Abstract
EphB receptors and their ephrinB ligands transduce bidirectional signals that mediate contact-dependent axon guidance primarily by promoting growth cone repulsion. However, how EphB receptor-mediated forward signaling induces axonal repulsion remains poorly understood. Here, we identify Nck and Pak proteins as essential forward signaling components of EphB2-dependent growth cone collapse in cortical neurons. We show that kinase-active EphB2 binds to Pak and promotes growth cone repulsion via Pak kinase activity, Pak-Nck binding, RhoA signaling and endocytosis. However, Pak’s function in this context appears to be independent of Rac/Cdc42-GTP, consistent with the absence of Rac-GTP production after ephrinB treatment of cortical neurons. Taken together, our findings suggest that ephrinB-activated EphB2 receptors recruit a novel Nck/Pak signaling complex to mediate repulsive cortical growth cone guidance, which may be relevant for EphB forward signaling-dependent axon guidance in vivo.
Keywords: Eph receptor, ephrin, axon guidance, growth cone, signal transduction
INTRODUCTION
The Eph family of receptor tyrosine kinases (Ephs) function with their membrane-bound ligands, the ephrins, to regulate the proper organization and connectivity of the developing nervous system (Egea and Klein, 2007; Pasquale, 2005; Shen and Cowan, 2010). Eph-ephrin binding requires cell-cell contact and can stimulate both forward and reverse signaling events that facilitate complex attractive and adhesive or repulsive cell responses. The Ephs comprise two subgroups in mammals, the EphA subclass (A1–A8, A10) and EphB subclass (B1–B4, B6), based in part on their binding affinities for ephrinA or ephrinB proteins, respectively (Pasquale, 2005). Eph forward signaling plays a major role in several repulsive axon guidance events during normal brain and spinal cord development, including roles in retinal ganglion cell (RGC) midline repulsion at the optic chiasm (Williams et al., 2003), topographic mapping of RGC axons within the thalamus and superior colliculus (Feldheim et al., 1998; Hindges et al., 2002), midline repulsion of corticospinal tract axons (Kullander et al., 2001a; Kullander et al., 2001b; Yokoyama et al., 2001), and thalamocortical axon topographic mapping (Dufour et al., 2003; Torii and Levitt, 2005).
In the developing brain, EphB receptors are highly expressed in the developing cortical plate, and are known to contribute to aspects of cortical axon guidance in vivo, including the formation of the corpus callosum (CC), an interhemispheral tract that connects layer 2/3 and layer 5 cortical neurons from one hemisphere to the other (Innocenti et al., 1995; Mendes et al., 2006), and the posterior branch of the anterior commissure (ACpp), an interhemispheral tract that connects cortical axons from temporal regions of the cerebral cortex (Henkemeyer et al., 1996; Orioli et al., 1996). While several studies have characterized EphB receptor-associated proteins, the critical signaling and cellular events that mediate Eph receptor signaling-dependent axonal repulsion remain poorly understood.
Here, we studied embryonic cortical neurons in culture to characterize EphB2 forward signaling pathways that are required for ephrinB2-induced cortical growth cone collapse. Interestingly, we find that several intracellular signaling proteins, including Nck, Pak, RhoA and Rho kinase, are all required for EphB2-dependent cortical growth cone repulsion. Interestingly, Pak’s novel kinase-dependent function in this process is dependent on Nck binding, but independent of Rac/Cdc42 binding or activity, suggesting a novel role and regulation for Pak in EphB2-mediated growth cone collapse.
MATERIALS AND METHODS
Animals
The EphB receptor knockout and knock-in mice used in this study, including EphB1−/−, EphB2−/−, EphB2Tr/Tr and EphB3−/− mice, were previously described (Genander et al., 2009; Henkemeyer et al., 1996; Orioli et al., 1996; Williams et al., 2003) and maintained in a CD1 background. EphB2Tr/Tr mice express C-terminal truncated fusion proteins containing the extracellular and trans-membrane domain of EphB2 conjugated to β-galactosidase (Henkemeyer et al., 1996). The resulting EphB2-βgal fusion proteins lack their respective intracellular tyrosine kinase catalytic domain and PDZ binding motif. For PCR genotyping, DNA was extracted from tails samples of dissected mice embryos with 100% isopropanol after an intervening lysis step. PCR was performed using the extracted DNA in a total volume of 15ul using the primer sets described previously (Henkemeyer et al., 1996; Orioli et al., 1996; Williams et al., 2003).
DNA Constructs
Expression plasmids for wild-type and mutant Pak1, Pak5, Rac1, Cdc42, and RhoA were described previously (Cotteret et al., 2003; Galisteo et al., 1996; Manser et al., 1994; Manser et al., 1998; Sells et al., 1997; Zhang et al., 1995) and obtained from Addgene. The compound mutant Pak1 (K299R/P13A) was generated by standard subcloning. The Pak1 shRNA plasmid and vector control for rodent Pak1 was previously described and validated (Yi et al., 2008). Expression plasmids for Nck1 and Dynamin K44A were also described previously (Hu et al., 2009; van der Bliek et al., 1993).
Antibodies
In most cases, the antibodies were commercially available and all were used at a 1:1000 dilution for immunoblotting (except where noted): Pak1 (CST), Nck (BD), anti-T7 epitope (Novagen), anti-M2 Flag epitope (Sigma), anti-Myc (MBL), anti-HA (Roche) (1:285 dilution), anti-Rac1 (Millipore), anti-pY490 TrkA (CST), anti-pY, 4G10 clone (Millipore). Antibodies against P-Eph receptors and anti-P-S198/S203 Pak1 were described previously (Shamah et al., 2001).
Dissociated Cortical Neuron Cultures
Embryonic cortical neurons were cultured from embryonic day 18 Long Evans Rats (CRL) or CD-1 mice at E16.5. The cortices were dissected and treated with 100 U papain (Worthington) for 3 min. The digestion was terminated by the addition of trypsin inhibitor (Sigma). The tissue was washed for a total of 3X with trypsin inhibitor, followed by 3 washes with plating medium consisting of DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% L-glutamine (Sigma), and 1% penicillin-streptomycin (Sigma). Neurons were mechanically dissociated with a pipet and plated on Poly-D-Lysine (Sigma)- and Laminin (Invitrogen)-coated coverslips in a 24-well plate at a density of 100,000 viable cells per dish. The culture medium was replaced with Neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen), L-glutamine (Sigma), and 1% penicillin-streptomycin (Sigma) at 24 hr after plating. For experiments analyzing EphB mutant mice, heterozygous crosses were used, and individual embryos were processed for cortical cultures, and PCR genotyping was performed the next day using tail snip DNA.
Cortical neuron transfections at 0 DIV
Following dissociation of rat or mouse cortical neurons with papain, and before plating, the neurons were co-transfected in solution with indicated expression vectors together with a plasmid expressing mCherry (1/10th of total DNA in transfection) using Lipofectamine 2000 (protocol available upon request). The total amount of plasmid DNA in the transfection was held constant, such that empty vector was included to increase levels where appropriate.
