Abstract
Neonatal spinalized (NST) rats can achieve autonomous weight supported locomotion never seen after adult injury. Mechanisms that support function in NST rats include increased importance of cortical trunk control, and altered biomechanical control strategies for stance and locomotion. Hindlimbs are isolated from perturbations in quiet stance and act in opposition to forelimbs in locomotion in NST rats. Control of roll and yaw of the hindlimbs is crucial in their locomotion. The biomechanics of the hind limbs of NST rats are also likely crucial. We present new data showing the whole leg musculature scales proportional to normal rat musculature in NST rats, regardless of function. This scaling is a prerequisite for the NST rats to most effectively use pattern generation mechanisms and motor patterns that are similar to those present in intact rats. Pattern generation may be built into the lumbar spinal cord by evolution and matched to the limb biomechanics, so preserved muscle scaling may be essential to the NST function observed.
Introduction
The mechanisms of plasticity that operate in the locomotion of intact rats may also contribute to recovery after injury.1,2,3 However, following injury, the control problems are much more severe. Injury alters motor organization: some pathways are lost and sprouting may add novel and unusual connnections.4 Neural plasticity and motor learning mechanisms must adapt this novel and reduced neural system structure to best restore function.5,6,7 How does developmental reorganization, motor learning and plasticity achieve function after injury?8,9 A particularly interesting and informative example occurs in complete spinal cord injury in very young rats or kittens.5,7,10,11,12,13,14,15,16,17
Paralysis of adults ST animals is always complete but some neonatal ST animals walk
Adult mammals are very severely paralyzed after complete spinal transection (ST). Only perineal stimulation, epidural stimulation18 or drug delivery therapies19,20,21 can initiate stepping. Rehabilitation training 19,22,23,24,25 and/or therapeutic interventions improve such stepping, but do not restore autonomy.26 However, in stark contrast to this limited recovery, some neonatal spinal transected (NST) rats or cats develop autonomous weight-supported stepping as adults.5,7,10,11,12,13,14,15,16,17
How do neonatal spinalized rats walk? Understanding this may be a roadmap to improve animal model therapies. The results of research on rehabilitating quadrupeds may translate to significant but lesser gains in man.27,28
In this paper, we review studies of the biomechanics and control operating in weight supporting neonatal spinalized (WSNST) rats, compared to non-weight supporting neonatal spinalized (NWSNST) rats, and intact control rats. We then describe new data on muscle scaling's role in these rats' function.
A definition of functional walking
Spinal cord contains the circuitry to organize complex movements using pattern generators and reflexes local to spinal cord.29 Most postnatal day 1 (P1) to 5 (P5) NST rats can, as adults, generate stepping motions on a treadmill without additional stimuli. A subset of ∼20% of NST rats also achieve what we term independent or autonomous weight support as adults.5,6,7,14,17,30,31 We define independent or autonomous weight supported stepping using a ‘sumo-wrestling’ like criterion for a weight-supported step. It involves ground contact of nothing except some part of the feet through the full progression of swing and stance. We then measure the percentage of such steps in locomotion. In NST rats the distribution of this measure of function is bimodal14 with peaks centered on ∼20% and 75% allowing the division of NST rats into two groups, of weight supporting rats (WSNST, with BBB32 12-16) and non-weight-supporting rats (NWSNST, BBB <8). WSNST rats all have better than 50% of their steps classified as weight-supported steps. The WSNST rats recover from falls and can balance the majority of steps.
How might function be achieved through the integration of two autonomous pieces of CNS connected to the same body? - a model of function
The NST rat has two autonomous pieces of CNS controlling its body: 1. the brain and cervical spinal system, and 2. the lumbosacral spinal enlargement. These two pieces of CNS cannot directly communicate with one another in NST rats. They develop separately, although they control a single mechanical system, the rat's body, in a piecemeal fashion. From this piecemeal control, some rats develop a cooperative process that supports autonomous weight supported stepping. The closest intuitive analogies to the problem that the transected rat faces may be childrens' wheelbarrow races, and the ‘pantomime horse’ used in musical theater. In the former, as two children form a ‘wheelbarrow’, the arms of the front child and legs of the rear child must be coordinated and balanced while the front child's body is supported cooperatively by both. In the pantomime horse, two actors form the front and rear of the horse. The rear actor has no vision, and only knows how to step through the mechanical actions and communications of the front actor. The NST rat is an amalgam of these: the lumbar CNS is ‘blinded’ and lacks vestibular information, and all communication is through the mechanical coupling of the body parts, mediated primarily through the trunk. The thoracic axial musculature is partly shared by both. A natural hypothesis is thus that trunk control will play a central role in the function of these rats, as do the mechanical couplings and coordinations used in the wheelbarrow race and pantomime horse. To what extent are these ideas validated experimentally?
Sites of possible plasticity and compensation in the neonatal model of SCI
There are many points of plasticity in NST rats. Compensations may involve the cortex,13,30,31,33,34,35,36,37,38 in cooperation with cerebellum and basal ganglia, the spinal central pattern generators,2,8,24 primary afferents, autonomic pathways, the trunk, and the hindlimb biomechanical plant.39-45 Development in NST rats occurs without many normal targets and inputs, and the functional and task contexts differ. Spinal cord development occurs without the normal descending neuromodulation from above the lesion.46 Spinal pattern generation develops separated from its usual coordinating neural inputs, and in an unusual mechanical context.47 Differing development of limb muscles could also contribute.40-45 Mechanical and muscle changes can follow change in neural systems, drive patterns, or use of very different kinematic and kinetic behaviors.40-45
What is the rat cortex like after neonatal spinalization?
Trunk motor representations in relation to function
Trunk cortex changes after spinal cord injury (SCI). The motor representation of hindlimb and lumbar axial musculature in intact rats is in an area caudal to bregma and within 2.5mm of the midline. This area also contains a sensory representation of trunk and hindlimbs: a sensorimotor amalgam.48, 49,50 Both these motor and sensory representations are vulnerable to SCI.5,13,30,31,36 P1/P2 injuries occur before various critical periods in cortical organization. Sensory representations develop in this region in all P1/P2 rats but are lost in rats with ST after these critical periods.36 The NST sensory representations can be enhanced with exercise: Kao and colleagues13 showed that exercise increased both responses and the percentage of responding sensory cells in the hindlimb SI area in response to stimulation of dermatomes rostral to the transection.
