Abstract
In the tongue, distinct classes of taste receptor cells detect the five basic tastes, sweet, sour, bitter, sodium salt, and umami1,2. Among these qualities, bitter and sour stimuli are innately aversive, whereas sweet and umami are appetitive, and generally attractive to animals. In contrast, salty taste is unique in that increasing salt concentration fundamentally transforms an innately appetitive stimulus into a powerfully aversive one3–7. This appetitive-aversive balance helps maintain appropriate salt consumption3,4,6,8, and represents an important part of fluid and electrolyte homeostasis. We have previously shown that the appetitive responses to NaCl are mediated by taste receptor cells expressing the epithelial sodium channel, ENaC8, while the cellular substrate for salt aversion was unknown. Here we explore the cellular and molecular basis for the rejection of high concentrations of salts (>300 mM NaCl or KCl). We now show that high-salt recruits the two primary aversive taste pathways by activating the sour and bitter taste-sensing cells. We also demonstrate that genetic silencing of these pathways abolishes behavioral aversion to concentrated salt, without impairing salt attraction. Notably, mice devoid of salt-aversion pathways now exhibit unimpeded, continuous attraction even to exceedingly high concentrations of NaCl. We propose that the “co-opting” of sour and bitter neural pathways evolved as a means to ensure that high levels of salt reliably trigger robust behavioral rejection, thus preventing its potentially detrimental effects in health and well-being.
Sodium is an essential ion, and as such animals have evolved dedicated salt-sensing systems, including prominent detectors in the taste system. Salt taste in mammals can trigger two opposing behavioral responses. On the one hand, low concentrations of salt (<100 mM NaCl, referred to as “low-salt”) are generally appetitive and elicit behavioral attraction. On the other hand, high concentrations (>300 mM, referred as “high-salt”) are aversive, and provoke strong behavioral rejection. Notably, the attractive salt pathway is selectively responsive to sodium (underscoring the key requirement of NaCl in the diet), while the aversive one functions as a non-selective detector for a wide range of salts3,4,6,7.
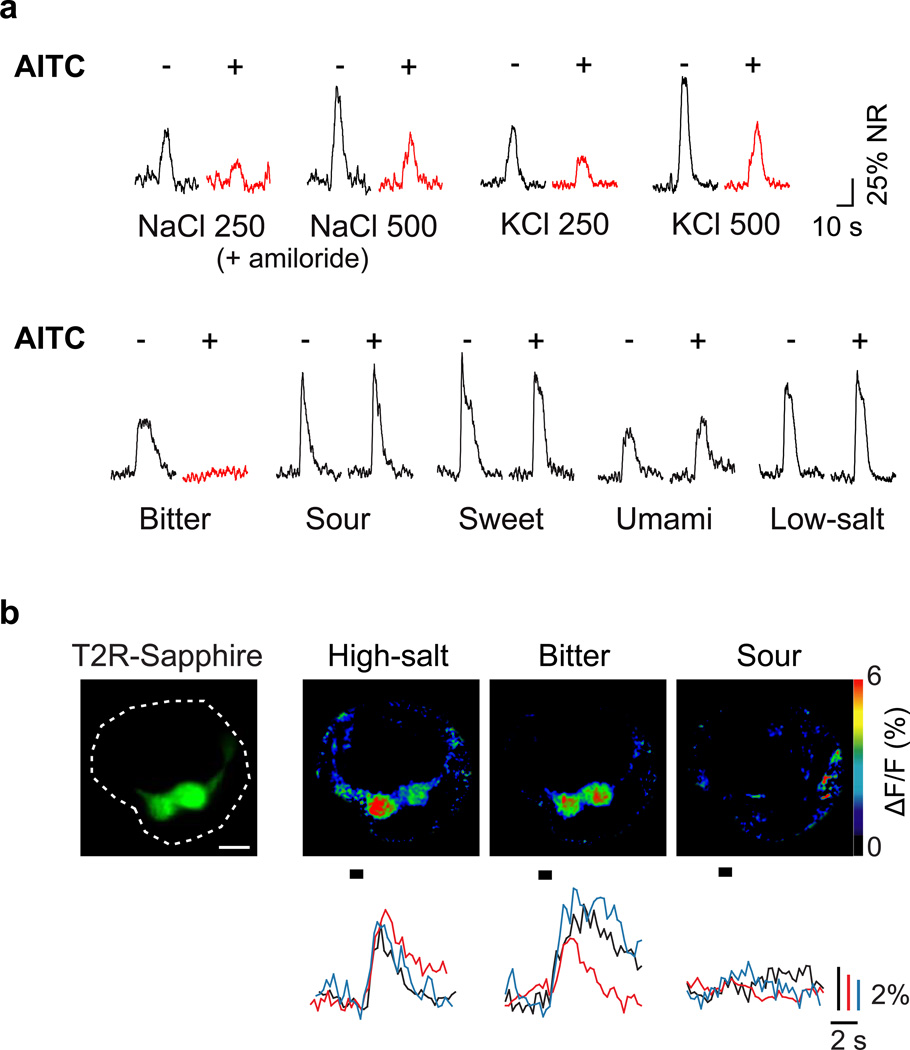
For many years, the sensitivity of ENaC to the diuretic amiloride9–12 has been used as a powerful means to block ENaC function and separate the contributions of the appetitive and aversive salt pathways8,10,13. We reasoned that if we could identify an equivalent pharmacological blocker for the high-salt sensing pathway, it might provide a valuable tool to dissect the cellular basis of high-salt taste. To this end, we recorded chorda tympani taste responses in the presence or absence of various compounds (Supplementary Table 1) known to affect ion channel function and found that allyl isothiocyanate (AITC), a