Immunoblotting
Samples were run on SDS-PAGE gels and transferred to PVDF membrane (GE Healthcare). The membranes were blocked in 10% milk/TBS-T (tween-20 at 0.05% v/v) for 1 hr and probed with 1° antibody for 2 hr at room temperature or overnight at 4 °C. The membranes were then incubated with a 2° antibody (1:10,000 G∞R IgG or G M IgG, Jackson ImmunoResearch Labs) for 1 hr at room temperature and developed with a homemade enhanced chemiluminescence (ECL) solution or Amersham™ ECL Plus Western Blotting Detection System (GE Healthcare).
GST-PBD Assay
At 6 days in culture, dissociated cortical neurons (plated at a density of 8 million cells per dish in a 10 cm dish) were treated with clustered EphrinB2/Fc or Fc alone or BDNF (0.1 μg/mL; Peprotech). GST-PBD assays were performed as described previously (Hale et al., 2011). In brief, the treated neurons were lysed in a buffer consisting of 50 mM Tris (pH 7.2), 1% (v/v) Triton X-100, 250 mM NaCl, and 10 mM MgCl2 and containing 10 μg GST-PBD per condition. Following incubation with 20 μL glutathione beads (50% slurry; GE Healthcare) for 1 hr at 4°C, beads were washed 3 times with a wash buffer consisting of 50 mM Tris (pH 7.2), 1% Triton X-100, 150 mM NaCl, and 5 mM MgCl2). Samples were eluted with 2X sample buffer, boiled and then loaded onto SDS-PAGE gels for Western blotting with anti-Rac antibodies (Santa Cruz).
Cell Transfections and Immunoprecipitations
HEK-293T cells were transfected using a calcium phosphate method. After ~24 hours, myc-Pak1 and HA-EphB2 transfected cells were lysed in a modified RIPA buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 5 mM NaF, 1mM activated Na3VO4, 1 mM PMSF). Pak1 was immunoprecipitated with 5 μl of anti-Myc epitope antibody (MBL) followed by incubation with 4 μl of protein-A agarose beads (Roche). Beads were washed 3 times with modified RIPA lysis buffer, prior to immunoblotting for anti-Pak1 or anti-HA.
Growth Cone Collapse Assays
Rat (E18) or mouse (E16.5) cortical neurons were prepared as previously described (Cowan et al., 2005) and cultured on PDL/Laminin-coated glass coverslips (100,000 neurons/well in a 24-well plate). After 48 hrs (rat) or 72 hrs (mouse) in culture, the neurons were treated with pre-clustered ephrinB2-Fc or Fc control (1–10 μg/ml final concentration) for 60 minutes at 37°C. Clustered ephrinB2/Fc, ephrinB1/Fc or Fc control (all from R&D systems) was prepared by incubation of 100 μg/ml ephrinB/Fc or Fc alone with 450 μg/ml goat anti-human Fc (Jackson ImmunoResearch Labs) for 60 min at room temp, then diluted to the final concentrations indicated in conditioned culture medium. Treated cortical neurons were fixed with paraformaldehyde (PFA) (4% PFA/2% sucrose/D-PBS) for 8–10 minutes and stained with Phalloidin-green (Molecular Probes) for 30 min. Collapsed or un-collapsed growth cones were scored under experimenter-blinded conditions using standard criteria (Shamah et al., 2001). In experiments with plasmid transfection, only neurons with detectable expression of mCherry fluorescence were analyzed. The RhoA inhibitor (Y-27632 - Sigma) (10 μM) and Rac inhibitor (NSC 23776 – Sigma) (10 μM) were added to conditioned medium for 2 hours prior to ephrinB2/Fc or Fc stimulation in indicated experiments.
RNA Isolation and Reverse Transcription
Whole cortices and retinae were dissected from E16.5 mice of either sex at various ages and processed immediately or snap frozen on ethanol/dry-ice. The samples were homogenized in TRIzol (Invitrogen) using a tissue homogenizer, and the RNA was precipitated with chloroform (Sigma). The remaining steps were carried out using the RNeasy® Micro kit (Qiagen). The RNA concentration of each sample was determined using a NanoDrop spectrophotometer and was reverse transcribed using the Superscript™ III First-Strand Synthesis System for RT-PCR (Invitrogen).
Quantitative Real-time PCR
All primers were designed to amplify a 150–200 base-pair product. The qRT-PCR primers for mouse Pak1-3 were as follows: (1) Pak1 forward, 5′-CTTGCTTCTCCCATTTCCTG-3′, and Pak1 reverse, 5′-GGGTAAACCCTTGCTCATCA-3′, (2) Pak2 forward, 5′-ACCGCGCTTGACGTTCGCATA-3′, and Pak2 reverse, 5′-AGAGGAAGGGAAGGTCACGAAGGA-3′, (3) Pak3 forward, 5′-GCCAGTAGTGAATCCCCTCA-3′, and Pak3 reverse, 5′-CGTTGGGTAAGGGATTTTGA-3′. The primers used to amplify mouse cyclophilin B were 5′-CATCTATGGTGAGCGCTTCCC-3′ (forward) and 5′-GCCTGTGGAATGTGAGGGGTG-3′ (reverse). The reactions were carried out using the SYBR Green PCR Master Mix (Ambion) and the ABI 7500 real-time PCR thermal cycler (ABI). Pak1-3 expression in the developing cortex and retina were determined by running reactions of 32 ng of RT-generated single-stranded cDNA. Fold changes relative to cyclophilin B were determined using the ΔΔCt method, in which mean fold change (2−Δ ΔCtAVE) and s.e.m. (abs(((2−Δ ΔCtAVE × 2−Δ ΔCtSEM) − (2−Δ ΔCtAVE/2−Δ ΔCtSEM))/2)) were determined.
Pak1 Kinase Assay
Primary cortical neurons (rat, E18) were cultured for 6 days prior to stimulation with pre-clustered ephrinB2-Fc or control Fc. Neurons were lysed in a modified RIPA buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 5 mM NaF, 1mM activated Na3VO4, 1 mM PMSF), and the soluble lysate fraction was incubated with 5 μl anti-Pak1 antibody (CST) to immunoprecipitate Pak1 with protein-A agarose beads (Roche). Immunoprecipitated Pak1 was then incubated with 5 μg MBP (myelin basic protein (Sigma)) plus gamma 32P-ATP (10 μCi/reaction) (75 μM ATP final) for 30 minutes at 30°C in kinase reaction buffer (25 mM HEPES, pH 7.5, 5 mM MgCl2, 1 mM MnCl2). Samples were run on a 12% SDS-PAGE gel to visualize MBP phosphorylation via Storm phosphorimager (Molecular Dynamics).
Data Analysis
Two-way ANOVA or Student’s t-test was used to determine statistical significance in growth cone collapse assay experiments.
