FDG uptake had negative BMB. These findings suggest that the probability of bone marrow involvement is low with a solitary FDG abnormality, whereas multifocal FDG abnormalities have a greater likelihood of yielding a positive BMB. To establish this concept, FDG-avid sites, other than the conventional site of BMB (i.e. posterior iliac spine in the pelvis) should be biopsied and characterised histopathologically [27]. However, this was beyond the scope of our current study. The use of combined PET/CT is helpful for correlating bone FDG uptake with CT findings and improves specificity [35]. In our study, CT was able to identify corresponding bony abnormality in 17/37 (46%) patients with abnormal bone/bone marrow FDG uptake. Routine reading of CT has a low yield in depicting bone/bone marrow lesions. Schaefer et al examined a selected population of 50 lymphoma patients (22 HL) with FDG-avid bone lesions on PET/CT and found only 32 of the 193 lesions on CT without the PET information [24]. In our study, we decided to report bone and bone marrow lesions together and did not try to make a distinction between bony involvement and bone marrow involvement in every patient and for each lesion. Even with FDG-PET/CT, it would be difficult to make a clear distinction in every patient and each lesion as it is known that one type of invasion may lead to the other by local spread, making distinction less easy, and sometimes both types of lesion do coexist at the same time [9]. In the meta-analysis carried by Pakos et al [1], the number of biopsies directed at PET/CT-positive lesions is low. In our cohort, only 1 (17%) out of 6 patients with unifocal FDG abnormality had positive BMB in contrast to 8/12 (67%) patients with multifocal FDG abnormalities. This observation highlights the significance of the “pattern of FDG abnormality” in the likelihood of bone/bone marrow disease involvement [27]. However, as no FDG-avid bone lesion was biopsied, the specificity of these findings cannot be calculated. “Response to treatment”, as seen on follow-up PET/CT, is also not a suitable criterion from which to draw a conclusion because, with the exception of 4/26, all patients with focal bone lesions and negative BMB had non-skeletal evidence of Stage IV disease and were treated accordingly. As BMB was the gold standard of staging and treatment in our cohort, the treatment strategy was not altered based on FDG uptake in the bone/bone marrow. MRI is a useful technique for assessing bone marrow [36]; however, so far the role of whole-body MRI is not well-established when compared with whole-body PET/CT.
Our study confirms the observation of many authors cited earlier that PET/CT has a high negative predictive value and is a useful tool in the evaluation of the bone/bone marrow along with BMB. Our findings support the emerging evidence that normal bone/bone marrow FDG uptake may eventually obviate the need for BMB. Additionally, the significance of multifocal abnormal bone/bone marrow FDG uptake, highlighted by cases of discordance between FDG uptake and BMB findings, warrants further investigation. Future studies might seek to confirm discordant findings with correlative imaging or targeted biopsy.
Conclusion
18F-FDG-PET/CT plays a complementary role with BMB in the staging of patients with HL. However, prospective studies are needed to further determine and validate the potential role of FDG-PET/CT in this arena.
References
- 1.Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med 2005;46:958–63 [PubMed] [Google Scholar]
- 2.Pelosi E, Penna D, Deandreis D, Chiappella A, Skanjeti A, Vitolo U, et al. FDG-PET in the detection of bone marrow disease in Hodgkin's disease and aggressive non-Hodgkin's lymphoma and its impact on clinical management. Q J Nucl Med Mol Imaging 2008;52:9–16 [PubMed] [Google Scholar]
- 3.Stumpe KD, Urbinelli M, Steinert HC, Glanzmann C, Buck A, von Schulthess GK. Whole body positron emission tomography using fluorodeoxyglucose for staging of lymphoma: effectiveness and comparison with computed tomography. Eur J Nucl Med Mol Imaging 1998;25:721–8 [DOI] [PubMed] [Google Scholar]
- 4.Edeiken-Monroe B, Edeiken J, Kim EE. Radiological concepts of lymphoma of bone. Radiol Clin North Am 1990;28:841–64 [PubMed] [Google Scholar]
- 5.Guermazi A, Brice P, de Kerviler EE, Fermé C, Hennequin C, Meignin V, et al. Extranodal Hodgkin disease: spectrum of disease. Radiographics 2001;21:161–79 [DOI] [PubMed] [Google Scholar]
- 6.Ponzoni M, Ciceri F, Crocchiolo R, Famoso G, Doglioni C. Isolated bone marrow occurrence of classic Hodgkin's lymphoma in an HIV-negative patient. Haematologica 2006;91:ECRO4. [PubMed] [Google Scholar]
- 7.Gronich N, Radnay J, Shapiro H, Manor Y, Lahav M, Lishner M. Clinical outcome of low-grade NHL patients with bone marrow involvement. Eur J Clin Invest 2007;37:305–9 [DOI] [PubMed] [Google Scholar]
- 8.Levis A, Pietrasanta D, Godio L, Vitolo U, Ciravegna G, Di Vito F, et al. A large-scale study of bone marrow involvement in patients with hodgkin's lymphoma. Clin Lymphoma 2004;5:50–5 [DOI] [PubMed] [Google Scholar]
- 9.Gerard Moulin R, Elif H, Xaviar C, Pauline B, Didier D, Myriam B, et al. F18-FDG PET/CT bone/bone marrow findings in Hodgkin's lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging 2010;37:1095–105 [DOI] [PubMed] [Google Scholar]