component of mustard oil (and the source of its pungency) significantly suppressed high-sodium responses (Figure 1a upper panel) without affecting responses to low concentrations of NaCl (see Methods); identical suppression was observed for KCl, which selectively activates the high-salt pathway (Figure 1a and Supplementary Figure 1). Interestingly, AITC also inhibited responses to bitter stimuli without significantly impacting any other taste modality (Figure 1a lower panel and Supplementary Figure 2; see Methods for details on conditions). These results suggested that bitter taste receptor cells might be the target of AITC, and a constituent of the high-salt sensing pathway. Thus, we next asked if bitter-sensing cells are activated by high-salt stimuli.
Figure 1. Bitter receptor cells mediate high-salt taste responses.
Allyl isothiocyanate (AITC) acts as a selective inhibitor of bitter and high-salt taste responses. (a) Shown are integrated chorda tympani responses to taste stimuli (see methods for details) before (−) and after (+) application of AITC; amiloride was used to selectively eliminate the contribution of the ENaC-dependent, low-salt pathways. AITC completely inhibited bitter responses (0.1 mM cycloheximide) and significantly suppressed high-salt (250 or 500 mM NaCl + amiloride and KCl) responses (highlighted in red) but did not affect responses to low salt (60 mM NaCl) or other taste qualities; representative responses from multiple animals are shown. See Supplementary Figure 1 for quantitation. (b) Calcium imaging of taste cell responses confirmed that T2R32-Sapphire positive taste cells respond to bitter stimuli (mixture of 1 mM cycloheximide, 1 mM quinine, and 10 mM denatonium) and high-salt (500 mM KCl) but not to sour stimuli (100 mM citric acid). Shown is a taste bud overlaid with Sapphire fluorescence (dotted circle, left) and pseudo-colored images depicting taste responses to high-salt, bitter and sour stimuli (right panels); scale bar, 10 µm. Below the imaging panels are representative ΔF/F traces for these tastants from three additional Sapphire-positive cells. In total, 15 and 12 Sapphire-positive cells were activated by bitter and KCl respectively; among these, 11 cells were activated by both compounds, but not by sour stimuli (see Supplementary Figure 4)
We directly examined salt responses using a peeled epithelium preparation that allows functional imaging of TRCs in response to tastant stimulation with single cell resolution8. In essence, TRCs from fungiform papillae were loaded with the calcium-sensitive dye, Calcium Green-1 in vivo, and then stimulated and imaged, ex vivo8. To ensure we focused on bitter-sensing cells, we used mice expressing a GFP-Sapphire reporter selectively in T2R-positive cells14 (Supplementary Figure 3). In these animals, high concentrations of salt indeed activated the GFP-positive cells, which in turn responded to bitter (Figure 1b and Supplementary Figure 4).