RESULTS
Cortical neuron EphB2 receptors are required for ephrinB2-induced growth cone collapse
To study the potential role of EphB receptors in cortical growth cone collapse, we cultured embryonic rodent cortical neurons and treated them with clustered ephrinB2/Fc, a major repulsive ligand for EphB receptors, or clustered Fc control (1 μg/ml) for various times (3–60 minutes) before assessing either endogenous EphB receptor autophosphorylation at juxtamembrane (JM) tyrosines or the extent of growth cone collapse (GCC) (Figure 1A–C). Compared to clustered Fc control, treatment with clustered ephrinB2 led to a time-dependent increase in autophosphorylated Eph receptors and robust GCC as early as 10 minutes after treatment (Figure 1A,C). In the absence of clustering anti-Fc antibody, ephrinB2/Fc failed to increase P-Eph levels (data not shown), consistent with many previous studies. Interestingly, treatment with clustered ephrinB1/Fc, which is also an EphB receptor ligand, led to a much weaker stimulation of Eph receptor phosphorylation than ephrinB2 and failed to induce GCC (Figure 1A and 1D), suggesting that ephrinB2 is a more potent repulsive cue, at least in vitro, than ephrinB1 when presented at similar concentrations or that ephrinB1 binding to EphB2 does not promote repulsive signaling. Even at 3- or 10-fold higher concentrations of clustered ephrinB1/Fc (10 μg/ml), we observed little or no cortical GCC (Figure 1E).
Figure 1. EphrinB2 stimulates EphB activation and growth cone collapse of cultured cortical neurons.
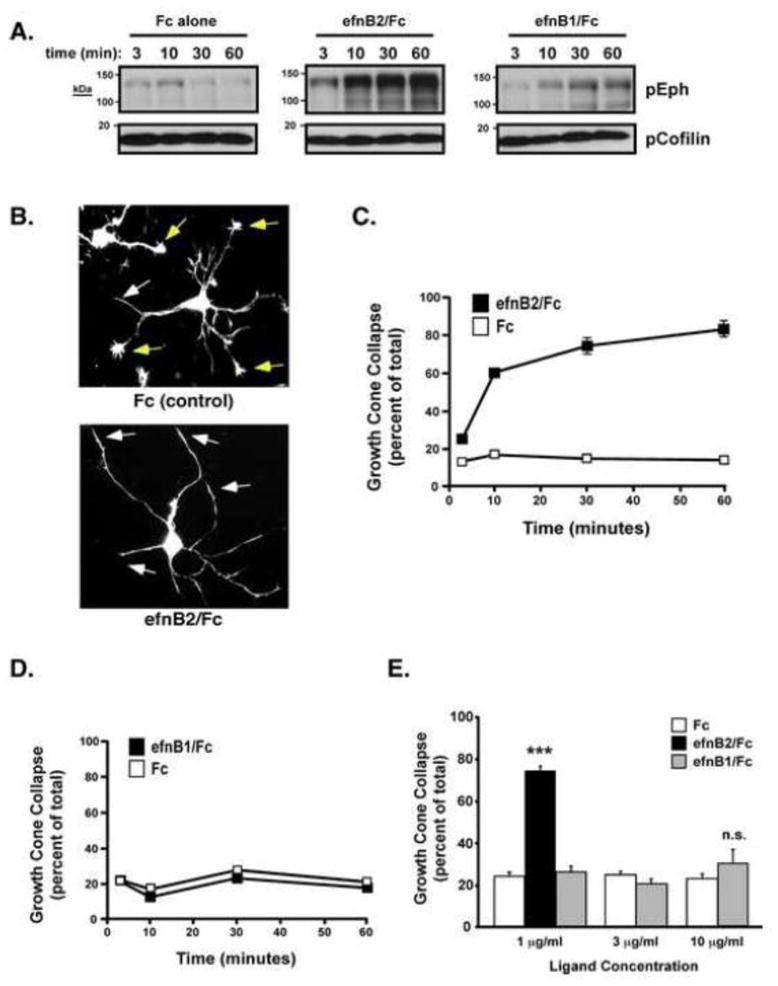
(A) E18 rat cortical neurons were stimulated with clustered ephrinB2 (efnB2), ephrinB1 (efnB1) or Fc control for 3, 10, 30 or 60 mins, and then lysates were immmunoblotted with a general anti-phospho-Eph antibody (Shamah et al, 2001) or a loading control anti-phospho-cofilin antibody.
(B) Growth cone morphology of Fc-control (top) and EphrinB2 (bottom) stimulation conditions. Uncollapsed (yellow arrows) and collapsed (white arrows) growth cones were visualized with phalloidin (Oregon-green).
(C) Time-course analysis of cortical (E18 + 2 DIV) neuron growth cone collapse upon preclustered Fc-control or ephrinB2/Fc addition (mean +/− SEM, n=9 from 3 independent experiments).
(D) Time-course analysis of cortical (E18 + 2 DIV) neuron growth cone collapse upon preclustered Fc-control or ephrinB1/Fc addition (mean +/− SEM, n=9 from 3 independent experiments).
(E) Cortical GCC (E18 + 2DIV) after 1 hr treatment with indicated preclustered ephrins or Fc control (mean +/− SEM, n=3, ***p<0.001 ephrin/B2 vs. Fc control, Student’s t-test).
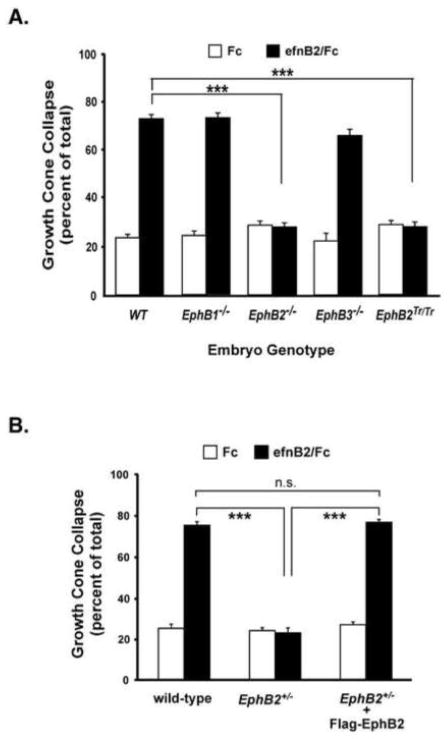
To determine which EphB receptors mediate the ephrinB2-induced growth cone collapse in cortical neuron cultures, we cultured E16.5 cortical neurons from wild-type, EphB1−/−, EphB2−/−, or EphB3−/− mutant mice (Henkemeyer et al., 1996; Orioli et al., 1996; Williams et al., 2003), or combinations of these mutant alleles (data not shown). We then treated the neurons with clustered ephrinB2/Fc (1 μg/ml) and measured GCC. EphrinB2 induced robust GCC in all genotype combinations, except for neurons cultured from EphB2−/− mice (Figure 2A), which indicates the EphB2 receptor is required to mediate ephrinB2-induced GCC in this context. As expected, neurons cultured from EphB2Tr/Tr forward signaling mutant mice also failed to undergo GCC in response to ephrinB2 treatment (Figure 2A, right), indicating that EphB2 forward signaling is essential for mediating ephrinB2-induced GCC in the cultured cortical neurons. Interestingly, even EphB2+/− cortical neurons had profound defects in ephrinB2-induced GCC (Figure 2B), and this could be rescued by transiently expressing Flag-EphB2 in the neurons (Figure 2B). Together these data suggest that ephrinB2-induced GCC is highly dependent on normal levels of EphB2 expression and forward signaling in the cortical neuron cultures.
