Abstract
Previous studies using animal models and human clinical trials have demonstrated that the use of low oxygen transmissible contact lens materials produce corneal epithelial surface damage resulting in increased Pseudomonas aeruginosa (PA) adhesion and raft-mediated internalization into surface corneal epithelial cells. These findings led to the testable clinical predictions that: (1) microbial keratitis (MK) risk is expected to be greatest during the first 6 months of wear; (2) there is no difference between 6 and 30 night extended wear; and (3) that wear of hyper-oxygen transmissible lenses would reduce the reported incidence of infection. Subsequent epidemiological studies have confirmed the first two predictions; however, increased oxygen transmissibility with silicone hydrogel (SiHy) lens wear has not altered the overall incidence of MK. In this review, more recent clinical and basic studies that investigate epithelial alterations and bacterial adhesion to corneal epithelial cells following wear of SiHy lenses with and without concomitant exposure to chemically preserved multipurpose solutions (MPS) will be examined. The collective results of these studies demonstrate that even in the absence of lens-related hypoxia, MPS induce ocular surface changes during SiHy lens wear which are associated with a pathophysiological increase in PA adherence and internalization in the corneal epithelium, and therefore, predict an increased risk for PA-MK. In addition, new data supporting an interactive role for inflammation in facilitating PA adherence and internalization in the corneal epithelium will also be discussed.
Keywords: silicone hydrogel, corneal epithelium, microbial keratitis, Pseudomonas aeruginosa
Introduction
The corneal epithelium is a continuously renewing, stratified epithelial sheet that provides the frontline of defense against invading ocular pathogens and a smooth refractive surface essential for vision. In the absence of a contact lens, pre-existing ocular trauma or disease, the epithelium maintains an insurmountable defense against attacks from pathogenic microorganisms, affording a high level of resistance against microbial invasion. These innate defense mechanisms include antimicrobial proteins and surfactants in tear fluid, along with the inherent self-defense properties of the epithelium: apoptotic shedding of bacterially infected cells from the corneal surface, ZO-1 mediated tight junctions along apical epithelial cells, and the secretion of mucins which act in a “trapping” fashion to sequester and facilitate removal of microbial invaders. 1-7 The breakdown of these unique defenses through alterations in the natural biology of the epithelium, such as those reported during contact lens wear, predispose the normally resilient cornea to infection.
Contact lens-related microbial keratitis (MK) is the most visually devastating complication associated with contact lens wear and for more than three decades, Pseudomonas aeruginosa (PA) has been consistently identified as the primary infectious pathogen.8-11 Early studies in the late eighties established the annualized incidence of contact lens-related MK to be 4.1 per 10,000 wearers per year for daily wear and 20.9 per 10,000 wearers per year for extended wear with overnight wear on either a regular or occasional basis identified as the leading risk factor.12, 13 These findings were re-confirmed ten years later by a study in the Netherlands that had similar results with 3.5 per 10,000 wearers per year for daily wear and 20 per 10,000 wearers per year for extended wear.14 The collective results of this work allowed for the establishment of a hierarchy of clinical risk of infection stratified by lens type and wearing mode with extended wear > daily wear soft lenses > daily wear, rigid gas permeable (RGP) lenses.12, 14-16 This also led to the recognition that chronic hypoxia arising from low-oxygen transmissibility lens materials was a critical mediator in contact lens-induced epithelial damage and stagnation, which coincided with the development of new and improved lens polymers and designs in the quest to improve corneal physiology and maximize lens safety.
While the widespread acceptance of hyper-oxygen transmissible silicone hydrogel (SiHy) lenses have eliminated several of the complications previously associated with hypoxia, a robust population-based surveillance study in Australia recently re-evaluated the absolute risk of MK with the conclusion that the optimal enhancement of oxygen to the corneal surface failed to produce the much anticipated reduction as had been hoped.17 Moreover, the incidence and risk of inflammatory events have been shown to be greater with SiHy lens wear,18-20 underscoring a critical need for the evaluation of the independent and interactive effects of the mechanical, chemical and inflammatory stressors imposed by lens materials and care solutions on the corneal epithelium. The goal of this review is to re-visit previous data evaluating the effects of lens-induced hypoxia on the corneal epithelium and to investigate the effects of SiHy lenses and interactions with care solutions, using PA adherence and cellular internalization as outcome measures indicative of corneal epithelial damage and increased risk of microbial keratitis.