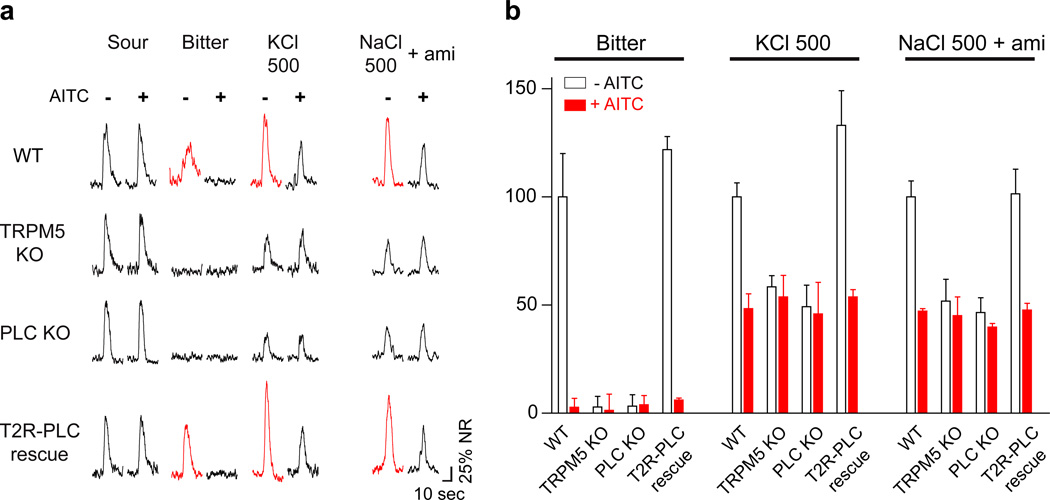
The finding that high-salt activates bitter-sensing cells and the observation that high-salt and bitter stimuli are both blocked by AITC suggest that bitter and high-salt may share a common pathway (e.g. through the T2R pathway). If so, we would expect TRPM5 or PLCβ2 knockout (KO) mice15, which lack key components for bitter taste signaling, to be also defective in high-salt sensing. Indeed, Figure 2 shows this to be the case: the nerve responses of the knockout animals to high-salt are significantly reduced, and are no longer sensitive to AITC. To rigorously demonstrate that the TRPM5- and PLCβ2-dependent high-salt responses are mediated by bitter receptor cells, we conducted a selective-rescue experiment whereby PLC function was restored only to bitter taste receptor cells of PLCβ2 knockout mice. As hypothesized, expressing a wild-type PLC transgene in bitter receptor cells fully rescued the electrophysiological responses to both bitter and high-salt (e.g. KCl) to levels indistinguishable from those in wild type mice (Figure 2a bottom panel and 2b). These results demonstrate that bitter sensing cells mediate the PLCβ2-dependent high-salt responses, and support the proposal that the aversion to high-salt is mediated, at least in part, by activation of the bitter-sensing pathway.
Figure 2. High-salt responses in bitter cells are TRPM5/PLCβ2 dependent.
(a) Representative chorda tympani responses from control (WT), TRPM5-KO, PLCβ2-KO (PLC KO) and T2R32-PLCβ2 (T2R-PLC) rescue mice before (−) and after (+) application of AITC. Note that both TRPM5-KO and PLCβ2-KO mice lose bitter responses and significant part of their response to high-salt together with all sensitivity to AITC. Expressing PLCβ2 in just the bitter cells of PLCβ2-KO mice (T2R-PLC rescue) fully restores normal bitter and high-salt responses as well as AITC sensitivity of these responses (denoted by red traces); note responses to NaCl and amiloride were from different animals from the other responses shown. (b) Quantification of normalized responses, before (open bars) and after (red bars) application of AITC (mean ± s.e.m, n ≥ 3 animals, see methods for normalization). AITC treatment almost completely suppressed responses to 0.1 mM cycloheximide and reduced by half the responses to 500 mM KCl and 500 mM NaCl in the presence of 10 µM amiloride in control and T2R-PLC rescue animals (Student’s t-test, P < 0.05).