Figure 2. EphB2 is required for ephrinB2-induced cortical neuron growth cone collapse.
(A) Growth cone collapse assay from EphB1−/−, EphB2−/−, EphB3−/−, EphB2T-lacZ/T-lacZ, and wild-type cultured mouse cortical neurons. Cultured neurons (E16.5 + 3 DIV) were treated with 1 μ/ml clustered Fc or ephrinB2/Fc for 1 hour prior to fixation, staining and blinded scoring (***p<0.001, Two-way ANOVA).
(B) Expression of EphB2 rescues the EphB2 deficit in ephrinB2/Fc induced GCC. EphB2−/− cortical neurons were transfected with pcDNA3-Flag-EphB2 or vector alone following dissociation. After ~72 hours in culture, the neurons were treated with clustered ephrinB2/Fc for 1 hr prior to fixation and analysis of GCC as in 3A (***p<0.001, Two-way ANOVA).
EphB2 receptor-mediated growth cone collapse requires the adaptor protein, Nck
Since the cultured cortical neurons provided a tractable, reductionist system in which to study EphB2-dependent GCC in primary neurons, we next tested candidate forward signaling molecules in ephrinB2-induced GCC. The Nck adaptor proteins have previously been implicated in EphB2 receptor forward signaling (Holland et al., 1997). Nck contains one Src homology type II (SH2) domain, which mediates binding to phosphorylated tyrosines, and three SH3 domains, which mediate physical interactions with proteins containing proline-rich regions. The second SH3 domain has been shown to mediate binding to a number of signaling proteins, including the p21-activated kinases (Paks) (Buday et al., 2002; Li et al., 2001). To test whether Nckα is required for EphB2-mediated GCC, we transiently transfected plasmids expressing wild-type or function-blocking, dominant negative Nck proteins (i.e. a SH2 domain (ΔSH2) deletion mutant or a middle SH3 domain deletion mutant (ΔSH3-#2)) into freshly-dissociated embryonic cortical neurons just prior to plating. This approach allows for rapid plasmid-directed protein expression for 2–3 days prior to ephrinB2 treatment. Transfected primary cortical neurons were treated with clustered ephrinB2/Fc (1 μg/ml) or Fc alone for 1 hour, and then the transfected neurons (detected by co-transfected mCherry expression) were analyzed for F-actin content (phalloidin) and general growth cone morphology. Whereas expression of vector alone or wild-type Nck had no effect on basal or ephrinB2-induced GCC, the expression of either the ΔSH2 Nck or the ΔSH3-#2 Nck significantly blocked ephrinB2-induced GCC without altering basal GCC (Figure 3A). In addition, we also analyzed the basal outgrowth of neurite extensions of these transfected neurons in the Fc-alone control condition. Here, we observe a modest but significant decrease in neurite length between vector control and Nck mutant conditions (Supplementary Figure 1). Together, these findings indicate that Nck is an essential downstream signaling molecule that mediates growth cone collapse and basal neurite outgrowth through functions requiring both its SH2 and middle SH3 domain.
Figure 3. Role for Nck1 and Pak1 in ephrinB2-induced growth cone collapse.
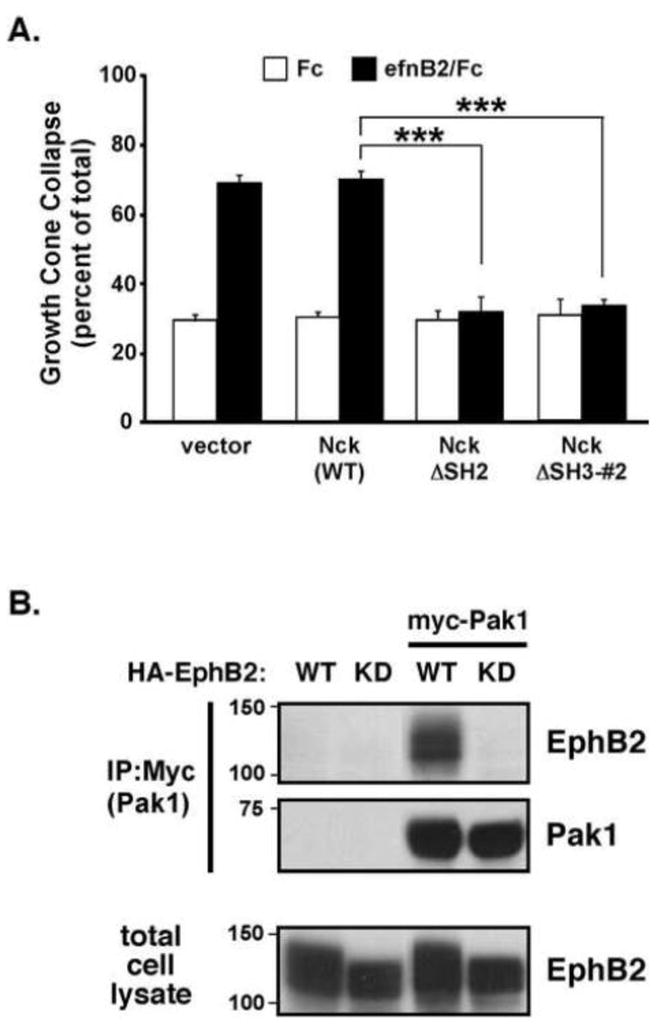
(A) Cultured rat cortical neurons (E18 + 2 DIV) were transfected with wild-type Nck1, the ΔSH2 deletion Nck1 mutant (Nck ΔSH2), a Nck1 mutant lacking a functional second SH3 domain (Nck ΔSH3-#2), or vector control and then treated with clustered Fc control (white bars) or ephrinB2/Fc (black bars) for 1 hr prior to analysis of GCC. (***p<0.001, Two-way ANOVA, n=9, data are mean of 3 independent experiments).
(B) Coimmunoprecipitation of Pak1 and EphB2 co-expressed in HEK293T cells. Cells were transfected with HA-tagged wild-type or kinase-dead (K643M) EphB2 with or without myc-tagged Pak1 prior to anti-myc IPs. IP and total cell lysates (30 μg) were Western blotted with anti-HA (EphB2) or anti-Pak1 antibodies.
Since the second Nck SH3 domain binds Pak-family proteins, which are known to regulate the F-actin cytoskeleton, we tested whether EphB2 receptors might physically interact with Pak. When co-expressed in HEK-293T cells, Pak1 (myc-tagged) was co-immunoprecipitated by wild-type EphB2 receptor (HA-tagged). However, a kinase-inactive mutant EphB2 receptor (K643M) failed to co-immunoprecipitate Pak1 (Figure 3B). Together, these data suggest that activated EphB2 receptors bind to Nck and Pak to form a functional signaling complex necessary for GCC.