The effects of silicone hydrogel lens wear on the corneal epithelium
In contrast to the non-lens wearing eye, the presence of a contact lens significantly alters the normal biology of the corneal epithelium. At the cellular level, all forms of lens wear, regardless of lens type or modality of wear, have been shown to impart differential effects on epithelial homeostasis, particularly with extended wear.21-30 This includes the inhibition of basal cell proliferation within the central cornea,23, 24, 31 delayed vertical migration as cells move towards the ocular surface,22 and a reduction in apoptotic-mediated surface cell desquamation.25, 26, 28-30 These changes are detailed in a series of studies that were conducted to systematically investigate the effects of lens wear on the corneal epithelium. Importantly, this work stratified the experimental lens as a function of oxygen and lens material and established for the first time that central corneal epithelial proliferation is mediated in part by lens oxygen transmissibility, with silicone hydrogel lenses exerting the least response on short term proliferation (−33.8% SiHy compared to −40.8% with hydrogel lens wear).23 Interestingly, as the authors point out in their report, both rigid and soft lenses consisting of hyper-oxygen transmissible lens materials negatively impacted central corneal epithelial proliferation rates, indicating that the mechanical presence of the lens, irrespective of oxygen, is enough to alter the epithelial homeostatic response when compared to the no lens wear, open-eye condition.
Clinical studies have further reported on the effects of SiHy lenses on epithelial desquamation, surface epithelial cell size, and corneal sublayer thickness following daily and extended wear.28-30, 32 These studies employed the use of an ocular irrigation chamber to gently collect exfoliated cells from the human ocular surface and tandem scanning confocal microscopy for quantitative assessment of changes in surface epithelial cell area and central corneal epithelial thickness. The findings from this work demonstrated that all soft lens wear, hydrogel and SiHy, adversely affected the corneal epithelium. While greater epithelial thinning was reported with extended hydrogel lens wear, indicating partial dependence on oxygen transmission, the increase in surface epithelial cell size and corresponding reduction in surface cell desquamation were greatest in the SiHy lens group. When compared with the even greater reductions seen in the RGP lens group, these effects indicate a more mechanical etiology likely related to changes in lens modulus of the first generation SiHy lens materials, as opposed to hypoxia. A summary of these findings is detailed in Table 1.
Table 1.
Summary of central epithelial thickness and surface epithelial cell size changes as a function of lens wear.28-30
| Central Epithelial Thickness (% decrease compared to baseline) | |||
|---|---|---|---|
|
| |||
| Lens Type | 4 WK DW | 1M EW | 12M EW |
| Tisilfocon A | 10% | 17% | 10% |
| Etafilcon A | no change | 5% | 6% |
| Balafilcon A | no change | 4% | 3% |
| Lotrafilcon A | no change | 4% | 4% |
|
| |||
| Surface Epithelial Cell Size (% increase compared to baseline) | |||
|
| |||
| Tisilfocon A | 111% | 123% | 129% |
| Etafilcon A | 103% | 109% | 111% |
| Balafilcon A | 102% | 106% | 115% |
| Lotrafilcon A | 102% | 105% | 117% |
|
| |||
| Surface Cell Desquamation (% decrease compared to baseline) | |||
|
| |||
| Tisilfocon A | 51% | 59% | 63% |
| Etafilcon A | 62% | 75% | 31% |
| Balafilcon A | 46% | 59% | 49% |
| Lotrafilcon A | NR* | 52% | 60% |
Not Reported
While relatively uncommon, contact lens wear remains a causative factor in patients presenting with limbal stem cell deficiency.33-37 Importantly, within this select patient group, contact lens-induced keratopathy has only been reported to occur with soft contact lenses and not RGP lens wear. This is likely due to the inherently larger size of the soft lens as it crosses the limbal region. Confocal analysis suggests that soft contact lenses may alter the limbal basal cell morphology through an increase in cell size (1.58 fold increase).38 These effects were not apparent in the central cornea. Tight fitting lenses, reduced tear flow under the soft lens, and increased buildup of inflammatory debris are all likely contributors. A more recent report evaluated the effect of silicone hydrogel lenses on the rabbit limbal epithelium when used in a therapeutic modality for up to 30 days. In this study the authors failed to detect any cellular changes in limbal epithelia; however, the short duration of lens wear in this study prohibits any meaningful long term changes.39
Unfortunately, very little work has been done in recent years to further our knowledge on the effects of SiHy lens wear on corneal epithelial homeostasis. Thus it remains to be answered, how, in the absence of lens-induced hypoxia, does the resultant mechanical and inflammatory stress imposed by SiHy lens wear result in an uncoupling of homeostatic events (ie: proliferation and desquamation with resultant epithelial thinning) and the differential phenotypes seen as a result of lens wear. Moreover, questions remain, regarding the relationship between contact lens-induced alterations in corneal epithelial homeostasis and susceptibility or predisposition of the cornea to microbial infection.