AITC and TRPM5/ PLCβ2 knockouts eliminate only ~50% of the high-salt neural responses (Figure 2). Not surprisingly, these animals still retain strong behavioral aversion to high-salts15. Which additional cells mediate the remaining neural responses and behavior? Given that high-salt is strongly aversive, and recruits one of the primary aversive taste pathways, we wondered whether sour, the other principal aversive pathway may mediate the remaining responses.
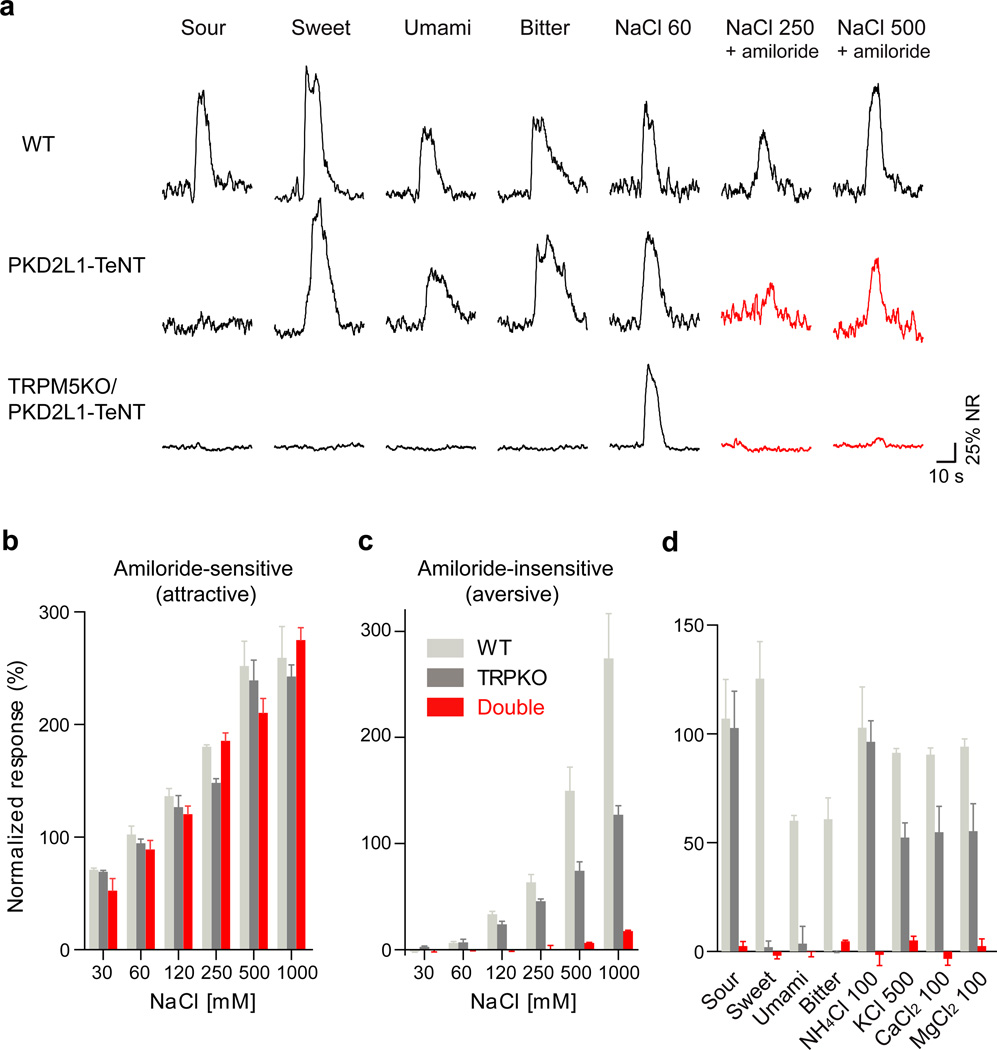
To examine the involvement of sour-sensing cells in high-salt detection, we inactivated the sour TRCs (i.e. PKD2L1-expressing cells16) by silencing their synaptic machinery. In essence, we engineered animals in which tetanus toxin light chain (TeNT) was targeted to PKD2L1-expressing cells17,18 and then assayed their tastant-evoked neural activity in response to salt stimulation. As shown previously17, silencing PKD2L1-expressing cells eliminates acid-evoked taste responses (Figure 3a). However, as shown in figure 3, these animals also display a major reduction in their high-salt electrophysiological responses, and further treatment with AITC effectively abolished their remaining high-salt (KCl) responses (Supplementary Figure 5). We thus considered that high-salt taste responses are most likely mediated by the combined action of bitter and sour-sensing cells, and hypothesized that genetically blocking both pathways should abolish high-salt responses. Indeed, double mutant mice expressing PKD2L1-TeNT and harboring a TRPM5 mutation exhibit a near complete loss of electrophysiological taste responses to a variety of high-salts (Figure 3), including concentrations of NaCl as high as 1000 mM.