Pak kinase activity is required for ephrinB2/EphB2-mediated growth cone collapse
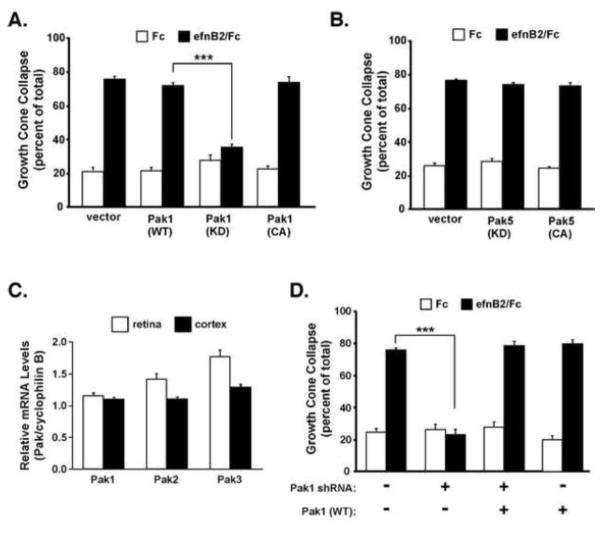
We next tested whether Pak kinase activity was important for ephrinB2-induced GCC in the cortical neurons. Similar to the Nck findings, we found that transfection of an empty vector or wild-type Pak1 plasmid into primary cortical neurons had no effect on ephrinB2-induced GCC, but transfection of a kinase-inactive Pak1 (K299R) plasmid significantly blocked GCC (Figure 4A). Interestingly, overexpression of constitutively-active Pak1 (King et al., 2000) had no effect on basal or ephrinB2-induced GCC (Figure 4A, right). These results indicate that Pak1 kinase activity is necessary for EphB2-mediated GCC, but expression of a hyperactive Pak1 kinase alone was not sufficient to promote GCC in the absence of ephrinB2 treatment. To test whether all Pak proteins are involved in this process, we expressed kinase-inactive or constitutively-active Pak5, a member of the group II Pak genes (Pak4-6), and measured ephrinB2-induced GCC. Unlike the Pak1 kinase-inactive mutant, expression of the kinase-inactive Pak5 mutant did not alter normal ephrinB2-induced GCC (Figure 4B), suggesting that ephrinB2-induced GCC may preferentially involve the group I Pak genes (Pak1-3).
Figure 4. EphrinB2-induced growth cone collapse requires Pak1 kinase activity.
(A) Growth cone collapse assay of E18 cultured rat cortical neurons transfected with wild-type Pak1, a kinase-dead Pak1 mutant (K299R), a constitutively active Pak1 mutant (L107F), or vector control (***p<0.001, Two-way ANOVA, n=9 from 3 independent experiments).
(B) Growth cone collapse assay of cultured cortical neurons transfected with WT or kinase activity mutants of Pak5.
(C) Relative mRNA expression of Pak1, Pak2 and Pak3 in whole retina (white bars) or cerebral cortex (black bars) from indicated developing mouse tissues (E16.5) and normalized to mRNA levels of the housekeeping gene, cyclophilin B (PpiB).
(D) Growth cone collapse assay of cultured cortical neurons transfected with a Pak1 shRNA plasmid with or without a wild-type, RNAi-resistant human Pak1 plasmid (***p<0.001, Two-way ANOVA, n=9 from 3 independent experiments).
To determine if other group I Pak genes are expressed in cortical neurons, we performed quantitative real-time PCR (qRT-PCR) on total RNA isolated from E16.5 cortex and retina, and compared relative expression of all three group I Pak genes in the cortex and retina (Figure 4C). Our findings indicated that all group one Paks are expressed at similar levels. To test the importance of Pak1, we transfected plasmids expressing a pre-validated Pak1 shRNA to reduce endogenous Pak1 mRNA and protein levels (Yi et al., 2008). We found that reducing Pak1 dramatically blocked ephrinB2-induced GCC in a Pak1 shRNA plasmid concentration-dependent manner (Figure 4D and data not shown). Furthermore, the induced GCC phenotype could be rescued by co-transfection with an RNAi-resistant human Pak1 expression plasmid (Figure 4D, right), suggesting that the Pak1 shRNA effects are not due to off-target RNAi effects. These data suggest an important functional role for Pak1 in EphB2 receptor-mediated GCC in cultured cortical neurons.
EphrinB2 stimulation does not regulate basal Pak1 kinase activity
Having established a role for Pak1 kinase activity in ephrinB2-induced GCC, we next tested whether ephrinB2/EphB2 activation regulates Pak1 kinase activity. Previous studies have shown that Rac-GTP-activated Pak1 induces autophosphorylation of two Pak1 serines (S198/S203) (Bokoch, 2003). Using a phosphorylation site-specific antibody to these autophosphorylation sites (Tolias et al., 2007)), we treated cultured neurons with clustered ephrinB2/Fc (1 μg/ml) for 3–60 minutes prior to analysis of autophosphorylated Pak1 (P-Pak1, Figure 5A). Unlike treatment with brain-derived neurotrophic factor (BDNF), which induced a rapid and robust induction of P-Pak1 (Figure 5A and (Hale et al., 2011)), treatment with clustered ephrinB2/Fc failed to induce a detectable increase in P-Pak1 levels at any time point analyzed (Figure 5A). Using these same experimental conditions, we also performed a kinase assay in cultured neurons to analyze Pak1 kianse activity in response to clustered ephrinB2 treatment. Kinase activity, as measured by MBP phosphorlyation, was relatively high in control conditions and only modestly increased upon ephrinB2 stimulation (Supplementary Figure 2). In addition, ephrinB2 treatment did not increase Rac-GTP levels at any time point (3–60 minutes) in the cultured cortical neurons using a standard GST-PBD pulldown assay (Figure 5B). Therefore, despite the importance of basal Pak1 kinase activity for ephrinB2-induced GCC (Figure 4A and 5C), ephrinB2 treatment does not appear to modulate Rac-GTP or Pak1 kinase activity levels under these conditions.
Figure 5. EphrinB2-stimulated cortical GCC requires Rac/Cdc42-independent Pak1 kinase activity and Nck and PIX/COOL binding to Pak1.