The role of hypoxia on PA adherence and internalization
A significant body of evidence exists supporting a role for lens-induced hypoxia in mediating corneal epithelial surface damage. Early animal studies in the rabbit have shown that contact lens wear creates corneal surface damage proportional to the oxygen transmissibility of the lens.40 These studies further showed that rigid lenses inherently produce more damage to surface corneal cells than soft lenses at the same level of oxygen transmission.40 Paradoxically, despite less evident surface damage, soft lenses induce significantly greater bacterial binding than RGP lenses, confirming that hypoxia alone is not the only driving factor in bacterial adherence to the cornea. Later studies using the same rabbit model demonstrated that discrete membrane domains known as lipid rafts formed in the surface epithelium in response to hypoxic contact lens wear.41-44 Quantitative analysis of PA invasion following 24 hours of PMMA lens wear resulted in a 3 fold increase in raft-mediated internalization of PA, compared to the control eye.41 A similar small, but significant effect was also seen with prolonged hypoxia following wear of a low Dk lens over three days, compared to eyelid closure in the absence of a lens, which resulted in a similar equivalent oxygen percentage at the corneal surface.42
Fleiszig et al were the first to demonstrate PA adherence to surface epithelia during extended contact lens wear.45 Utilizing similar methodology, a series of robust prospective clinical trials evaluated the effect of lens-oxygen transmissibility on the corneal epithelium and PA binding to exfoliated corneal epithelial cells as a function of lens type (RGP vs soft).28-30 In these trials, neophytes and established lens wearers following a 1 month washout period underwent for 4 weeks of daily lens wear using a PHMB-based (Renu, Bausch & Lomb) multipurpose solution (MPS). An important caveat however, as will be addressed in the following section, is that patients whom successfully completed the initial daily lens wear test arm were subsequently switched into extended wear and followed for 12 months without an additional washout period.
The cumulative results of these studies demonstrated that all soft lens wear significantly increased PA binding in daily and extended wear compared to RGP lenses, which was consistent with the proposed risk hierarchy (extended wear > daily wear soft lenses > daily wear RGP); however, the increase in PA adherence was greater following lower oxygen-transmissible hydrogel lens wear than that seen with either silicone hydrogel lens tested. Unexpectedly, when followed over 1 year, after 6 months of extended lens wear, all lenses began to show signs of adaptation back to baseline values. These findings led to the testable predictions that (1) MK risk is greatest during the first 6 months of wear; (2) there is no difference between 6 and 30 night extended wear; and (3) that increased lens oxygen transmissibility would reduce the reported incidence of infection. More recent epidemiological studies have confirmed the first two predictions; however, increased lens oxygen disappointedly failed to lower the overall incidence of MK as predicted.17
Silicone hydrogel contact lenses and chemically-preserved care solutions
Due to the high potential for interactions between SiHy lenses and care solutions, solution effects must be taken into account when evaluating the effects of SiHy lenses on the corneal epithelium. Epithelial toxicity stemming from the use of chemically preserved MPS and buffered lens packing solutions, both of which undergo uptake into the lens for later release on the corneal epithelium, have been heavily evaluated in both in vitro cell culture models and human clinical trials. 46-60 Similarly, experiments using SV-40 transformed corneal epithelial cell monolayer cultures have assessed PA adherence to corneal epithelial cells following incubation with a panel of MPS.59 Using quantitative PA adhesion assays, the authors concluded that specific MPS increase PA adherence to corneal cells under these in vitro conditions. These findings were further supported by a small, crossover clinical trial that evaluated PA adherence to exfoliated cells following direct instillation of MPS in the absence of a lens, which also suggested a potential role for MPS in mediating PA adherence.53
More recently, a 12 month clinical trial evaluated PA adherence during both daily and extended wear of silicone hydrogel lenses with identical outcome measures to those reported in earlier studies.32 Following a one month washout period, patients were dispensed lenses for bilateral wear according to FDA approved wearing schedules. In contrast to the prior studies, a peroxide based solution (Clear Care, CIBA Vision) was used in place of a PHMB-based MPS product. When evaluated following either daily or extended lens wearing schedules, no significant increase in PA adherence was detected with any of the three silicone hydrogel lenses tested regardless of wearing modality, indicating the absence of any clinically significant epithelial surface damage. The failure to detect an increase in PA adherence with extended SiHy lens wear differed from the prior studies using Balafilcon A and Lotrafilcon A lenses. Since the Lotrafilcon A lens worn over 30 days of continuous wear was evaluated in both the current and previous clinical trial, 12 month binding curves were re-plotted for direct comparison (Figure 1).
