Abstract
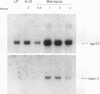
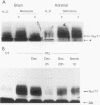
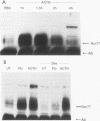
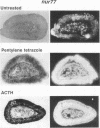
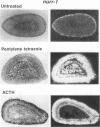
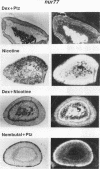
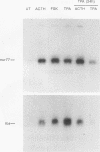
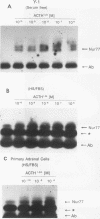
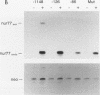
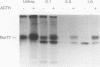
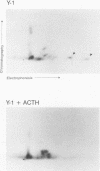
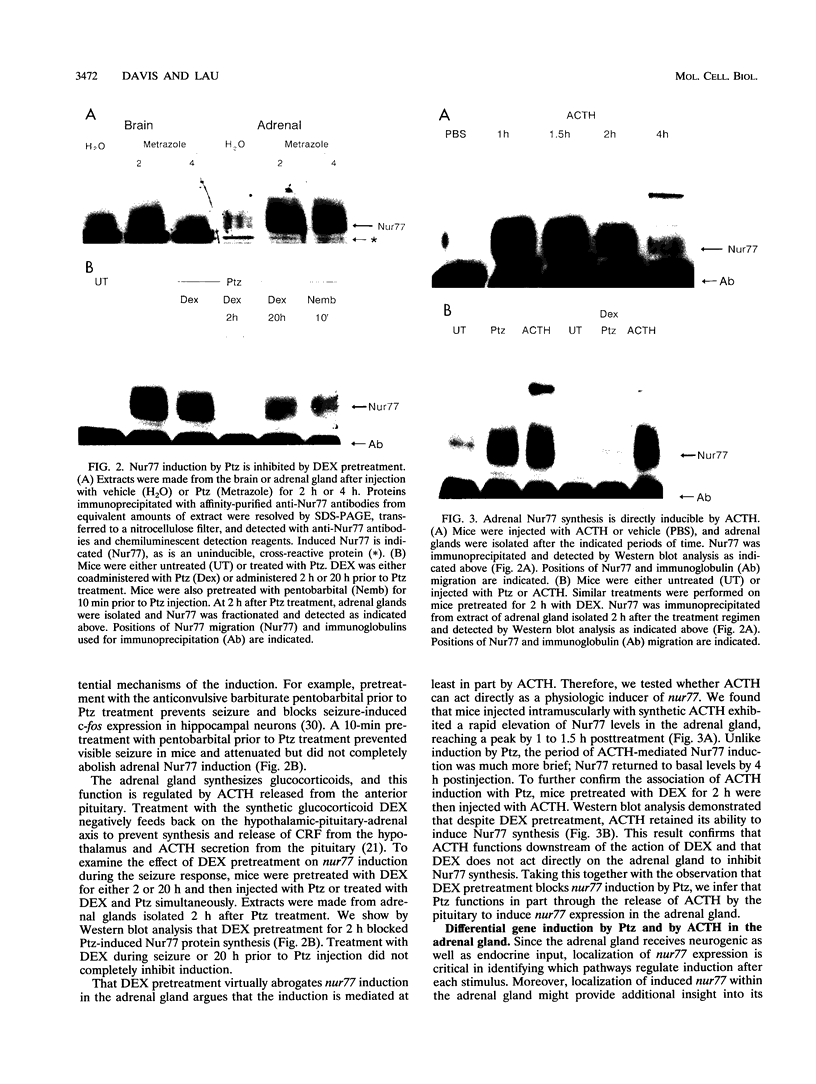
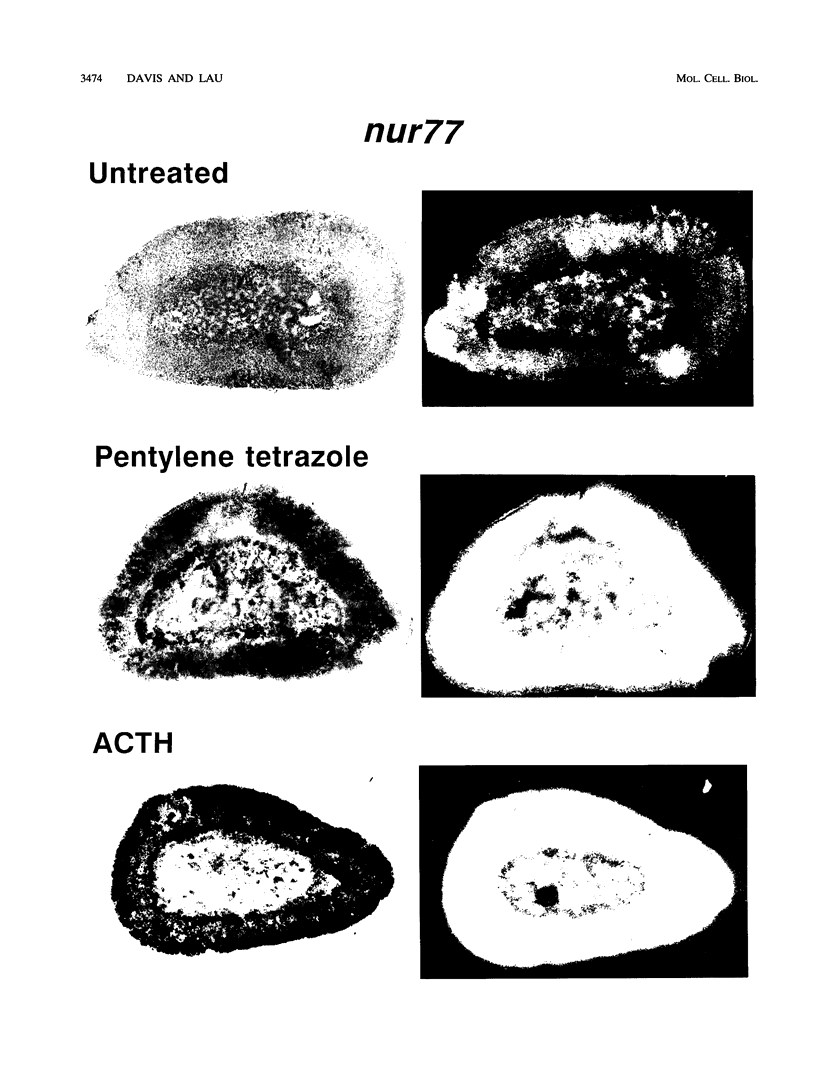
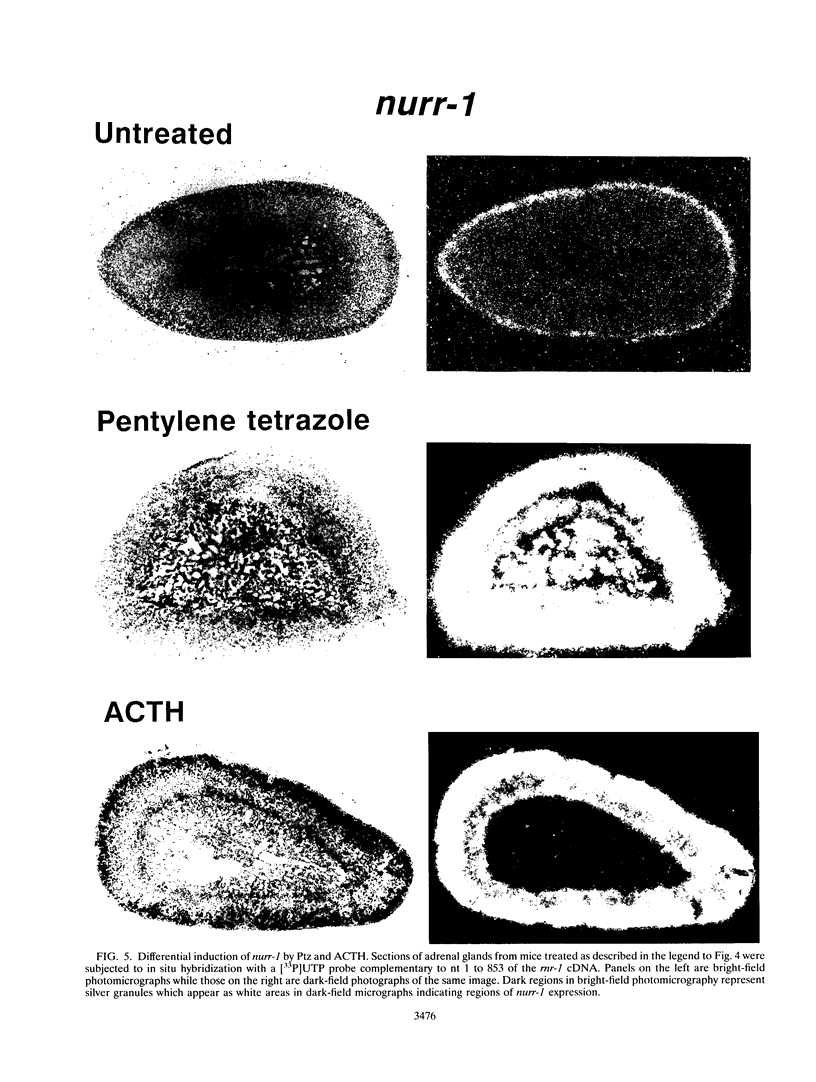
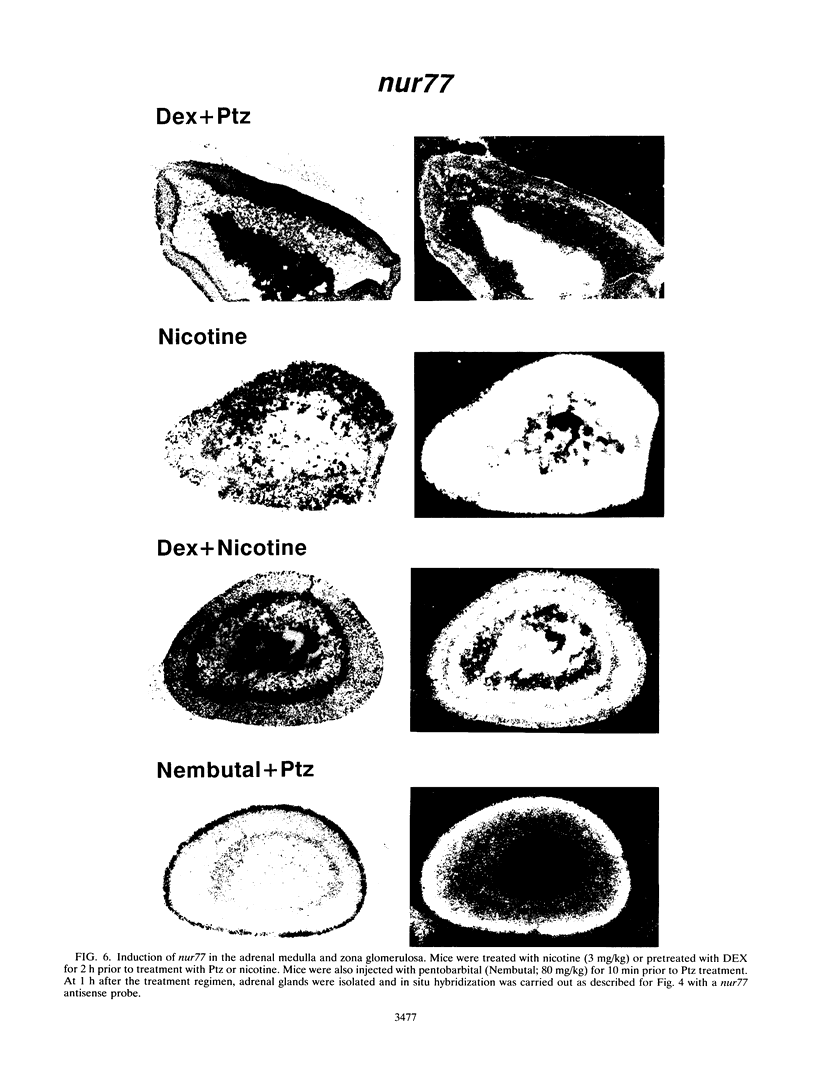
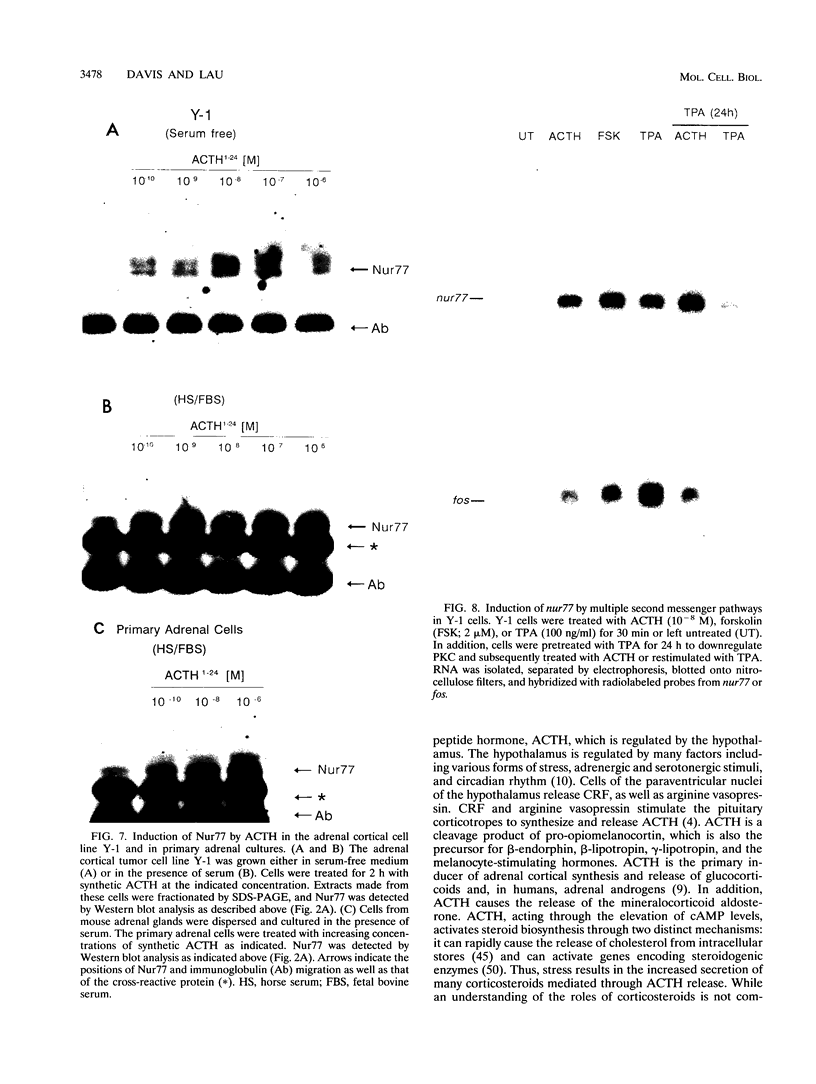