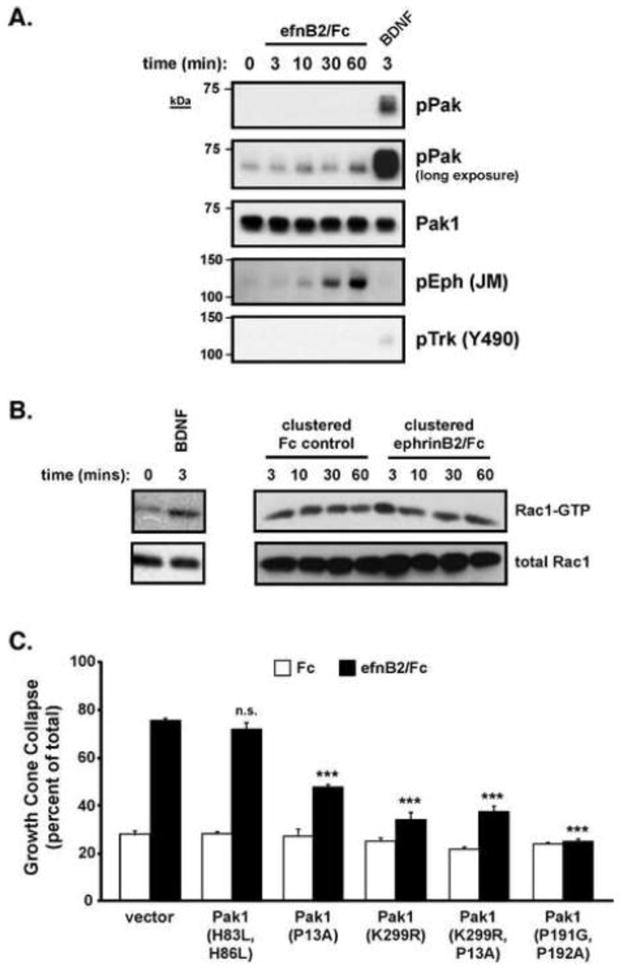
(A) Cultured cortical neurons (E18) were stimulated with clustered ephrinB2/FC for 0, 3, 10, 30 and 60 mins or human BDNF for 3 mins. Total cell lysates were immunoblotted with anti-phospho-Pak1 (S198/S203), anti-total Pak1, anti-phospho-Eph (JM) and anti-phospho-Trk (Y490) antibodies.
(B) Cultured rat cortical neurons were stimulated with clustered ephrinB2/Fc or Fc control for the indicated times prior to performing GST-PBD assays. GST-PBD bound Rac1-GTP or total cellular Rac1 levels were assessed by Western blotting with anti-Rac1 antibodies. Data are representative of 6 independent experiments.
(C) Standard GCC assay of cultured cortical neurons (rat E18 + 2 DIV) transfected with a Rac/Cdc42-GTP binding-deficient Pak1 mutant (H83L/H86L), a Nck-binding deficient Pak1 mutant (P13A), a kinase-dead Pak1 mutant (K299R), a double Pak1 mutant (P13A/K299R), a PIX/COOL-binding deficient mutant (P191G/P192G), or a vector only control (***p<0.001, Two-way ANOVA, n=9 from 3 independent experiments).
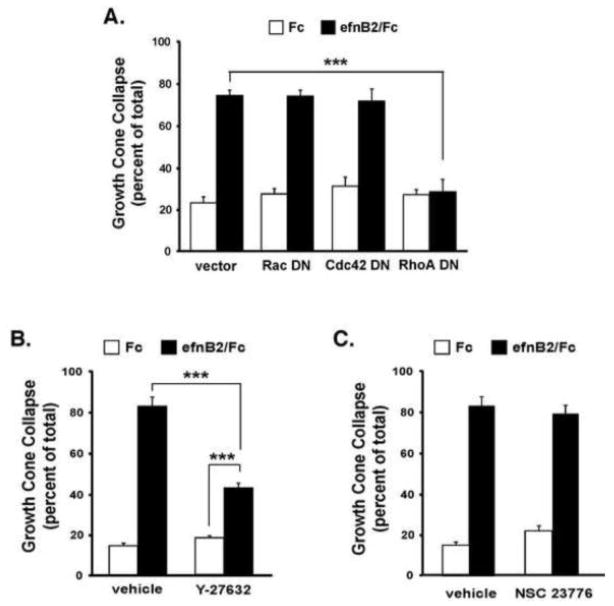
Since basal Rac-GTP levels are not altered by ephrinB2 treatment, we sought to test whether the function of Pak1 in GCC was independent of Rac/Cdc42 GTPase regulation. Unlike the kinase-inactive Pak1 mutant, overexpression of a Pak1 mutant (H83L/H86L) that cannot bind Rac/Cdc42-GTP (Sells et al., 1997) did not alter either basal or ephrinB2-induced GCC (Figure 5C). Consistent with this finding, expression of Rac1 (T17N) or Cdc42 (T17N) dominant negative mutants (Nofer et al., 2003; Palmieri et al., 2000) did not reduce ephrinB2-induced GCC (Figure 6A). This suggests that Rac/Cdc42 GTPase activities are not regulated by ephrinB2 and may not be required for ephrinB2-induced GCC in cortical neurons. In contrast, expression of the Nck binding-deficient Pak1 mutant (P13A) or the kinase-inactive Pak1 mutant (K299R) significantly reduced ephrinB2-induced GCC (Figure 5C). Moreover, a double Pak1 mutant that contained both the kinase-inactivating mutation and the Nck-binding mutation (K299R/P13A) did not result in a more severe dominant negative effect than either mutant alone (Figure 5C), indicating that Pak-Nck binding and Pak1 kinase activity likely function in the same molecular pathway to mediate ephrinB2-induced GCC. Interestingly, overexpression of a Pak1 mutant (P191G/P192A, (Manser et al., 1998)) that disrupts binding to the constitutive binding partner, PIX/COOL (a Rho-family GEF), dramatically reduced ephrinB2-induced GCC (Figure 5C). Taken together, these findings suggest that ephrinB2-induces the recruitment of Nck and kinase-active Pak1, and possibly PIX/COOL, to activated EphB2 receptors to promote cortical GCC. However, Rac/Cdc42 binding to Pak1 and activation of Rac/Cdc42-GTP appear to be non-essential for ephrinB2-induced GCC in this context.
Figure 6. RhoA and Rho-kinase activity are required for EphrinB2-induced growth cone collapse.
(A) Growth cone collapse assay of cultured rat cortical neurons (E18 + 2 DIV) transfected with dominant-negative Rac1 (T17N), Cdc42 (T17N), RhoA (T19N) mutants, or a vector only control (***p<0.001, Two-way ANOVA, n=3).
Growth cone collapse assay of E18 cultured cortical neurons pre-treated with either (B) the Rho kinase inhibitor (Y-27632, 10 μM) or (C) the Rac inhibitor (NSC 23776, 10 μM). Both assays were performed with appropriate vehicle control conditions. Data represent the mean +/− SEM of 3 independent experiments. (***p<0.001, Two-way ANOVA) (Y-27632 inhibitor: ***p<0.001 ephrin/B2 vs. Fc control, Student’s t-test).
The RhoA/Rho Kinase signaling cascade is required for EphrinB2-induced GCC
Numerous studies have reported an essential role for RhoA-GTP, and one of its effectors, Rho kinase, in the process of GCC in culture (e.g. Dickson, 2001; Schmandke and Strittmatter, 2007; Wahl et al., 2000). To test the importance of RhoA GTPases, we expressed dominant-interfering forms of RhoA (T19N) and measured the extent of ephrinB2-induced GCC. Consistent with previous studies, the dominant-negative RhoA robustly blocked ephrinB2-induced GCC (Fig. 6A). Since RhoA-GTP is required for GCC, we next sought to test the importance of one of its known downstream effectors, Rho kinase (ROCK), which has been reported previously to be important for ephrin-induced GCC and axon retraction ((Harbott and Nobes, 2005). Incubation of the cortical neurons with the Rho kinase inhibitor, Y-27632 (10 μM), resulted in a partial reduction in ephrinB2-induced GCC (Figure 6B), indicating that RhoA/Rho kinase signaling is required for EphB2 receptor-mediated GCC. In contrast, incubation with the “Rac inhibitor”, NSC23776 (10 μM), did not alter ephrinB2-induced GCC (Figure 6C), generally consistent with our findings that Rac-GTP is not a critical mediator of soluble ephrinB2 GCC in culture.