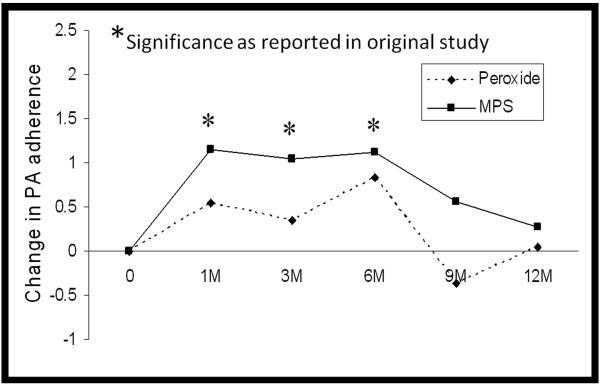
Figure 1.
PA adherence to exfoliated corneal epithelial cells over 12 months of extended wear. Composite graph showing data re-plotted from original studies investigating changes in PA binding during 30-night extended wear of the Lotrafilcon A lens.29, 32 Solid line represents data from the 2001 study which incorporated MPS. Dotted line represents data from the 2008 study which utilized a peroxide care solution. A significant increase was reported from baseline to 1 month of extended wear in the MPS study; this difference was not detected in the peroxide study. Comparison of study protocols suggests that an additional 4 weeks of daily wear with MPS usage prior to initiating extended lens wear may contribute to the increase in PA adherence detected in the MPS study.
When evaluating differences in these data, there is a critical distinction between the two curves in the 0 to 1 month time period. For the top curve, the extended wear study which incorporated MPS, the 1 month extended wear data is obtained following 4 weeks of daily lens wear and concurrent MPS use. This becomes important when interpreting this binding curve as daily use with MPS may contribute to the increase in PA adherence detected when switched into extended wear. In contrast to this, all patients initiating overnight wear in the bottom curve, representing the extended wear study which utilized peroxide, began lens wear directly following a 30 day washout period and thus had not been exposed to MPS. Subsequent comparison of PA binding rates over 4 weeks of daily silicone hydrogel lens wear further demonstrate significant increases when used with MPS compared to peroxide, indicate that removing MPS from the care regimen eliminated preservative-induced epithelial damage associated with daily silicone hydrogel lens wear (Figure 2). In support of this view, an early pilot study evaluating PA adherence to exfoliated corneal epithelial cells that also used a peroxide-based solution (AOSEPT, CIBA Vision) reported similar results, with an increase in PA binding seen with wear of a hydrogel lens but no corresponding increase with wear of the Balafilcon lens material.61 Collectively, these data indicate that MPS use during the initial phase of lens wear in the prior hypoxia studies may represent a confounding variable contributing to the increase in PA adherence reported with silicone hydrogel lens wear, nullifying the expected decrease in PA-MK expected with silicone hydrogel lenses.