Figure 3. PKD2L1-expressing cells mediate the residual TRPM5/PLCβ2-independent high-salt responses.
Integrated chorda tympani recordings show that (a) silencing PKD2L1 sour-cells affects high-salt taste responses. PKD2L1-TeNT mice have severe deficits in their responses to high-salt while TRPM5-KO / PKD2L1-TeNT double mutant animals completely lose all amiloride insensitive NaCl (high-salt) responses (highlighted as red traces). (b) Quantification demonstrates that TRPM5-KO / PKD2L1-TeNT (Double, shown as red bars) mice exhibit normal responses to low salt, but (c) lack responses to high-salt (Student’s t-test, P< 0.001). (d) The double mutant animals also fail to respond to sweet, bitter, sour, umami as well as non-sodium salts. Data (b–d) were normalized to the response of 60 mM NaCl and are means ± s.e.m, n ≥ 3 animals.
Importantly, if these two cellular pathways are the mediators of behavioral aversion to high-salt, then simultaneously silencing both the T2R and PKD2L1-expressing cells should abolish rejection of concentrated salt solutions. As shown in Figure 4 and Supplementary Figure 6, single mutant mice (i.e. TRPM5−/− or PKD2L1-TeNT) still retain strong aversion to high-salt, demonstrating that activation of either pathway on its own is sufficient to trigger behavioral rejection to salt. However, double mutant animals exhibit no salt aversion even at concentrations where controls are strongly repelled. Remarkably, these double mutants are not simply indifferent to high-salt, but now exhibit unimpeded attraction, even to exceedingly high concentrations of salt (e.g. levels equivalent to ocean water; ~500 mM NaCl; Figure 4b). Thus, under normal conditions the appetitive-aversive balance to salt, which collectively tunes the animal’s behavioral response to sodium salts, must be orchestrated by the combined activity of the attractive ENaC pathway (which remains in the bitter/sour double mutants) and the repulsive T2R and sour pathways.
Figure 4. TRPM5-KO / PKD2L1-TeNT mice exhibit no taste aversion to high-salt.
Immediate lick assays were used to measure behavioral responses to KCl (aversive, panel a) and NaCl (attractive, panel b). (a) Control mice (WT, solid black line) exhibit robust dose dependent behavioral aversion to increasing concentrations of KCl. In contrast, TRPM5-KO / PKD2L1-TeNT double mutant animals (red line) do not avoid high-salt stimuli; single mutants (dotted lines) behave as control animals. Two-way ANOVA with post hoc test for individual concentrations revealed significant differences at 500 mM KCl between the double mutants and other genotypes (P< 0.001), and at 250 mM KCl between the double mutants and control or PKD2L1-TeNT mice (P<0.001). (b) After sodium depletion, control mice (black line) exhibit powerful attractive responses to NaCl (see also Supplementary Figure 7) but the attraction is considerably reduced at higher concentration (500 mM). In contrast, double mutant animals (red line) show a continuous increase in attraction even at concentrations as high as 500 mM NaCl (two-way ANOVA with post hoc test, P< 0.001). Values are means ± s.e.m., n ≥ 6 mice.