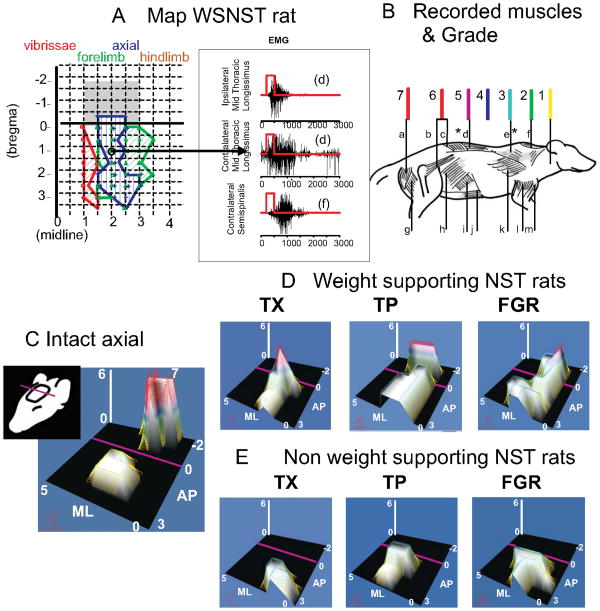
Extensive trunk motor changes also occur in NST rats.30,31 (Figure 1.) There is no cortical hindlimb representation in WSNST rats. However, all WSNST rats developed low trunk motor representations. NWSNST rats lack them. All WSNST rats' motor cortex had a representation of mid to low trunk, and these matched 1:1 with achievement of autonomous weight-support: i.e., rats with good weight-support all possessed caudal trunk motor representations and vice versa.
Figure 1.
Trunk representation in intracortical microstimulation (ICMS) maps of cortex is compared across function and intervention in NST rats. Note the orientation of the maps indicated by the cartoon on the left in C. A. Cortical microstimulation example from rats transected as neonates with weight support, along with sample EMGs from their mapping. Rats with weight-support all showed mid to low trunk motor representations (in blue regions) when mapped using 50 microAmps current pulses in 300ms trains. In intact rats these representations would in general occur caudal to bregma (in the grey shaded regions in A, and see C). The midthoracic ipsilateral and contralateral latissimus dorsi and contralateral suprspinatus were activated at the site circled. B. Muscle diagram. To create microstimulation maps and assess trunk control at different segmental levels the following muscles were recorded: a: semitendinosus, b: iliopsoas, c: multifidus, d: longissimus, e: trapezius, f: supraspinatus, g: biceps femoris, h: external oblique, i: internal oblique, j: rectus abdominis, k: latissimus, l: triceps brachii, m: biceps brachii. Leg muscles (a,g) were never recruited in spinalized rats. Mid to low trunk muscles (c,d and i,j,k and very rarely b) could cause observable pelvic motion either directly or through reflex and mechanical couplings. Trunk or hind-leg segmental level found in ICMS maps was scored from 1 to 7 as shown in (1 upper cervical, 2 upper back, 3 upper shoulder/thorax, 4 mid back, 5 mid to low back, 6 low back / lumbar, 7 legs). The scored values for trunk alone were represented in panels D,E and F in the figure in two ways: they were used as the height parameter for the surface and a false color mesh was applied to the surface with color related to height. The values were interpolated across the ICMS map so as to construct a continuous surface in which height represents the segmental level score and thus the caudal extent of motor recruitment of trunk from each site in the map. In the false color mesh red represents low trunk (color assignments as shown). C. For the normal rat hindlimb recruitment (level 7) is achieved in the caudal region of the map behind bregma (AP coordinate 0 and purple line in each map, grey shaded region in Panel A). D. In WSNST spinalized rats the maximum height was always 6 or less. Weight supporting spinalized rats show peaks at level 5-6 but in TX (spinal transection alone, NST) rats these peaks are rostral to bregma, while in TP (fetal transplant repair NST) and FGR (fibrin glue repair NST) rats these are behind bregma, in the normal intact rats location. (Reproduced from Giszter et al. 2008b). E. Non-weight supported rats are unresponsive to microstimulation in the area behind bregma and the purple line regardless of intervention and show low axial scores of 3-4 (indicated by low hill peaks). Figure redrawn and re-arranged from Figure 6 in Giszter et al. 2008b, J. Neurophysiology, 100(2): 839-51. Epub 2008 May 28.31
How do spinalized rats walk: Stance, locomotion or both?
Usually, adult spinalized cats trained to walk do not stand well, and those that are trained to stand do not walk well.8,22,24 However, with special efforts, spinalized cats may accomplish both, without autonomous weight support.40 Cats and rats that are spinalized as neonates 10,11,12,15,16,17 can sometimes perform both autonomous locomotion and stance tasks competently as adults.43 We trained WSNST rats to both walk on a treadmill and to stand quietly for rewards. They successfully managed both, and this allowed us to explore the biomechanics of fully spinalized rats that accomplish both tasks.5,7
Control of quadrupedal stance in spinalized rats
Our biomechanical testing for stance was similar to the mutual jostling of rats in cages.5 We examined how stresses applied at the torso using a robot ‘saddle’ were resisted, how the applied stress was distributed to the limbs, and how the stance center of pressure (CoP) was controlled. Both normal and WSNST rats adapted to the predictable occurence of perturbations. Each moved its resting CoP to produce a more even load distribution between fore- and hindlimbs during perturbation testing. The CoP for the normal rats was shifted forward, while the CoP for the WSNST rats moved caudally, actually increasing the load borne by the hindlimbs.
The robot interaction forces were examined for perturbations in 8 directions.5,51 During perturbations the robot applied force to the rats which rose smoothly to a plateau and the rats actively opposed this. In normal rats, during rostral perturbations, the opposing horizontal forces were larger in the forelimbs, and during caudal perturbations, the opposing horizontal forces were larger in the hindlimbs. In contrast, in WSNST rats the distinction between rostral and caudal perturbations was largely absent. Forelimb forces changed in all directions while hindlimb forces were little different from initial resting forces (Figure 2). The way in which normal and WSNST rats compensated for perturbation forces thus differed. WSNST rats isolated the hindlimbs from the perturbation as much as possible.
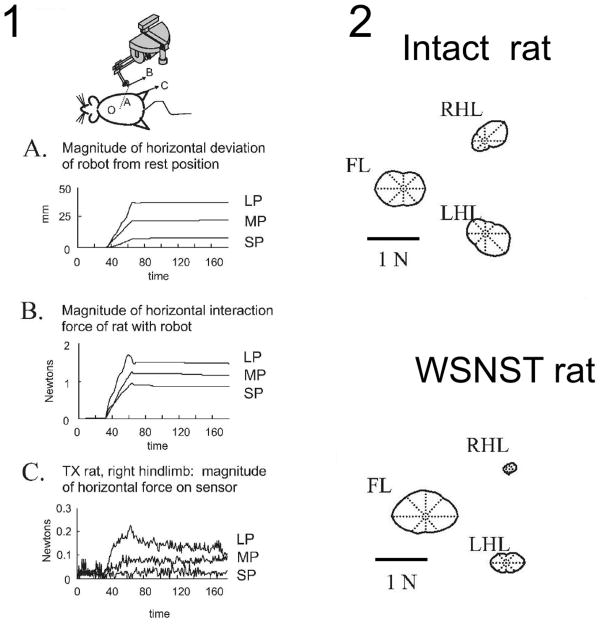
Figure 2.
Panel 1. Plots of the responses in perturbation trials for a single direction perturbation applied to an operate rat's stance using the robot and saddle (cartoon above). Shown are :(A) the distance over time of the position of the phantom tip from rest (B), the magnitude of the horizontal plane interaction force between an operate rat and the Phantom, and (C) the magnitude of the horizontal plane ground reaction force at the right hindlimb force-plate sensor. Rats picked a strategy for how to distribute horizontal (‘shear’) forces among the individual leg's ground reaction forces. Data is shown for a WSNST rat (indicated TX in figure). Data are plotted during each size perturbation, all in direction D. Note the small hindlimb force responses in panel C relative to the applied force in panel B in this rat.