nurr77 and nurr-1 are growth factor-inducible members of the steroid/thyroid hormone receptor gene superfamily. In order to gain insight into the potential roles of nur77 in the living organism, we used pharmacologic treatments to examine the expression of nur77 in the mouse adrenal gland. We found that nur77 and nurr-1 are induced in the adrenal gland upon treatment with pentylene tetrazole (Ptz; Metrazole). This induction is separable into distinct endocrine and neurogenic mechanisms. In situ hybridization analysis demonstrates that nur77 expression upon Ptz treatment in the adrenal cortex is localized primarily to the inner cortical region, the zona fasciculata-reticularis, with minimal induction in the zona glomerulosa. This induction is inhibitable by pretreatment with dexamethasone, indicating involvement of the hypothalamic-pituitary-adrenal axis in the activation of adrenal cortical expression. When mice were injected with adrenocorticotrophic hormone (ACTH), nur77 expression in the adrenal gland spanned all cortical layers including the zona glomerulosa, but medullary expression was not induced. Ptz also induces expression of both nur77 and nurr-1 in the adrenal medulla. Medullary induction is likely to have a neurogenic origin, as nur77 expression was not inhibitable by dexamethasone pretreatment and induction was seen after treatment with the cholinergic neurotransmitter nicotine. nur77 is also inducible by ACTH, forskolin, and the second messenger analog dibutyryl cyclic AMP in the ACTH-responsive adrenal cortical cell line Y-1. Significantly, Nur77 isolated from ACTH-stimulated Y-1 cells bound to its response element whereas Nur77 present in unstimulated cells did not. Moreover, Nur77 in ACTH-treated Y-1 cells was hypophosphorylated at serine 354 compared with that in untreated cells. These results, taken together with the previous observation that dephosphorylation of serine 354 affects DNA binding affinity in vitro, show for the first time that phosphorylation of Nur77 at serine 354 is under hormonal regulation, modulating its DNA binding affinity. Thus, ACTH regulates Nur77 in two ways: activation of its gene and posttranslational modification. A promoter analysis of nur77 induction in Y-1 cells indicates that the regulatory elements mediating ACTH induction differ from those required for induction in the adrenal medullary tumor cell line PC12 and in 3T3 fibroblasts.