How does high-salt activate the bitter and sour taste receptor cells? The answer to this question is not known. However, given that the primary effectors of T2R signaling in bitter cells, PLCβ2 and TRPM5, are also required for high-salt sensing by the bitter cells, we suggest that either a signaling component in bitter cells (for example an ion channel), or one or more of the three dozen T2R-receptors might be sensitive to high concentrations of salt (i.e. perhaps causing the serendipitous transition between the receptor’s inactive and active state)19,20. What about sour-sensing cells? A salient feature of sour cells is the prominent expression of carbonic anhydrase 4 (CA417), a membrane-bound isoform of carbonic anhydrase. CA4 is likely involved in buffering the pH around taste receptor cells (CO2 + H2O <−> HCO3− + H+), and therefore its activity may directly impact local proton concentration and acid sensing. Notably, carbonic anhydrases are known to be sensitive to high ionic strength environments, with high-salt concentrations strongly inhibiting their enzymatic activity21,22. This raises the possibility that CA4 may function as a “translator” of external salts into local pH changes, and thus operate as an important component of high-salt receptor in sour-sensing cells. Indeed, supporting this proposal, our results (Supplementary Figures 8 and 9) demonstrate that pharmacological inhibition of tongue carbonic anhydrases, or the knockout of CA4, greatly impair high-salt sensing by the sour taste receptor cells.
Taken together, our studies demonstrate that salts activate 3 different classes of TRCs: the appetitive responses are mediated through the sodium selective ENaC pathway1, while the rejection of high-salt results from the recruitment of the sour and bitter pathways. At a cellular level, these results explain the conundrum of a “valence change” by reducing the problem to simply having distinct cell types with well-defined but opposing valences responding to salt. And, at a physiological level, these findings now provide a simple explanation for the long-standing observation that bitter and sour afferent fibers behave as “generalists”, responding not only to bitter and acid stimuli, but also to a variety of salts23. Importantly, we note that the fact that T2R- and PKD2L1-cells are also activated by high-salt does not imply a change in the logic of taste coding, or in the valence/quality encoded by these TRCs. In fact, if we assume that rodents and humans use similar taste signaling mechanisms, then the bitterness24 and “ionic” taste associated with high concentrations of non-sodium salts in humans may indeed be mediated by the concurrent activation of T2R- and PKD2L1- expressing cells. But why doesn’t high-salt taste like a mix of bitter and sour? Sourness represents the detection of protons by at least two separate signaling pathways in the oral cavity: taste (via PKD2L1-cells) and non-taste (via TRPV1-, ASIC-, etc.)25–27, thus we suggest that the activation of PKD2L1 cells, in the absence of the non-taste acid sensing pathway may instead evoke the ionic taste characteristic of high concentrations of non-sodium salts. This proposal recasts PKD2L1 cells, and their corresponding (labeled) neural line as sensors of ions (protons, potassium, etc), orchestrating different percepts whether activated alone (e.g. ionic taste) or in combination: PKD2L1 + non-taste acid sensors = sourness while PKD2L1 + T2Rs = the taste of KCl and other non-sodium salts.
Future studies using specific inhibitors and activators of each pathway should help address the contributions of the ENaC-, T2R- and PKD2L1-expressing taste cells to human salt taste perception, and possibly serve as a springboard for the development of selective receptor cell modulators to help control (and even satisfy) the strong appetite of the Western world for a high-salt diet, but without the potential ill effects of too much sodium.
Online Methods
Mice
All procedures followed the NIH Guidelines for the care and use of laboratory animals, and were approved by the Columbia University or National Institute of Dental and Craniofacial Research Animal Care and Use Committees. T2R32-Sapphire mice are transgenics engineered to express the blue shifted GFP-derivative, Sapphire28, under the control of the T2R32 (also referred to as Tas2R139, PubMed gene #NM_181275.1) promoter. These mice, generated by Ken Mueller (UCSD Thesis, 2004), contained 10 kbp upstream of the T2R32 start codon fused to the GFP reporter. Expression of Sapphire in taste tissue was characterized using double label in situ hybridization (see Supplementary Figure 3). All other mouse strains have been described previously14–17.