Panel 2. Polar plots of tuning curves of the magnitude of the horizontal response forces for different directions of perturbations in the forelimbs and hindlimbs. The polar plots are centered on the compass center. Notice the much reduced hindlimb responses in the WSNST rat. Figure redrawn and rearranged from Figures in Giszter et al. 2007a, J. Neurophysiology. 97(4):2663-75.5
Conceivably, the local lumbar circuitry and resistance reflexes could provide some additional useful hindlimb responses after initial loading. When perturbations were routine WSNST rats adjusted the resting CoP and the hindlimbs became more loaded than before perturbations. Why were the hindlimbs in WSNST rats not used dynamically? We believe that the strategy of minimizing transmission of perturbation forces to the hindlimbs reduced the likelihood of inappropriate stepping or reflex motions. Autonomous local pattern generators and reflexes may play a central role in weight supported locomotion in spinally-injured rats (see next section). However, their spurious activations could disrupt quiet stance.
Control of locomotion in spinalized rats
Stepping in WSNST rats is likely initiated through reflexes and through the available mechanical and reflex couplings and controlled voluntarily via trunk. To explore this we next compared kinetic features of locomotion in intact and WSNST rats.7 WSNST rats exhibited a gait which was too variable to allow standard gait analysis, which requires averaging of many cycles of constant velocity locomotion.52-58 WSNST gait was rarely if ever constant velocity. To compare statistically between normal and WSNST rats we examined and compared unconstrained locomotion on a runway. Although rats crossed the runway at various speeds, there were significant statistical differences in limb force coordination, net force and CoP between WSNST and normal rats tested in this way.
Normal rats crossed the runway with a diagonal trot, with 45% body weight on hindlimbs 55% on forelimbs. Forelimbs and hindlimb acted synergistically- both limb pairs generated similar decelerative and propulsive rostrocaudal forces. Figure 3 panel 1. These forces averaged about 15% of the antigravity forces. Occasional maximums were about 50% of body weight. Normal rats thus expended substantially less effort on control of forward progression than on weight support. The peak absolute mediolateral forces were substantially smaller than the other force components, averaging only 3-4% of antigravity forces. CoP progressed in jumps along a straight line. Mean lateral deviations of CoP were <1 cm. The normal rats were very well-balanced.
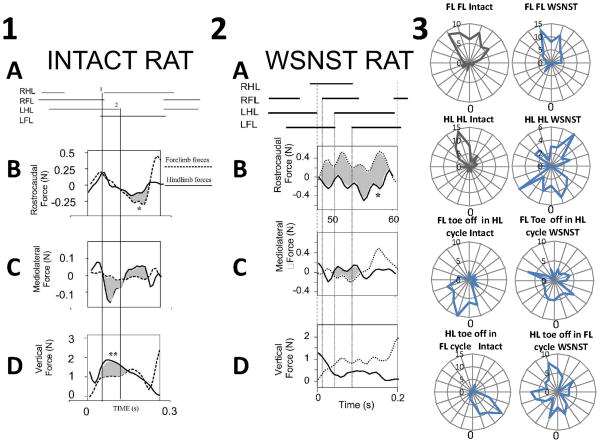
Figure 3.
Forces when a rat spanned two force plates during locomotion, allowing separation of forelimb and hindlimb contributions.
1. Normal rat. Forelimb and hindlimb force contributions to propulsion, stabilization and weight support. Top : stance phase of limbs during transition across plates. A : gait pattern. B: synergistic decelerative and then propulsive actions of forelimbs (dotted line) and hindlimbs (solid line) is shown by overlapping and similarly directed forces. Rostrocaudal forces correlate well. Individual peak contributions (∼0.25N) of forelimbs or hindlimbs are under 10% of body weight (2.7N). In the trial shown forelimbs play a larger part in acceleration (shaded and *). This is within the range of variability from the normal pattern observed in our runway task in which speed was not tightly controlled. C: Lateral forces: most mediolateral force (∼0.15N peak, ∼5% body weight) is exerted in hindlimbs (solid line). Difference of forelimb and hindlimb contributions are shaded. D: Antigravity forces: Hindlimbs (solid line) carry about 60% more body weight than forelimbs (dotted line). The difference is shaded, and indicated by **. Lines 1 and 2 indicate RHL foot strike and LHL lift. Panel redrawn and rearranged from Figure in Giszter et al. 2008a Experimental Brain Research 190(1):53-69.7
2. NWS ST rats. Forelimb and hindlimb propulsive forces are coordinated in opposition in injured rats. Phase II forces are shown and differences shaded. Top : stance phase of limbs during transition across plates. A : gait pattern. B: antagonistic decelerative actions of forelimbs (dotted line) and propulsive forces from the hindlimbs (solid line). Rostrocaudal forces correlate negatively and in a manner significantly different from normal. Individual peak contributions (∼0.5N) of forelimbs or hindlimbs are ∼25% of body weight (<2N). The forces show several peaks per cycle. Not all can be related to stance transitions, e.g. peak at *. C: Lateral forces: mediolateral force (∼0.4N peak) is exerted in both forelimbs (dotted line) and hindlimbs (solid line). D: Antigravity forces: Forelimbs (dotted line) carry about 60% of total body weight here, close to the typical mean of our ST rats, and significantly more than normal rats. Panel redrawn and rearranged from Figure in Giszter et al. 2008a Experimental Brain Research 190(1):53-69.7
3. Phase relations in gait of WSNST rats. Examples of phase distributions of swing onsets calculated for each girdle, and for hindlimb and forelimb on the same side. (FL FL: forelimb swing onset phase in contralateral forelimb cycle. HL HL: hindlimb swing onset phase in contralateral hindlimb cycle. FL in HL: forelimb swing onset phase in ipsilateral hindlimb cycle. HL in FL: hindlimb swing onset phase in ipsilateral forelimb cycle.) Intact rats are in left column of panel 3, WSNST rats are in right column of panel 3. FL FL phase is less variable in WSNST rats, while other phase show more unusual relationships. Note that the phase plotted may be associated with ‘winding numbers’ of one or more for WSNST data, while this was never true in intact rats, i.e. the rat may show multiple steps of one limb, before the measured limb phase is expressed by its toe-off motion. The average gait ratio was around 7:3 forelimb to hindlimb stepping, but there is more phase patterning in the WSNST stepping than this ratio might suggest. New analysis using data from runway experiments shown in panels 1 and 2.