Endocytosis is required for ephrinB2-induced cortical GCC
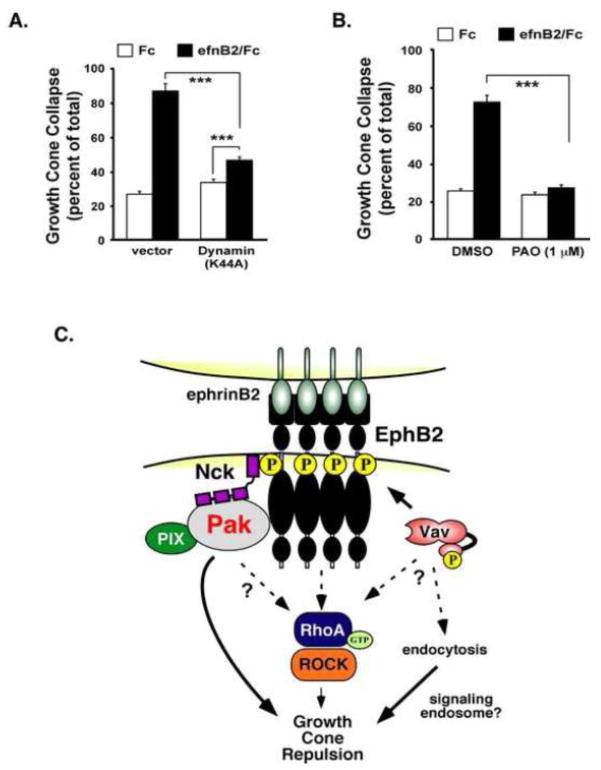
Several previous studies have indicated an important role for Eph receptor endocytosis in the process of cell and growth cone retraction (Cowan et al., 2005; Marston et al., 2003; Zimmer et al., 2003). To test the importance of endocytosis, we transiently-transfected a dominant-interfering mutant of Dynamin (K44A), a small GTPase required for receptor endocytosis, and measured ephrinB2-induced GCC. We observed that neurons expressing dynamin (K44A), a GTP binding deficient mutant that blocks endocytosis (Huang et al., 2010), had a significant reduction of ephrinB2-induced GCC (Figure 7A), suggesting that endocytosis is an important cellular process for ephrinB2-induced growth cone collapse. We observed similar effects by treating the neurons with phenylarsine oxide (PAO) (Figure 7B), a chemical inhibitor of clathryn-mediated receptor endocytosis (Gray et al., 2001; Hertel et al., 1985; Xia et al., 2009). These combined findings suggest an important role for endocytosis in ephrinB2-induced GCC.
Figure 7. Endocytosis is required for ephrinB2-induced cortical growth cone collapse.
(A) Growth cone collapse assay of cultured rat cortical neurons (E18 + 2 DIV) transfected with dominant-negative Dynamin (K44A) or a vector control. Neurons were stimulated with clustered Fc control (white bars) or ephrinB2/Fc (black bars) for 60 mins prior to analysis of GCC. Data represent the mean of 3 independent experiments. (***p<0.001, Two-way ANOVA, n=9) (Dynamin K44A: ***p<0.001 ephrin/B2 vs. Fc control, Student’s t-test).
(B) Growth cone collapse assay of E18 cultured rat cortical neurons pre-treated with PAO (1 M) or vehicle alone. (***p<0.001, Two-way ANOVA, n=6, from 2 independent experiments).
(C) Working model for the EphB2 forward signaling complexes required for growth cone collapse.
DISCUSSION
In this study, we identified several key signaling molecules and cellular processes required for endogenous EphB2-mediated cortical neuron GCC (Figure 7C), including a novel role for Pak in this process. We report a novel interaction between kinase-active EphB2 and Pak1, which likely occurs via interaction with the EphB2-interacting adaptor protein, Nck, and we show that Pak kinase activity and Nck are required for ephrinB2-induced GCC. However, our data suggest that ephrinB2 binding to EphB2 does not elevate Rac1-GTP to levels detectibly above baseline, which is consistent with a lack of effect by Rac/Cdc42 dominant negative expression on EphB2-mediated GCC. We also provide evidence that Pak1’s function in EphB2-dependent GCC requires binding to Nck, Pak kinase activity, and residues that are known to mediate binding to the PIX/COOL GEFs. However, a direct interaction between Pak1 and Rac/Cdc42-GTP does not appear to be required. These data suggest that recruitment of Pak to the ephrinB2/EphB2 receptor complex is necessary and sufficient to mediate its role in GCC. Consistent with previous studies, RhoA-GTP, Rho Kinase and endocytosis are all required for GCC, suggesting that the ephrinB2/EphB2 complexes recruit multiple key signaling components to orchestrate growth cone F-actin remodeling during repulsive guidance.
While our findings reveal a novel role for Pak signaling in EphB2-mediated GCC, these findings also raise many new questions for future study. Classically, Pak is thought to be an effector of Rac/Cdc42-GTP, and subsequent enhancement of Pak kinase activity results in F-actin remodeling through sequential activation of Lim kinase and phosphorylation of the F-actin binding protein, cofilin (Edwards et al., 1999). However, this pathway often results in generation of lamellapodia, membrane ruffles and focal adhesions (reviewed in (Bamburg, 1999)), which is in apparent conflict with presumed dynamics in growth cone repulsion. In our study, we did not observe any evidence that ephrinB2 treatment stimulates this Rac/Pak/LimK signaling cascade, as we do not observe an increase P-cofilin levels in response to ephrinB2 (Figure 1A & Supplementary Figure 2). Rather, we now report an important role for Pak1 and Nck in ephrinB2-induced GCC largely independent of Rac/Cdc42-GTP, which suggests that recruitment of Pak1/Nck to the activated EphB2 receptor complex has a distinct cellular function than Pak activated in response to upregulated Rac/Cdc42-GTP.
Previous work has reported a critical role for drosophila Pak and the Nck homolog, Dock, in the process of photoreceptor axon guidance in vivo (Garrity et al., 1996; Hing et al., 1999). In loss-of-function Dock or Pak mutant flies, R photoreceptor cell axons are mistargeted and disorganized within the lamina and medulla of the brain. Rescue studies in vivo revealed a critical role for Pak kinase activity, binding to PIX and Dock, and Rac/Cdc42-GTP binding (Hing et al., 1999). However, in the fruit fly, Rac/Cdc42 binding to Pak is required for R photoreceptor cell axon guidance (Hing et al., 1999), and as such, represents a significant departure from the mechanisms of EphB2-mediated cortical GCC in rodents. Interestingly, in both flies and mammals, the recruitment of Pak to the cell membrane appears to be sufficient to activate Pak kinase activity (Hing et al., 1999; Lu et al., 1997), so it is possible that Nck-mediated recruitment of Pak1 to the activated EphB2 receptor at the cell surface increases its intrinsic kinase activity, but Pak1 autophosphorylation of the S/T sites only occurs upon Rac/Cdc42-GTP binding. As such, we speculate that either: (1) the basal Pak kinase activity, recruited to the EphB2 receptors, is sufficient for its role in GCC or (2) the recruitment of Pak to the activated EphB2 receptors at the plasma membrane increases Pak1 kinase activity in a Rac-independent manner. We observed that Pak1 isolated from ephrinB2-treated neurons possesses increased kinase activity toward a known substrate, MBP (Supplemental Figure S2), suggesting that Pak activity has indeed been altered in response to ephrinB2 treatment.