Calcium imaging
Calcium imaging from fungiform TRCs was performed as previously described8,29. Briefly, fungiform TRCs were loaded in vivo with Calcium Green-1 dextran 3 kD (Invitrogen) by electroporating individual taste buds. Tongues were removed 24 – 36 h after dye loading and the epithelium was peeled enzymatically and placed in a custom recording chamber. The apical surface of the preparation was bathed in a constant flow of artificial saliva, and taste stimuli were delivered by focal application to individual taste buds. Tastants were applied for 1 s, with a minimum of 10 s of artificial saliva between stimuli. Changes in [Ca2+]i were monitored using a 5-Live confocal microscope (Zeiss) equipped with a ×40 C-Apochromat 1.20W objective; images were captured at 4 Hz, and ΔF/F from individual TRCs analyzed and pseudo-colored as described previously8. To identify sapphire positive cells in T2R32-Sapphire mice, we used 405-nm excitation laser to separate sapphire and Calcium Green-1 fluorescence. Mean cellular fluorescence intensity (F) was calculated for the individual TRCs and basal fluorescence (Fo) was assigned to each cell by averaging fluorescence intensity over 3 s just before tastant application. ΔF/F was calculated as [F − Fo] / Fo; taste cells were considered responders when ΔF/F exceeded 3 standard deviations above Fo within 5 s of tastant application.
Nerve recordings
Lingual stimulation and recording procedures were performed as previously described8,30; data analysis used the integrated response during the 5 s of tastant stimulation. Compounds used for nerve recordings were: 0.03 – 1 M NaCl (with and without 10 µM amiloride) or 0.03 – 1 M KCl (salty); 20 mM acesulfameK (sweet); 50 mM monopotassium glutamate (MPG) plus 0.5 mM inosine monophosphate (IMP) (umami); 0.1 mM cycloheximide (bitter); and 20 mM citric acid (sour). For pharmacological blocking experiments, 3 mM AITC was applied to the tongue for 5 min prior to initiation of the recording session; use of higher doses and/or repeated application of AITC often led to less specific inhibition of responses. Responses to 20 mM citric acid (Figure 1 and Supplementary Figure 1), 60 mM NaCl (Figure 3, Supplementary Figure 7, 8b and 9) or 250 mM KCl before AITC application (Supplementary Figure 8a) were used to normalize responses for each experimental series. For Figure 2, data were normalized to 20 mM citric acid and then scaled to WT responses before AITC application. Data were analyzed for statistical significance using an unpaired, one-tailed Student’s t-test and 95% confidence limits.
To compute the amiloride-sensitive salt component, the stimulation regime involved sequential applications of NaCl solutions first without and then with amiloride (5 sec pre- or pre-/post- incubation and co-application with NaCl solution) in the same experimental series. The amiloride-insensitive component was defined as the response in the presence of amiloride. The fraction of the response inhibited by amiloride was defined as the amiloride-sensitive component (amiloride-sensitive component = response without amiloride − response with amiloride).
For pharmacological inhibition studies using allyl isothiocyanate (AITC), responses to a series of taste stimuli were measured. Then 3 mM AITC (Aldrich, 377430-5G) was applied to the tongue at a rate of 6 ml / min for 5 min. The tongue was washed with artificial saliva for 1 min and nerve responses to the same series of taste stimuli measured; responses before and after AITC were compared for each animal. To minimize effects of recovery, responses after AITC were recorded within 15 min of AITC treatment.
For pharmacological studies using bicarbonate, taste responses were measured in the presence or absence of 30 mM KHCO3 (pH7.4) (5 sec pre-incubation and co-application with stimuli). In dorzolamide (DZA) experiments, responses were monitored before and after incubation of the tongue with 0.5% DZA (w/v) for 5 min. To study effects of pH on nerve responses, we adjusted the pH of artificial saliva (7.4) to 5.5 with hydrochloric acid.
Behavioral assays
Behavioral assays used a custom-made gustometer to measure immediate lick responses as described previously8,14,15. For salt-attraction assays, mice were injected with furosemide (50 mg/kg) and were placed in their home cage for 3 h without food or water before testing. For salt aversion assays, mice were water deprived for 24 h before testing. Three or four (attraction assay) or two (aversion assay) different concentrations of tastant and water were presented to animals in each experimental session. Differences between knockout and control mice were analyzed for statistical significance using a two-way ANOVA with a Bonferroni post hoc test.