WSNST rats' hindlimbs bore significantly less weight than intact rats' hindlimbs (37% body weight on hindlimbs, 63% on forelimbs). WSNST rats showed similar mean rostrocaudal forces, but had significantly larger maximum fluctuations ranging up to 80% of body weight (p<0.05). Joint force-plate recordings showed that in WSNST rats the forelimbs and hindlimb rostrocaudal forces acted in opposition, rather than synergistically, differing significantly from intact rats (p<0.05). Figure 3 panel 2. Mediolateral forces (∼20% of body weight), were significantly larger than normal rats (p<0.05). WSNST CoP zig-zagged, with mean lateral deviations of ∼2cm, (double those of intact rats), and a significantly larger range (p<0.05). The WSNST rats' gait was highly variable, near a 7 to 3 stepping ratio (forelimbs to hindlimbs). WSNST rats had much more variable forelimb-hindlimb and hindlimb-hindlimb phasing (see Figure 3, panel 3) but slightly more precise forelimb-forelimb step phasing. The haunches rolled much more than normal rats. The locomotor strategy of WSNST rats, using fore and hind-limbs in opposition, was inefficient but their complex gait was statically stable. Because forelimbs and hindlimbs acted in opposition, the trunk was held compressed. Injured rats contrasted strongly with normal rats in gait, control of ground reaction forces, and motion of the CoP.
These observations fit with the notion of the forelimbs and trunk acting as brakes and/or initiators and stabilizers for the hind-limb generated forces, and movements driven by pattern generation in the injured rats. Trunk control from cortex could be critical to manage, couple and direct the hindlimb generated forces.
The role of cortex in trunk control and SCI
Some trunk muscles physically span the lesioned segments, and these may have distributed motor pools spanning the lesion. Trunk muscles may be coordinated across a lesion by reflex chaining, in which mechanical interactions through the trunk elicit reflexes below the lesion which coordinate the muscle contraction patterns that occur below the lesion. For example, emetic responses remain coordinated and effective in thoracic spinalized cats, with segmental trunk muscles both above and below the lesion contracting in concert.59 Cortical motor control of trunk may thus provide several ways of interacting with the autonomous lumbar stepping. The trunk cortex might help coordinate forelimb-hindlimb mechanical transmissions, and thus shape the mechanical environment in which lumbar stepping occurs. Such mechanical shaping is known to play a role in pattern generator function after SCI.1,8,16,24,25,41,43,60
Cortical motor representations of mid/low trunk only occur in WSNST rats with high weight support as noted,30 and Kao and colleagues' study13 of S1 showed exercise altered cortex representations in NST rats. Motor cortex might be engaged differently in locomotion developed by the WSNST rats because they were injured preceding critical periods in cortical and cerebellar wiring. However, alternatively, perhaps the trunk cortex motor representations were an outcome of function rather than the cause. To test the role of trunk cortex in WSNST rats, we used intracranial microstimulation to guide focal lesions placed in the trunk area of cortex.31 We lesioned the normal hindlimb/trunk area in all rats, i.e. the area representing low trunk and hindlimb in normal rats. Injured rats could vary in their pre-lesion weight-support level, depending on their body weight.
In 4 intact control rats, lesions of hindlimb/trunk cortex caused no treadmill deficits. However, all NST rats lesioned in trunk cortex lost an average of ∼40% of their weight support, which did not recover. Although the role of hindlimb/trunk motor cortex in intact rats may be modest in normal locomotion, cortex must have become more significant after spinalization in NST rats. Trunk cortex became an essential participant in the weight supporting locomotion of these rats.
WSNST joint angle ranges were comparable to intact rats on the treadmill and to published data.61 WSNST hindlimb kinematic and joint parameters were also not significantly different pre- and post-lesion. (Figure 4.) However, the frequency of high roll (i.e., > 45 degree) events in the haunches was increased substantially by lesions, and more than doubled. A pre-lesion probability per step of high roll of 0.1 increased post-lesion to 0.25 (statistically significant, t-test, p< 0.005). Roll event probability correlated negatively with the percentage weight support measures (regression r2 = 0.81, p<0.0001), and correlated positively with the number of non-weight supporting step cycles (regression r2 = 0.83, p<0.0005). The hindlimb/trunk cortex lesions thus disrupted aspects of the control of roll, pelvic balance, and the integration of forelimbs and hindlimb mechanics. The data support a significant role of trunk cortex in locomotion after complete neonatal spinalization.
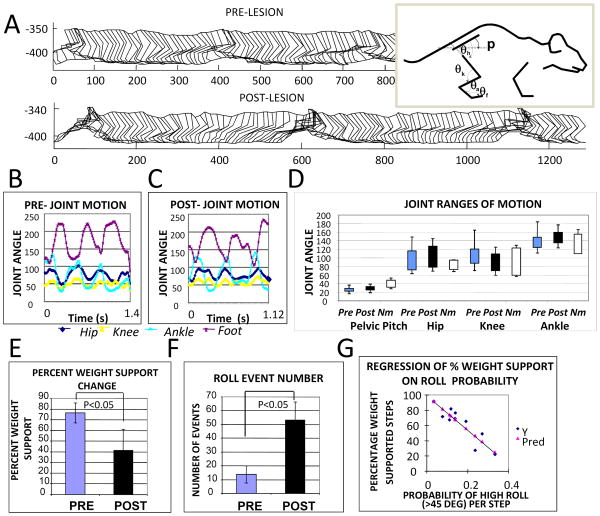
Figure 4.
Kinematics of hindlimbs and pelvis in rats before and after cortical lesions. A. Parasagittal stick figure motion was digitized for multiple step cycles before and after lesion. An example of data from one rat is shown. The measured internal angles are displayed to the right. B Pre-lesion and C Post-lesion joint angles. Pelvic pitch orientation and joint angles of hip, knee, ankle and foot were measured from the captured stick figures pre and post lesion. D. Range of motion for each angles time-series were compared pre-post for 8 WSNST rats (Pre, Post) and for normal rats (Nm). Ranges for data from B/C are shown, together with normal intact rat ranges measured similarly. The maximum and minimum joint angles and their standard deviations in the group of 8 rats tested in detail for pre and post lesion data were compared. In the NST 8 rats tested, statistical comparisons of the kinematic features measured in the parasagittal plane were not significantly different. Neither the ranges of motion, the basic pattern of coordination among joints, nor the period of the hindlimb stepping were significantly altered by the cortical lesions (n=8, p>0.1). E. After lesions, the group percent weight-support decreased significantly (paired t-test, p<0.05). F. After lesion there were increased numbers of pelvic roll events where roll clearly exceeded 45 degrees (paired t-test, p<0.05). The probability of 45 degree roll for each step was calculated from these data and more than doubled post-lesion (paired t-test, p<0.05). The number of non-weight supporting steps in rats was also linearly related to the number of roll events (r2 =0.83, slope coefficient ∼2 and significance p<0.0005). G The percentage of weight-supported steps in rats was negatively correlated to the probability of roll per step (r2=0.81, slope coefficient significance p<0.0001). Thus Hindlimb kinematics were not altered significantly, but pelvic roll was increased and related to quality of weight support. Reworked from Figure 6 in Giszter et al., 2008, J. Neurophysiology, 100(2):839-51, Epub 2008, May 28.31
Muscles masses, the biomechanics of pattern generation and their intimate relationships in effective locomotion
How much do altered limb muscle balances contribute to the autonomous weight-bearing of NST rats? The muscle balances in the WSNST and NWSNST rats have not previously been explored. From first principles in biomechanics, it can be shown that if the limb's muscle masses differ in their proportions, then effects of identical pattern generation and reflex synergies will be mechanically different. This is because the relative scaling of joint torques by coactive muscles will differ. As a result, the force magnitude and direction at the foot will necessarily be different following the transformation of differently scaled torques into force, through the limb linkage.6,62,63 In contrast, if muscles were scaled similarly, even after some atrophy, then similar motor patterns would cause similar balances of force and torque in the limb, and similar force directions at the foot, though more weakly. Human limbs in individuals of very different physical power and size are nonetheless similarly scaled.64,65 Do muscle proportions that occur in the hind-limbs in WSNST and NWSNST rats permit the ‘normal’ limb use? We set out to test this.