Full text
PDF




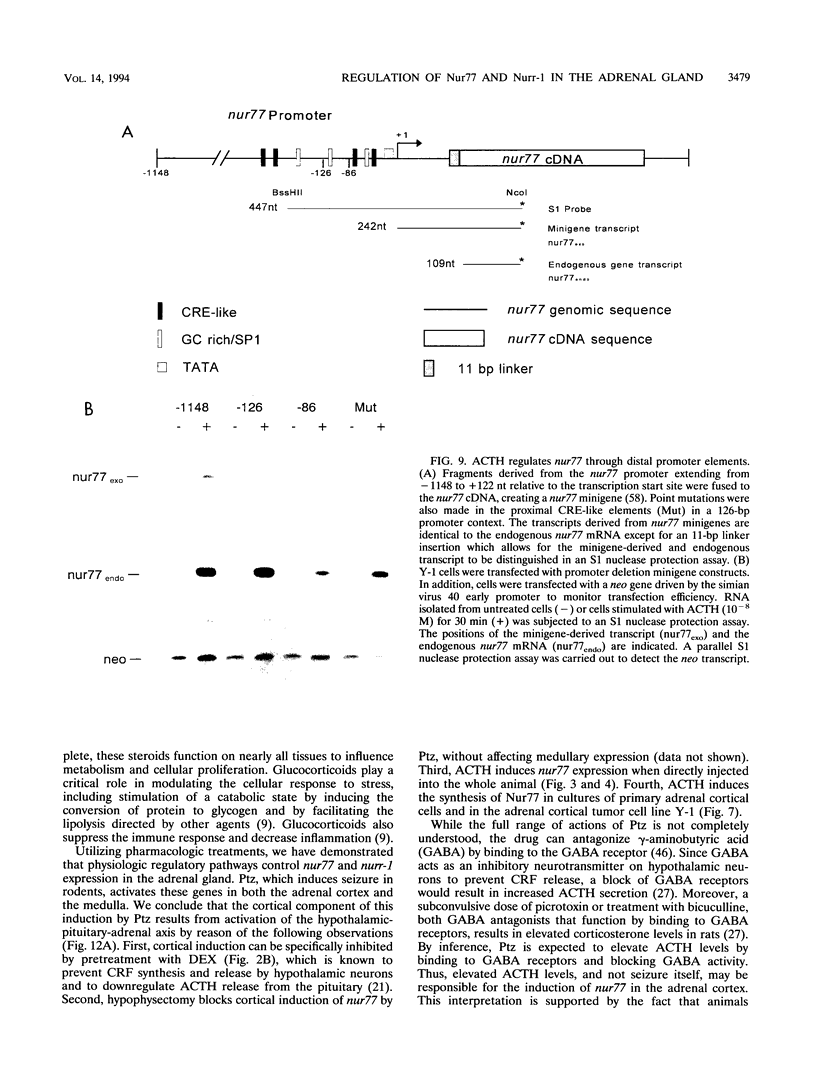

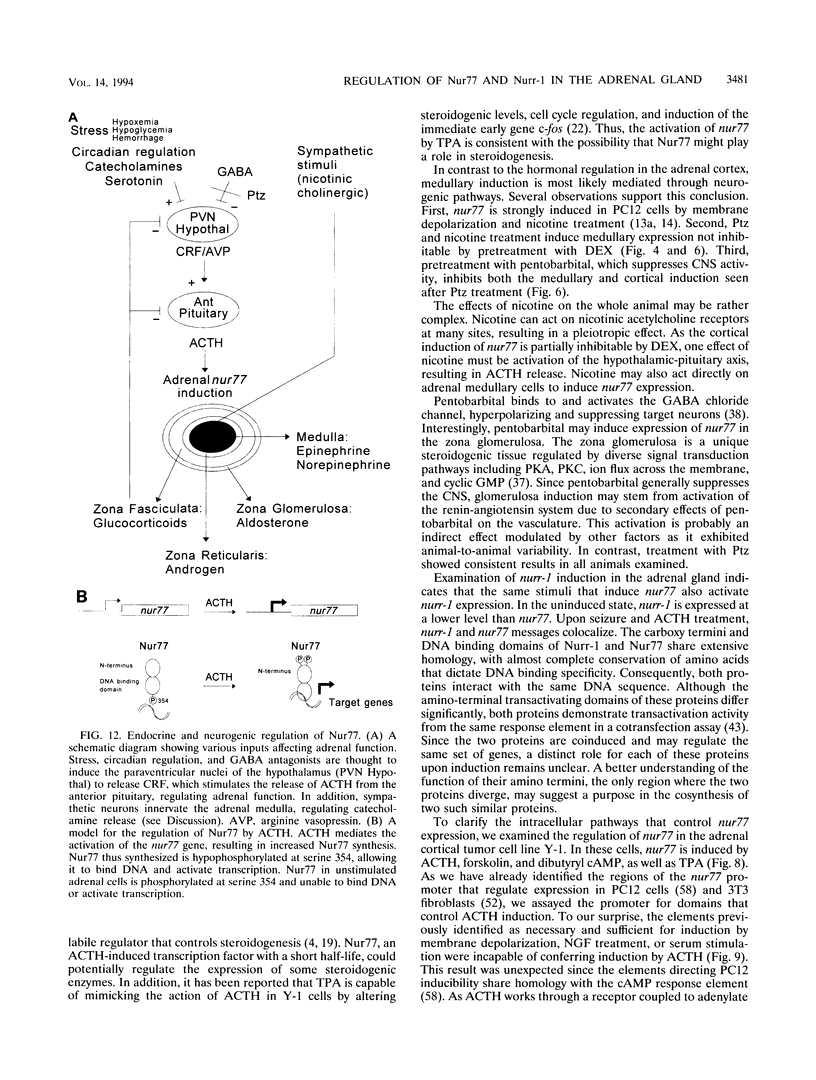


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Balla T., Jones M. R., Catt K. J. Stimulation of early gene expression by angiotensin II in bovine adrenal glomerulosa cells: roles of calcium and protein kinase C. Mol Endocrinol. 1992 Nov;6(11):1889–1898. doi: 10.1210/mend.6.11.1336125. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Akana S. F., Cascio C. S., Darlington D. N., Jacobson L., Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Davis I. J., Hazel T. G., Chen R. H., Blenis J., Lau L. F. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993 Aug;7(8):953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- Davis I. J., Hazel T. G., Lau L. F. Transcriptional activation by Nur77, a growth factor-inducible member of the steroid hormone receptor superfamily. Mol Endocrinol. 1991 Jun;5(6):854–859. doi: 10.1210/mend-5-6-854. [DOI] [PubMed] [Google Scholar]
- Fahrner T. J., Carroll S. L., Milbrandt J. The NGFI-B protein, an inducible member of the thyroid/steroid receptor family, is rapidly modified posttranslationally. Mol Cell Biol. 1990 Dec;10(12):6454–6459. doi: 10.1128/mcb.10.12.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo-Payet N., Escher E. Adrenocorticotropin receptors in rat adrenal glomerulosa cells. Endocrinology. 1985 Jul;117(1):38–46. doi: 10.1210/endo-117-1-38. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel T. G., Misra R., Davis I. J., Greenberg M. E., Lau L. F. Nur77 is differentially modified in PC12 cells upon membrane depolarization and growth factor treatment. Mol Cell Biol. 1991 Jun;11(6):3239–3246. doi: 10.1128/mcb.11.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hinson J. P., Vinson G. P., Whitehouse B. J., Price G. Control of zona glomerulosa function in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1985 Mar;104(3):387–395. doi: 10.1677/joe.0.1040387. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Kiuchi K., Chen H. C., Milbrandt J., Guroff G. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J Biol Chem. 1993 Nov 25;268(33):24808–24812. [PubMed] [Google Scholar]