Supplementary Material
Acknowledgements
We thank Ethia Vitalis and Nadia Propp for generation and maintenance of mouse lines and Ken Mueller for the construction of T2R-Sapphire lines. We also thank Drs. Jayaram Chandrashekar, William Sly and Abdul Waheed for useful advice and discussions, and Kristin Scott and members of our labs for valuable comments. Y.O. was supported by Japan Society for Promotion of Science, This research was supported in part by the intramural research program of the National Institutes of Health and National Institute of Dental and Craniofacial Research to N.J.P.R. C.S.Z. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author contributions Y.O. designed the study, carried out electrophysiological, biochemical, pharmacological and behavioral experiments, analyzed data and wrote the paper; M.B. performed nerve recordings and behavioral studies; L.v.B. performed nerve recordings and localization studies; N.J.P.R. and C.S.Z. designed the study, analyzed data and wrote the paper
References
- 1.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 2.Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp GK, Bertino M, Burke D, Engelman K. Experimental sodium depletion and salt taste in normal human volunteers. Am J Clin Nutr. 1990;51:881–889. doi: 10.1093/ajcn/51.5.881. [DOI] [PubMed] [Google Scholar]
- 4.Duncan CJ. Salt preferences of birds and mammals. Physiol. Zool. 1962;35:120–132. [Google Scholar]
- 5.Eylam S, Spector AC. Taste discrimination between NaCl and KCl is disrupted by amiloride in inbred mice with amiloride-insensitive chorda tympani nerves. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1361–R1368. doi: 10.1152/ajpregu.00796.2004. [DOI] [PubMed] [Google Scholar]
- 6.Contreras RJ. Gustatory mechanisms of a specific appetite. In: Cagan RH, editor. Neural Mechanisms in Taste. Boca Raton, FL: CRC; 1989. pp. 119–145. [Google Scholar]
- 7.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doolin RE, Gilbertson TA. Distribution and characterization of functional amiloride-sensitive sodium channels in rat tongue. J Gen Physiol. 1996;107:545–554. doi: 10.1085/jgp.107.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern BP. Amiloride and vertebrate gustatory responses to NaCl. Neurosci Biobehav Rev. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 11.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 12.Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- 13.Spector AC, Guagliardo NA, St John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 16.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekar J, et al. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, et al. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao ZG, Jiang Q, Jacobson KA, Ijzerman AP. Site-directed mutagenesis studies of human A(2A) adenosine receptors: involvement of glu(13) and his(278) in ligand binding and sodium modulation. Biochem Pharmacol. 2000;60:661–668. doi: 10.1016/s0006-2952(00)00357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990;265:8795–8801. [PubMed] [Google Scholar]
- 22.Baird TT, Jr, Waheed A, Okuyama T, Sly WS, Fierke CA. Catalysis and inhibition of human carbonic anhydrase IV. Biochemistry. 1997;36:2669–2678. doi: 10.1021/bi962663s. [DOI] [PubMed] [Google Scholar]
- 23.Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees II: single chorda tympani fibers. Physiol Behav. 1997;61:829–841. doi: 10.1016/s0031-9384(96)00562-8. [DOI] [PubMed] [Google Scholar]
- 24.Breslin PA, Beauchamp GK. Suppression of bitterness by sodium: variation among bitter taste stimuli. Chem Senses. 1995;20:609–623. doi: 10.1093/chemse/20.6.609. [DOI] [PubMed] [Google Scholar]
- 25.Hallock RM, Tatangelo M, Barrows J, Finger TE. Residual chemosensory capabilities in double P2X2/P2X3 purinergic receptor null mice: intraoral or postingestive detection? Chem Senses. 2009;34:799–808. doi: 10.1093/chemse/bjp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohkuri T, Horio N, Stratford JM, Finger TE, Ninomiya Y. Residual Chemoresponsiveness to Acids in the Superior Laryngeal Nerve in "Taste-Blind" (P2X2/P2X3 Double-KO) Mice. Chem Senses. 2012;37:523–532. doi: 10.1093/chemse/bjs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ugawa S, Ueda T, Yamamura H, Nagao M, Shimada S. Coexpression of vanilloid receptor subtype-1 and acid-sensing ion channel genes in the human trigeminal ganglion neurons. Chem Senses. 2005;30(Suppl 1):i195. doi: 10.1093/chemse/bjh181. [DOI] [PubMed] [Google Scholar]
- 28.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 29.Oka Y, et al. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.