Muscle Mass Comparison Methods
Muscle experiments were conducted with IACUC oversight according to PHS and USDA guidelines. Animals were spinalized as described in Giszter et al. (1998).30 We examined both NST and normal rats : 9 normal rats, 11 NWSNST rats and 10 WSNST rats. Rats were treadmill trained 3 times weekly as described by Giszter,5,7,31 similarly to the NST rats described in preceding sections. To compare masses among the groups 11 muscles were excised from each perfused rat and weighed: biceps femoris, vastus lateralis, rectus femoris, gracilis, semimembranosus, semitendinosus, gastrocnemius group, tibialis anterior, iliopsoas, forelimb triceps and biceps brachii. We combined medial and lateral gastrocnemius (the gastrocnemius group). We did not examine soleus or plantaris which were difficult to dissect accurately in the perfused NST rats. In a separate analysis of 9 NST and 4 normal rats we confirmed general symmetry of muscle masses measured bilaterally, between the limbs in these rats. Their mean difference in matched muscle mass measurements between the two sides was under 5%.
Estimated muscle cross-section areas
Cross-sectional area (CSA) and physiological cross-sectional area (PCSA) predict force producing capacity of muscle. CSA is a significant contributor to PCSA. We estimated CSA of muscles from their muscle mass. We calculated CSA as mass raised to the 2/3 power, as used in allometric scaling.66,67 We then tested this algorithm as a relative PCSA estimate by using data in the literature where both mass and PCSA, (which includes pennation angle, and sarcomere length) were measured in muscles after SCI.44 We found that the regression coefficient between published PCSA measures and our estimate of CSA from our algorithm was >0.96 for all muscles reported.
Scaling measures
What is important in muscle scaling in NST rats and normal? Neonatal spinalized NST rats are often lighter than intact littermates. We thus examined several scalings. To compare scaling of muscles of interest we used raw data and also normalized to: 1. total body mass, 2. combined leg muscle mass, and 3. combined estimated muscle area. Normalizations 2 and 3 relate an individual muscle to the rest of the ensemble of hind limb muscles within the leg, ignoring overall rat mass.
Scaling statistical tests
To test differences in scaling we used ANOVA, principal components analyses (PCA), regression and post-hoc statistical t-tests. These were performed in the MINITAB statistical package, Excel, or Statview. Earlier work in this area examined smaller numbers of muscles, where t-tests were appropriate, so here we also examined results of post-hoc t-test comparisons, with and without the Bonferroni correction, although such unadjusted t-tests may overestimate differences.
Muscle Scaling Results
Masses were always largest in normal and smallest in NWSNST rats. Mean body mass in Normals was 251g, WSNST rats 194.6g, and NWSNST rats 167.6g. However, WSNST and NWSNST rats body weight did not differ significantly (t-test, p=0.513), while both NST groups and the normal rats differed (all t-tests p<0.001). Leg masses differed between all 3 groups (t test comparing NWSNST and WSNST groups, p<10−5). Total mass of muscles mattered: In a subset of 5 WSNST rats, with total measured muscle mass > 4.5g, we found that percent weight support showed a positive relationship to leg muscle mass (adjusted linear regression coefficient 0.952, slope significant at p<0.005, N=5). All these significant differences held for estimated CSAs.
A two-way fixed effects ANOVA of raw muscle masses showed significant effects of level of function, muscle and their interaction (Table 1A). The masses of most individual muscles differed significantly (p<0.05) between each NST rat group and normal, excepting forelimb biceps and triceps, Figure 5A. Spinalization thus reduced muscle mass in the hindlimbs but not the forelimbs compared to normal. Post-hoc tests (Bonferroni correction) showed that vastus lateralis, semitendinosus and tibialis anterior also differed significantly between WSNST and NWSNST groups (t-tests, p<0.05). Thus, weight support in NST rats apparently correlated with increased limb mass, and significant differences in a subset of individual muscles.
Table 1. Repeated measures ANOVAs of raw muscle masses and muscle measures scaled to the other muscles.
| 1A. Raw Hind Leg Muscle Masses | ||||||
|---|---|---|---|---|---|---|
| ANOVA | ||||||
|
| ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Function level / group | 1.386384 | 2 | 0.693192 | 75.98011 | 2.51E-21 | 3.080388 |
| Muscle | 21.08176 | 8 | 2.63522 | 288.844 | 3.15E-69 | 2.025246 |
| Interaction | 1.49519 | 16 | 0.093449 | 10.2429 | 3.06E-15 | 1.738002 |
| Within | 0.98532 | 108 | 0.009123 | |||
| Total | 24.94865 | 134 | ||||
| 1B. Muscle Masses Normalized to Measured Hind Leg Muscle Mass | ||||||
|---|---|---|---|---|---|---|
| ANOVA | ||||||
|
| ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Function level / group | 0.001304 | 2 | 0.000652 | 1.857689 | 0.158826 | 3.042963 |
| Muscle | 0.973129 | 7 | 0.139018 | 396.0888 | 1.9E-110 | 2.057533 |
| Interaction | 0.005978 | 14 | 0.000427 | 1.216509 | 0.265868 | 1.743594 |
| Within | 0.067388 | 192 | 0.000351 | |||
| Total | 1.047799 | 215 | ||||
| 1C. Hind Leg Muscle Ratios (without iliopsoas) | ||||||
|---|---|---|---|---|---|---|
| ANOVA | ||||||
|
| ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Function level / group | 5.672938 | 2 | 2.836469 | 4.36662 | 0.013056 | 3.009127 |
| Muscle Pair ratio | 1691.575 | 27 | 62.65091 | 96.44835 | 1.1E-210 | 1.502368 |
| Interaction | 47.53946 | 54 | 0.88036 | 1.355276 | 0.050322 | 1.356053 |
| Within | 436.5177 | 672 | 0.64958 | |||
| Total | 2181.305 | 755 | ||||
Figure 5.