Nck genes are critical for proper cortical axon guidance in vivo such that the conditional deletion of both Nck1 and Nck2 genes leads to deficits in corticospinal tract axon guidance and a reduced posterior anterior commissure (Fawcett et al., 2007). Interestingly, individual Nck1 or Nck2 KO mice appeared normal, suggesting that these two genes serve largely redundant functions during development or can compensate for the loss of the other gene. Similar to this study, we found that expression of the Nck2 (ΔSH3-2) mutant also blocked ephrinB2-induced GCC (N. Srivastava, unpublished observations), suggesting functional redundancy in EphB2-mediated GCC in vitro. Interestingly, Nck2 recruits Pak1 to mediate ephrinB3 reverse signaling-dependent axonal pruning of hippocampal dentate gyrus neurons during development (Xu and Henkemeyer, 2009), suggesting that Nck/Pak may be a common signaling complex for both forward (Eph receptor) and reverse (ephrinB) repulsive axon guidance signaling.
In addition to ephrin-induced recruitment of signaling proteins to the clustered Eph receptors, ephrin-induced endocytosis has emerged as an important step for forward signaling and GCC (Cowan et al., 2005; Fournier et al., 2000; Jurney et al., 2002; Marston et al., 2003; Zimmer et al., 2003). Our present findings that both dynamin (K44A) mutant and PAO block ephrinB2-induced GCC (Figure 7A–B) are consistent with a key role for EphB2 and/or plasma membrane internalization in GCC. Surprisingly, we do not observe a key role for Rac-GTP in ephrinB2-induced GCC, which is distinct from findings of ephrinB2/EphB4-mediated cell-cell repulsion in a Swiss 3T3 cultures (Marston et al., 2003). However, it is interesting to note that Pak1 kinase activity, independent of Rac/Cdc42 binding, has been linked to a form of endocytosis, macropinocytosis, in non-neuronal cells (Dharmawardhane et al., 2000). It is admittedly difficult to interpret a negative result from the Rac dominant negative experiments in our study, but taken together with the absence of ephrinB2-induced Rac-GTP and P-Pak production (Figure 5A,B), the failure of the Rac/Cdc42-binding mutant of Pak (H83L,H86L) to reduce ephrinB2-induced GCC (Figure 5C), and the failure of the NSC “Rac inhibitor” to block GCC, these combined findings suggest that Rac-GTP function is likely dispensable for Pak’s role in ephrinB2-induced GCC in this context.
Taken together, our findings reveal important EphB2 receptor forward signaling mechanisms that are required for ephrinB2-induced cortical growth cone collapse. Considering the importance of axon guidance for proper cortical neuronal connectivity in vivo, the discovery of new repulsive signaling factors, such as Nck and Pak, may reveal new therapeutic targets for the treatment of neurodevelopmental disorders or axonal regeneration following brain injury.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Michael Greenberg for generously sharing various published antibodies and plasmids generated in his lab, and we acknowledge Addgene for numerous Pak1, Pak5, RhoA, Rac, and Cdc42 plasmids. We also thank Dr. Louise Larose for the generous gift of Nck1 WT and mutant expression plasmids. Purified GST-PBD protein was provided by the NEI-funded Recombinant Protein and Virus Core (P30 EY020799). M.A.R. was supported by a training grant from NIDA (T32 DA07290). We acknowledge the generous support of the Whitehall Foundation (C.W.C.), and grants from the National Institutes of Health (RO1 EY018207 to C.W.C.) and (RO1 MH66332 to M.H.).
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23:5526–5539. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Georgiou J, Ruston J, Bladt F, Sherman A, Warner N, Saab BJ, Scott R, Roder JC, Pawson T. Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci U S A. 2007;104:20973–20978. doi: 10.1073/pnas.0710316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisen J, Lu Q, Barbacid M, Flanagan JG. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol. 2000;149:411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Garrity PA, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky SL. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85:639–650. doi: 10.1016/s0092-8674(00)81231-3. [DOI] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Sheffler DJ, Bhatnagar A, Woods JA, Hufeisen SJ, Benovic JL, Roth BL. Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine(2A) receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- Hale CF, Dietz KC, Varela JA, Wood CB, Zirlin BC, Leverich LS, Greene RW, Cowan CW. Essential role for vav Guanine nucleotide exchange factors in brain-derived neurotrophic factor-induced dendritic spine growth and synapse plasticity. J Neurosci. 2011;31:12426–12436. doi: 10.1523/JNEUROSCI.0685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbott LK, Nobes CD. A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol Cell Neurosci. 2005;30:1–11. doi: 10.1016/j.mcn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Hertel C, Coulter SJ, Perkins JP. A comparison of catecholamine-induced internalization of beta-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J Biol Chem. 1985;260:12547–12553. [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Shi G, Larose L, Rivera GM, Mayer BJ, Zhou R. Regulation of process retraction and cell migration by EphA3 is mediated by the adaptor protein Nck1. Biochemistry. 2009;48:6369–6378. doi: 10.1021/bi900831k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng X, Zhuang J, Frohlich O, Klein JD, Cai H, Sands JM, Chen G. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae- and clathrin-coated pit pathways. Am J Physiol Renal Physiol. 2010;299:F1389–1395. doi: 10.1152/ajprenal.00718.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Aggoun-Zouaoui D, Lehmann P. Cellular aspects of callosal connections and their development. Neuropsychologia. 1995;33:961–987. doi: 10.1016/0028-3932(95)00033-y. [DOI] [PubMed] [Google Scholar]
- Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001a;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001b;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20:6403–6417. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Mendes SW, Henkemeyer M, Liebl DJ. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci. 2006;26:882–892. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofer JR, Feuerborn R, Levkau B, Sokoll A, Seedorf U, Assmann G. Involvement of Cdc42 signaling in apoA-I-induced cholesterol efflux. J Biol Chem. 2003;278:53055–53062. doi: 10.1074/jbc.M305673200. [DOI] [PubMed] [Google Scholar]
- Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996;15:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- Palmieri SJ, Nebl T, Pope RK, Seastone DJ, Lee E, Hinchcliffe EH, Sluder G, Knecht D, Cardelli J, Luna EJ. Mutant Rac1B expression in Dictyostelium: effects on morphology, growth, endocytosis, development, and the actin cytoskeleton. Cell Motil Cytoskeleton. 2000;46:285–304. doi: 10.1002/1097-0169(200008)46:4<285::AID-CM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48:563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7937. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108(Pt 6):2285–2292. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.