- 10.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–6, [Erratum in: J Clin Oncol 1990;8:1602] [DOI] [PubMed] [Google Scholar]
- 11.Franco V, Tripodo C, Rizzo A, Stella M, Florena AM. Bone marrow biopsy in Hodgkin's lymphoma. Eur J Haematol 2004;73:149–55 [DOI] [PubMed] [Google Scholar]
- 12.Gossmann A, Eich HT, Engert A, Josting A, Müller RP, Diehl V, et al. CT and MR imaging in Hodgkin's disease - present and future. Eur J Haematol Suppl 2005;66:83–9 [DOI] [PubMed] [Google Scholar]
- 13.Brennan DD, Gleeson T, Coate LE, Cronin C, Carney D, Eustace SJ. A comparison of whole-body MRI and CT for the staging of lymphoma. AJR Am J Roentgenol 2005;185:711–16 [DOI] [PubMed] [Google Scholar]
- 14.Spaepen K, Stroobants S, Dupont P, Thomas J, Vandenberghe P, Balzarini J, et al. Can positron emission tomography with [(18)F]-fluorodeoxyglucose after first-line treatment distinguish Hodgkin's disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity? Br J Haematol 2001;115:272–8 [DOI] [PubMed] [Google Scholar]
- 15.Gordon H, Sweet HH. A simple method for the silver impregnation of reticulin. American Journal of Pathology 1936;12:545. [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86 [DOI] [PubMed] [Google Scholar]
- 17.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–8 [DOI] [PubMed] [Google Scholar]
- 18.Kirby AM, Mikhaeel NG. The role of FDG PET in the management of lymphoma: What is the evidence base? Nucl Med Comm 2007;28:335–54 [DOI] [PubMed] [Google Scholar]
- 19.Buchmann I, Reske SN. Novel imaging techniques in NHL: Clinical results with PET imaging. Ann Hematol 2001;80(Suppl. 3):B54–7 [DOI] [PubMed] [Google Scholar]
- 20.Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med 2006;47:1326–34 [PubMed] [Google Scholar]
- 21.Howell SJ, Grey M, Chang J, Morgenstern GR, Cowan RA, Deakin DP, et al. The value of bone marrow examination in the staging of Hodgkin's lymphoma: a review of 955 cases seen in a regional cancer centre. Br J Haematol 2002;119:408–11 [DOI] [PubMed] [Google Scholar]
- 22.Vassilakopoulos TP, Angelopoulou MK, Constantinou N, Karmiris T, Repoussis P, Roussou P, et al. Development and validation of a clinical prediction rule for bone marrow involvement in patients with Hodgkin lymphoma. Blood 2005;105:1875–80 [DOI] [PubMed] [Google Scholar]
- 23.Brusamolino E, Bacigalupo A, Barosi G, Biti G, Gobbi PG, Levis A, et al. Classical Hodgkin's lymphoma in adults: guidelines of the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation on initial work-up, management, and follow-up. Haematologica 2009;94:550–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaefer NG, Strobel K, Taverna C, Hany TF. Bone involvement in patients with lymphoma: the role of FDG-PET/CT. Eur J Nucl Med Mol Imaging 2007;34:60–7 [DOI] [PubMed] [Google Scholar]
- 25.Brunning RD, Bloomfield CD, McKenna RW, Peterson LA. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med 1975;82:365–6 [DOI] [PubMed] [Google Scholar]
- 26.Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN. 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol 1998;16:603–9 [DOI] [PubMed] [Google Scholar]
- 27.Naumann R, Beuthien-Baumann B, Reiss A, Schulze J, Hänel A, Bredow J, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin's lymphoma. Br J Cancer 2004;90:620–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr R, Barrington SF, Madan B, O'Doherty MJ, Saunders CA, van derWalt J, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood 1998;91:3340–6 [PubMed] [Google Scholar]
- 29.Buchmann I, Reinhardt M, Elsner K, Bunjes D, Altehoefer C, Finke J, et al. 2-(Fluorine-18)fluoro-2-deoxy-D-glucose positron emission tomography in the detection and staging of malignant lymphoma: a bicenter trial. Cancer 2001;91:889–99 [PubMed] [Google Scholar]
- 30.Sasaki M, Kuwabara Y, Koga H, Nakagawa M, Chen T, Kaneko K, et al. Clinical impact of whole body FDG-PET on the staging and therapeutic decision making for malignant lymphoma. Ann Nucl Med 2002;16:337–45 [DOI] [PubMed] [Google Scholar]
- 31.Hong SP, Hahn JS, Lee JD, Bae SW, Youn MJ. 18F-Fluorodeoxyglucose-positron emission tomography in the staging of malignant lymphoma compared with CT and 67Ga scan. Yonsei Med J 2003;44:779–86 [DOI] [PubMed] [Google Scholar]
- 32.Partridge S, Timothy A, O'Doherty MJ, Hain SF, Rankin S, Mikhaeel G. 2-Fluorine-18-fluoro-2-deoxy-D glucose positron emission tomography in the pretreatment staging of Hodgkin's disease: influence on patient management in a single institution. Ann Oncol 2000;11:1273–9 [DOI] [PubMed] [Google Scholar]
- 33.Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin's disease. Haematologica 2001;86:266–73 [PubMed] [Google Scholar]
- 34.Fuster D, Chiang S, Andreadis C, Guan L, Zhuang H, Schuster S, et al. Can [18F]fluorodeoxyglucose positron emission tomography imaging complement biopsy results from the iliac crest for the detection of bone marrow involvement in patients with malignant lymphoma? Nucl Med Comm 2006;27:11–15 [DOI] [PubMed] [Google Scholar]
- 35.Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med 2007;37:206–22 [DOI] [PubMed] [Google Scholar]
- 36.Kwee TC, Kwee RM, Verdonck LF, Bierings MB, Nievelstein RA. Magnetic resonance imaging for the detection of bone marrow involvement in malignant lymphoma. Br J Haematol 2008;141:60–8 [DOI] [PubMed] [Google Scholar]