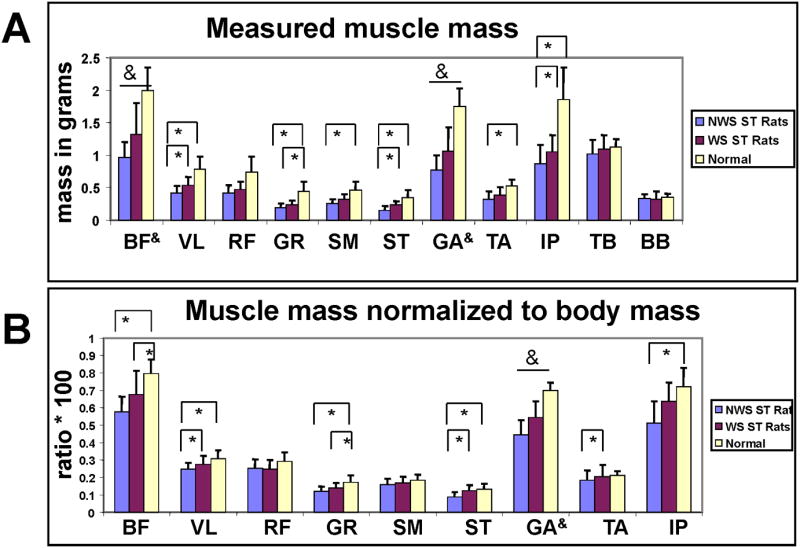
A. Measured muscle masses by weight-support group. NWS ST group (grey) WS ST group (black) N normal group (white). Muscles: 1. BF biceps femoris, 2. VL vastus lateralis, 3. RF rectus femoris, 4. GR gracilis, 5. SM semimembranosus, 6. ST semitendinosus, 7. GA gastrocnemius, 8. TA tibialis anterior, 9. IP iliopsoas, 10. TB forelimb triceps and 11. BB biceps brachii. Vertical bars : standard deviations. Bars and asterisks indicate significant differences in muscle masses (t-tests p<0.05). Ampersands on bars indicate all three comparisons were significant.
B. Muscle Masses of leg normalized to body weight. NWS ST group (grey) WS ST group (black) N normal group (white). Muscles labeled as in A. Vertical bars : standard deviations. Bars and asterisks indicate significant differences in muscle masses (t-tests p<0.05). Ampersands on bars indicate all three comparisons were significant. Data not previously published.
We next normalized the muscle masses to body mass, assessing muscle scaling with the whole body. All NWSNST and normal muscles were again significantly different. However, these differences all disappeared when we examined within limb scaling.
Muscle masses normalized to leg mass
We examined muscle masses normalized as percentages of total muscle mass measured within the leg (Figure 6A). Scaling to limb muscle mass examines local scaling within the limb. It is unaffected by fat, or bone density changes in the body or limbs. After the normalization to leg mass, none of the muscle masses were significantly different among the groups. We first performed an ANOVA of normalized data (Table 1B). There were no significant effects of group or interaction in the ANOVA with this normalization. In 27 post-hoc t-test comparisons (3 groups × 9 muscles) with the Bonferroni correction, none were significant. Variances relative to mean values in the data were decreased, not increased, as a result of normalization (compare Figures 5 and 6). Thus we cannot attribute the absence of significant differences to an increased variance of the data sets. Including a forelimb muscle in the ANOVA, it alone showed significant effects, showing the normalization had power to detect variations. Unsurprisingly, results were unaltered by a transformation of muscle mass to a cross-section area estimate (Figure 5B). Within leg muscle scaling was thus preserved between all 3 groups. This was surprising given the different levels of function and loading conditions in the three groups. However, note that this scaling provides the necessary mechanical basis for similar pattern generation to generate similar mechanics.63,63
Figure 6.
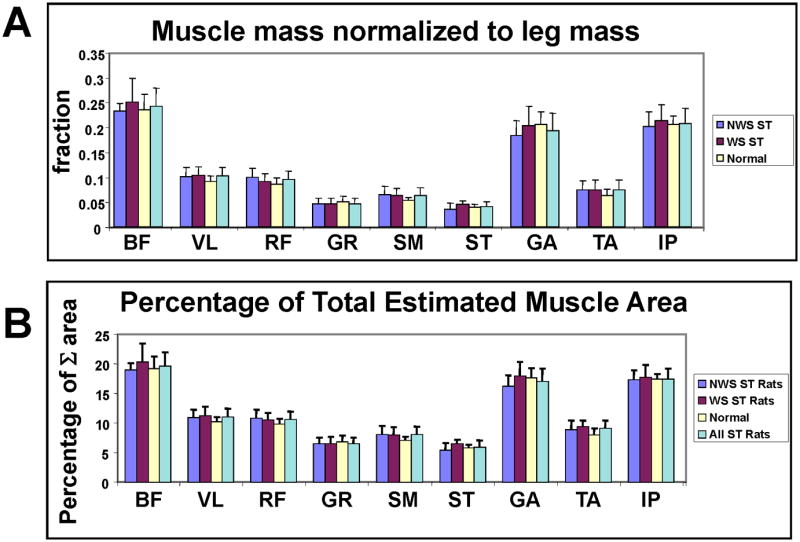
A. Muscle Masses of the leg normalized to leg total muscle mass. NWS ST group (dark grey) WS ST group (black) N normal group (white), All ST rats (light grey). Muscles: 1. BF biceps femoris, 2. VL vastus lateralis, 3. RF rectus femoris, 4. GR gracilis, 5. SM semimembranosus, 6. ST semitendinosus, 7. GA gastrocnemius, 8. TA tibialis anterior, 9. IP iliopsoas, 10. TB forelimb triceps and 11. BB biceps brachii. Vertical bars : standard deviations. There were no significant differences in post-hoc t-tests with Bonferroni corrections. Note lowered standard deviations and increased similarities compared to Figure 5.
B. Percentage of estimated muscle area as a fraction of summed leg muscle estimated areas, obtained applying a non-linear scaling. NWS ST group (dark grey) WS ST group (black) N normal group (white), All ST rats (light grey). All spinalized combined (cyan). Muscles labeled as in A. Vertical bars : standard deviations. Note similarity across groups and the reduced variance after these normalizations and scalings compared to Figure 5. Data not previously published.
Muscle mass and area ratio matrices are similar
To further test the proportionality of muscles, we calculated the 9×9 diagonally symmetric matrices of the ratios of muscle masses (and also ratios of area estimates) for each rat. We then compared these data among tested muscles in each rat group. These ratios did not involve any normalization steps; they were simply raw mass ratios. We tested statistically whether the balances among the ratios of any of the muscles differed significantly among the groups. We again used a two factor ANOVA of groups (NWS, WS, normal, Table 1C) using the 27 muscle ratios (we omitted iliopsoas ratios with only partial data), with a post-hoc Bonferroni/Dunn correction, testing at the 0.05 significance level. In the ratiometric analysis there was a significant effect of function / group. However, most variance was captured due to choice of muscle ratio (78%), and the function/treatment group provided only 0.2%. Interaction of treatment group and ratio combined provided 3%, with residual noise of 18.8%. Only two muscle ratios differed significantly among groups in post-hoc tests with the Bonferroni correction : (1) the ratio of semimembranosus and gastrocnemius differed between normal and NWS, and (2) the ratio of tibialis and gastrocnemius ratio differed between NWS and normal. The simple ratiometric analysis thus also supports the idea of a largely uniform scaling of limb muscle mass regardless of function, with the possible exception of ankle spanning muscles.