- John M. E., John M. C., Boggaram V., Simpson E. R., Waterman M. R. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4715–4719. doi: 10.1073/pnas.83.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M. E., Dallman M. F. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984 Winter;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kimura E., Armelin H. A. Phorbol ester mimics ACTH action in corticoadrenal cells stimulating steroidogenesis, blocking cell cycle, changing cell shape, and inducing c-fos proto-oncogene expression. J Biol Chem. 1990 Feb 25;265(6):3518–3521. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Conneely O. M., DeMayo F. J., O'Malley B. W. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992 Dec;6(12):2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Makara G. B., Stark E. Effects of gamma-aminobutyric acid (GABA) and GABA antagonist drugs on ACTH release. Neuroendocrinology. 1974;16(3-4):178–190. doi: 10.1159/000122564. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Habener J. F. Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993 Jun;14(3):269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Honda S., Inomata Y., Handa H., Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992 Sep 5;267(25):17913–17919. [PubMed] [Google Scholar]
- Nakai A., Kartha S., Sakurai A., Toback F. G., DeGroot L. J. A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily. Mol Endocrinol. 1990 Oct;4(10):1438–1443. doi: 10.1210/mend-4-10-1438. [DOI] [PubMed] [Google Scholar]
- Nathans D., Lau L. F., Christy B., Hartzell S., Nakabeppu Y., Ryder K. Genomic response to growth factors. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):893–900. doi: 10.1101/sqb.1988.053.01.102. [DOI] [PubMed] [Google Scholar]
- O'Brien T. P., Lau L. F. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 1992 Sep;3(9):645–654. [PubMed] [Google Scholar]
- Paulsen R. E., Weaver C. A., Fahrner T. J., Milbrandt J. Domains regulating transcriptional activity of the inducible orphan receptor NGFI-B. J Biol Chem. 1992 Aug 15;267(23):16491–16496. [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Quinn S. J., Williams G. H. Regulation of aldosterone secretion. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- Rice D. A., Mouw A. R., Bogerd A. M., Parker K. L. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol. 1991 Oct;5(10):1552–1561. doi: 10.1210/mend-5-10-1552. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Macdonald-Bravo H., Mattéi M. G., Ruppert S., Bravo R. Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. EMBO J. 1989 Nov;8(11):3327–3335. doi: 10.1002/j.1460-2075.1989.tb08494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce L. M., Laz T. M., Hazel T. G., Lau L. F., Taub R. RNR-1, a nuclear receptor in the NGFI-B/Nur77 family that is rapidly induced in regenerating liver. J Biol Chem. 1993 Apr 25;268(12):8855–8861. [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Saederup E., Crawley J. N., Skolnick P., Paul S. M. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984 Oct 1;35(14):1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Viard I., Hall S. H., Jaillard C., Berthelon M. C., Saez J. M. Regulation of c-fos, c-jun and jun-B messenger ribonucleic acids by angiotensin-II and corticotropin in ovine and bovine adrenocortical cells. Endocrinology. 1992 Mar;130(3):1193–1200. doi: 10.1210/endo.130.3.1311231. [DOI] [PubMed] [Google Scholar]
- Waterman M. R., Simpson E. R. Regulation of steroid hydroxylase gene expression is multifactorial in nature. Recent Prog Horm Res. 1989;45:533–566. doi: 10.1016/b978-0-12-571145-6.50016-9. [DOI] [PubMed] [Google Scholar]
- Watson M. A., Milbrandt J. The NGFI-B gene, a transcriptionally inducible member of the steroid receptor gene superfamily: genomic structure and expression in rat brain after seizure induction. Mol Cell Biol. 1989 Oct;9(10):4213–4219. doi: 10.1128/mcb.9.10.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Lau L. F. Activation of the inducible orphan receptor gene nur77 by serum growth factors: dissociation of immediate-early and delayed-early responses. Mol Cell Biol. 1993 Oct;13(10):6124–6136. doi: 10.1128/mcb.13.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Fahrner T. J., Johnston M., Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991 May 31;252(5010):1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- Wilson T. E., Mouw A. R., Weaver C. A., Milbrandt J., Parker K. L. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol Cell Biol. 1993 Feb;13(2):861–868. doi: 10.1128/mcb.13.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Paulsen R. E., Padgett K. A., Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992 Apr 3;256(5053):107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
- Yang G. A., Koistinaho J., Iadarola M., Shenhua-Zhu, Hervonen A. Administration of adrenocorticotropic hormone (ACTH) enhances Fos expression in the rat adrenal cortex. Regul Pept. 1990 Aug 21;30(1):21–31. doi: 10.1016/0167-0115(90)90132-g. [DOI] [PubMed] [Google Scholar]
- Yasumura Y., Buonassisi V., Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966 Mar;26(3):529–535. [PubMed] [Google Scholar]
- Yoon J. K., Lau L. F. Transcriptional activation of the inducible nuclear receptor gene nur77 by nerve growth factor and membrane depolarization in PC12 cells. J Biol Chem. 1993 Apr 25;268(12):9148–9155. [PubMed] [Google Scholar]