Principal Components Analysis shows muscles masses covary strongly among rats
Principal Components Analysis (PCA) examines the variance structure of data without any preconceived reference. The dimensionality of the overall muscle scaling was thus assessed more directly. The preceding analyses suggest the major source of variance in muscle masses, is simply whole limb mass scaling. If true, PCA should capture most variance in the first component. We thus combined all three groups' data and applied PCA. For measured mass, the first principal component captured 89.8% of variance with the second component capturing 3% and the third 2%. This result indicates that there was strong linear covariation of individual muscle masses between animals and groups.
The strong proportionality of muscles we found may be surprising given the differences in function of the rats. However, this scaling suggests that differences in the physical plant balance of muscles do not limit NWSNST rats, causing failure to achieve autonomous function. The balance of muscles significantly affects the extent to which similar central pattern generation, and feedback, can generate similar mechanics and stepping kinematics. Because muscle masses scale closely despite functional differences in the rats, biomechanical factors and muscle interactions intrinsic to the rat limb (i.e. largely independent of weight support, akin to scaling in utero) may cause and conserve the scaling. Similar proportionality across different individuals of greatly varying size, capacity, and activities have also been reported in man.64,65 Many factors may be critical to weight support in NST rats. The preserved muscle scaling reported here is one of these contributing factors, with overall leg mass then determining power capability.
Conclusions: Future questions, combined therapies and future needs
Transection of the spinal cord is an unambiguous lesion. Plastic reorganization, novel strategies and altered control by cortex probably allow the greater development of function seen in neonatally lesioned rats. Preserved muscle scaling may permit similar pattern generation in both NST rats and normal. It is not yet clear if the same reorganization of control and movement strategies as are seen in NST rats are possible in adult spinalized rats. NST rats likely learn their motor strategies in critical periods in development. Whether injured adults can re-learn these is an open question. However, the best experimental therapies may require this learning.68 Any interventions that assist in training the trunk controls will likely help. Rehabilitation should maximize trunk integration and enable the adult injured rats to explore novel control strategies.
Acknowledgments
Supported by NIH (grants NS24707, NS44564, NS54894).
References
- 1.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 2.Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21(10):3531–41. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolpaw JR. The education and re-education of the spinal cord. Prog Brain Res. 2006;157:261–280. doi: 10.1016/s0079-6123(06)57017-7. 2006. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue JP, Sanes JN. Peripheral nerve injury in developing rats reorganizes representation pattern in motor cortex. PNAS. 1987;84:1123–1126. doi: 10.1073/pnas.84.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giszter SF, Davies MR, Graziani VG. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J Neurophysiol. 2007a;97(4):2663–75. doi: 10.1152/jn.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giszter SF, Patil V, Hart CB. Primitives, Premotor Drives and Pattern Generation: a combined Computational and Neuroethological Perspective. Prog Brain Res. 2007b;165:325–349. doi: 10.1016/S0079-6123(06)65020-6. [DOI] [PubMed] [Google Scholar]
- 7.Giszter SF, Davies MR, Graziani V. Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates. Exp Brain Research. 2008a;190(1):53–69. doi: 10.1007/s00221-008-1451-4. Epub 2008 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgson JA, et al. Can the mammalian lumbar spinal cord learn a motor task? Medicine and Science in Sports and Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- 9.Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1647–71. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forssberg H, Grillner S, Sjostrom A. Tactile placing reactions in chronic spinal kittens. Acta Physiol Scand. 1974 Sep;92(1):114–20. doi: 10.1111/j.1748-1716.1974.tb05727.x. [DOI] [PubMed] [Google Scholar]
- 11.Howland DR, Bregman BS, Tessler A, Goldberger ME. Development of locomotor behavior in the spinal kitten. Exp Neurol. 1995a;135:108–122. doi: 10.1006/exnr.1995.1071. [DOI] [PubMed] [Google Scholar]
- 12.Howland DR, Bregman BS, Tessler A, Goldberger ME. Transplants enhance locomotion in neonatal kittens whose spinal cords are transected. Exp Neurol. 1995b;135:123–145. doi: 10.1006/exnr.1995.1072. [DOI] [PubMed] [Google Scholar]
- 13.Kao T, Shumsky JS, Murray M, Moxon KA. Exercise induces cortical plasticity after neonatal spinal cord injury in the rat. J Neurosci. 2009;29(23):7549–57. doi: 10.1523/JNEUROSCI.2474-08.2009. 2009 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miya D, et al. Fetal transplants alter the development of function after spinal cord transection in newborn rats. The Journal of Neuroscience. 1997;17(12):4856–4872. doi: 10.1523/JNEUROSCI.17-12-04856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. I. The infant lesion effect. Experimental Brain Research. 1986;62:373–386. doi: 10.1007/BF00238857. [DOI] [PubMed] [Google Scholar]
- 16.Smith JL, Smith LA, Zernicke F, Hoy M. Locomotion in exercised and nonexercised cats cordotomized at two and twelve weeks of age. Experimental Neurology. 1982;76:393–413. doi: 10.1016/0014-4886(82)90217-5. [DOI] [PubMed] [Google Scholar]
- 17.Stelzner DJ, Ershler WB, Weber ED. Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol. 1975;46(1):156–77. doi: 10.1016/0014-4886(75)90039-4. [DOI] [PubMed] [Google Scholar]
- 18.Gerasimenko YP, et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods. 2006;157(2):253–63. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.de Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J Neurotrauma. 2006;23(7):1147–63. doi: 10.1089/neu.2006.23.1147. 2006 Jul. [DOI] [PubMed] [Google Scholar]
- 20.Orsal D, et al. Locomotor recovery in chronic spinal rat: long-term pharmacological treatment or transplantation of embryonic neurons? Prog Brain Res. 2002;137:213–30. doi: 10.1016/s0079-6123(02)37018-3. [DOI] [PubMed] [Google Scholar]
- 21.Ribotta MG, et al. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20(13):5144–52. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Leon RD, et al. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82(1):359–69. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- 23.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80(1):83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- 24.Tillakaratne NJ, et al. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22(8):3130–43. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timoszyk WK, et al. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050(1-2):180–9. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Belanger M, Drew T, Provencher J, Rossignol S. A comparison of Treadmill Locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- 27.Dietz V. Do human bipeds use quadrupedal coordination. Trends Neurosci. 2002;25(9):462–7. doi: 10.1016/s0166-2236(02)02229-4. 2002 Sep. [DOI] [PubMed] [Google Scholar]
- 28.Dietz V, Michel J. Human bipeds use quadrupedal coordination during locomotion. Ann N Y Acad Sci. 2009;1164:97–103. doi: 10.1111/j.1749-6632.2008.03710.x. 2009 May. [DOI] [PubMed] [Google Scholar]
- 29.Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiological Reviews. 1975;55:247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- 30.Giszter SF, Kargo WJ, Davies M, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol. 1998;80(6):3021–30. doi: 10.1152/jn.1998.80.6.3021. 1998 Dec. [DOI] [PubMed] [Google Scholar]
- 31.Giszter SF, et al. Trunk sensorimotor cortex is essential for hindlimb weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J Neurophysiology. 2008b;100(2):839–51. doi: 10.1152/jn.00866.2007. Epub 2008 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basso DM, Beattie MS, Bresnehan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 33.Chakrabarty S, Martin JH. Motor but not sensory representation in motor cortex depends on postsynaptic activity during development and in maturity. J Neurophysiol. 2005;94(5):3192–8. doi: 10.1152/jn.00424.2005. [DOI] [PubMed] [Google Scholar]
- 34.Friel KM, Martin JH. Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol. 2005;485(1):43–56. doi: 10.1002/cne.20483. [DOI] [PubMed] [Google Scholar]
- 35.Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually-guided locomotion. J Neurophysiol. 2007;97(5):3396–406. doi: 10.1152/jn.00750.2006. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain N, Diener PS, Coq JO, Kaas JH. Patterned activity via spinal dorsal quadrant inputs is necessary for the formation of organized somatosensory maps. J Neurosci. 2003;23(32):10321–30. doi: 10.1523/JNEUROSCI.23-32-10321.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11(2):161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 38.Martin JH, Choy M, Pullman S, Meng Z. Corticospinal system development depends on motor experience. J Neurosci. 2004;24(9):2122–2132. doi: 10.1523/JNEUROSCI.4616-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont-Versteegden EE, et al. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279(6):C1677–84. doi: 10.1152/ajpcell.2000.279.6.C1677. 2000 Dec. [DOI] [PubMed] [Google Scholar]
- 40.Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am J Phys Med Rehabil. 2002;81(11 Suppl):S127–47. doi: 10.1097/00002060-200211001-00014. 2002 Nov. [DOI] [PubMed] [Google Scholar]
- 41.Ohira Y, et al. Dependence of normal development of skeletal muscle in neonatal rats on load bearing. J Gravit Physiol. 2000;7(2):P27–30. 2000 Jul. [PubMed] [Google Scholar]
- 42.Peterson CA, Murphy RJ, Dupont-Versteegden EE, Houle JD. Cycling exercise and fetal spinal cord transplantation act synergistically on atrophied muscle following chronic spinal cord injury in rats. Neurorehabil Neural Repair. 2000;14(2):85–91. doi: 10.1177/154596830001400201. 2000. [DOI] [PubMed] [Google Scholar]
- 43.Roy RR, Acosta L., Jr Fiber type and fiber size changes in selected thigh muscles six months after low thoracic spinal cord transection in adult cats: exercise effects. Exp Neurol. 1986;92(3):675–85. doi: 10.1016/0014-4886(86)90308-0. [DOI] [PubMed] [Google Scholar]
- 44.Roy RR, et al. Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle Nerve. 1999;22(2):230–41. doi: 10.1002/(sici)1097-4598(199902)22:2<230::aid-mus11>3.0.co;2-r. 1999 Feb. [DOI] [PubMed] [Google Scholar]
- 45.Roy RR, Zhong H, Siengthai B, Edgerton VR. Activity-dependent influences are greater for fibers in rat medial gastrocnemius than tibialis anterior muscle. Muscle Nerve. 2005;32(4):473–82. doi: 10.1002/mus.20369. 2005 Oct. [DOI] [PubMed] [Google Scholar]
- 46.Vinay L, et al. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Brain Res Rev. 2002;40(1-3):118–29. doi: 10.1016/s0165-0173(02)00195-9. 2002 Oct. [DOI] [PubMed] [Google Scholar]
- 47.Westerga J, Gramsbergen A. Development of locomotion in the rat: the significance of early movements. Early Human Development. 1993;34:89–100. doi: 10.1016/0378-3782(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 48.Donoghue JP, Sanes JN. Organization of Adult Motor Cortex representation Patterns following neonatal forelimb nerve injury in rats. J Neurosci. 1988;8:3221–3232. doi: 10.1523/JNEUROSCI.08-09-03221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall RD, Lindholm EP. Organization of motor and sensory neocortex in the albino rat. Brain Research. 1974;66:23–28. [Google Scholar]
- 50.Hummelsheim H, Wiesendanger M. Is the hind-limb representation of the rat's cortex a sensorimotor amalgam? Brain Res. 1986;346:75–81. doi: 10.1016/0006-8993(85)91096-0. [DOI] [PubMed] [Google Scholar]
- 51.Udoekwere UI, Mbi LT, Ramakrishnan A, Giszter SF. Robot applied elastic fields at the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation. Proceedings of the IEEE/EMBC Conference; New York, NY. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke KA. Differential fore- and hindpaw force transmission in the walking rat. Physiol Behav. 1995;58(3):415–9. doi: 10.1016/0031-9384(95)00072-q. [DOI] [PubMed] [Google Scholar]
- 53.Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol. 2006;95(3):1397–409. doi: 10.1152/jn.01300.2004. Epub 2005 Oct 5. [DOI] [PubMed] [Google Scholar]
- 54.Howard CS, et al. Functional assessment in the rat by ground reaction forces. J Biomech. 2000;33(6):751–7. doi: 10.1016/s0021-9290(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 55.Kaya M, Leonard TR, Herzog W. Control of ground reaction forces by hindlimb muscles during cat locomotion. J Biomech. 2006;39(15):2752–66. doi: 10.1016/j.jbiomech.2005.10.012. Epub 2005 Nov 28. [DOI] [PubMed] [Google Scholar]
- 56.Lavoie S, McFadyen B, Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Exp Brain Res. 1995;106(1):39–56. doi: 10.1007/BF00241355. [DOI] [PubMed] [Google Scholar]
- 57.Muir GD, Whishaw IQ. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav Brain Res. 1999;103(1):45–53. doi: 10.1016/s0166-4328(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 58.Muir GD, Whishaw IQ. Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. Eur J Neurosci. 2000;12(3):1113–22. doi: 10.1046/j.1460-9568.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 59.Iscoe S. Control of abdominal muscles. Progress in Neurobiology. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 60.de Leon RD, et al. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002a;40(1-3):267–73. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- 61.Thota AK, et al. Neuromechanical Control of Locomotion in the Rat. J Neurotrauma. 2005;22:442–465. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- 62.Asada H, Slotine J-JE. Robot Analysis and Control. Wiley Interscience; NY: 1986. [Google Scholar]
- 63.Kargo WJ, Giszter SF. Individual premotor drive pulses, not time-varying synergies, are the units of adjustment for limb trajectories constructed in spinal-cord. J Neuroscience. 2008;28(10):2409–25. doi: 10.1523/JNEUROSCI.3229-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holzbaur KR, Delp SL, Gold GE, Murray WM. Moment-generating capacity of upper limb muscles in healthy adults. J Biomech. 2007 doi: 10.1016/j.jbiomech.2006.11.013. 2007 Jan 22 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40(4):742–9. doi: 10.1016/j.jbiomech.2006.11.011. 2007. Epub 2007 Jan 22. [DOI] [PubMed] [Google Scholar]
- 66.Lindstedt SL, Schaeffer PJ. Use of allometry in predicting anatomical and physiological parameters of mammals. Laboratory Animals. 2002;36:1–19. doi: 10.1258/0023677021911731. [DOI] [PubMed] [Google Scholar]
- 67.Payne RC, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giszter SF. Spinal Cord Injury: Present and Future Therapeutic Devices and Prostheses. Neurotherapeutics. 2008;5(1):147–162. doi: 10.1016/j.nurt.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]