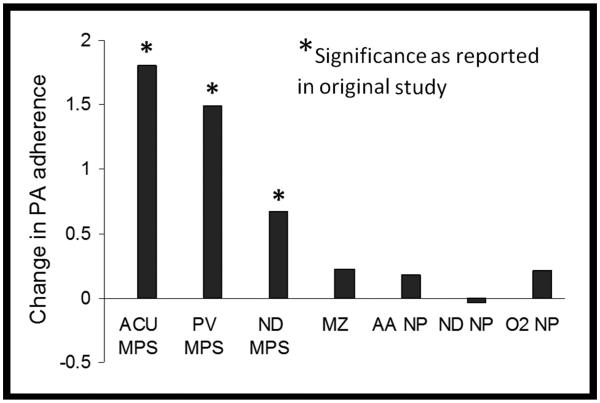
Figure 2.
PA adherence to exfoliated corneal epithelial cells during daily wear. Composite graph showing data re-plotted from original studies investigating changes in PA binding following four weeks of daily wear.30, 32 Daily wear of both hydrogel and silicone hydrogel lenses showed a significant increase in PA adherence to exfoliated corneal epithelial cells when used in conjunction with MPS. In contrast, a significant increase in PA adherence was not detected following wear of silicone hydrogel lenses in conjunction with peroxide-based care products, similar to daily RGP lens wear. ACU: Acuvue 2; PV: PureVision; ND: Night and Day; MZ: Menicon Z, alignment fit; AA: Acuvue Advance; O2: O2 Optics; NP: non-preserved care solution.
To further investigate the effects of MPS during SiHy lens wear on the corneal epithelium, recent studies in our laboratory have used the rabbit contact lens model to monitor intracellular invasion by PA in response to lens-solution-induced corneal epithelial damage. The results of these studies indicate that 2 hours of SiHy lens wear in conjunction with certain MPS will induced a significant increase in PA internalization into the rabbit corneal epithelium, the response of which appeared much greater than the earlier effects reported by hypoxia (unpublished data). Unexpectedly, these studies further suggest the potential for a sympathetic response with use of a specific MPS on the ocular surface which may be important when designing clinical trials, although significantly more work is required to confirm that finding. When coupled with the findings from the human clinical trials, these data provide strong evidence to support that some chemically-preserved care solutions increase PA adherence and internalization into the corneal epithelium. This results in an increased infectious burden in the corneal epithelium and indicates that solution-induced chemical damage is a risk factor for MK. This is further supported by recent epidemiological data, supporting that peroxide lowers risk for MK approximately 2.5× (OR 0.41, 95% CI 0.22 – 0.75, p=0.004).6
The clinical significance of PA adherence to exfoliated corneal epithelial cells or intracellular invasion by PA, as performed in these studies, are that they provide an objective measure of epithelial damage that facilitates PA uptake into the epithelium, and by extension, increased risk for MK. However, while clinical data predicts that elimination of hypoxia and concurrent MPS usage would result in a reduction in MK, since PA challenge was performed ex vivo in these assays, these findings do not take into account alterations in tear fluid, the accumulation of cellular byproducts in the post lens environment, and the corresponding effects of inflammation in the presence of a lens with bacterial challenge.
Inflammation: a permissive traveler or contributing co-conspirator?
Neutrophils are the primary effector cells of the innate immune system that are recruited to the site of infection to remove pathogenic bacteria. Estimated to live only a few hours in inflamed tissue,62 the short life span of neutrophils is essential to reduce proteinase-induced tissue damage that can result in scarring and vision loss. In the contact lens wearing eye, animal studies have demonstrated robust neutrophil infiltration in response to bacterial challenge during lens wear.63 While a deficient or inadequate neutrophil response is associated with severe corneal infection, a prolonged or deregulated response results in the accumulation of necrotic neutrophil debris in the post lens environment.44, 64 Recently, necrotic neutrophil debris has been reported to enhance PA adherence to both hydrogel and silicone hydrogel contact lens surfaces in vitro.65, 66 This occurs through electrostatic interactions generated between negatively charged F-actin and DNA and positively charged histones that are released by dying cells, forming a scaffold that promotes PA adherence.67-69 Importantly, PA adherence to the lens surface is thought to be an initial event in infection, thus the ability of PA to exploit the neutrophil response to colonize the contact lens and/or inhibit bacterial clearance may contribute to the pathobiology of the disease process.
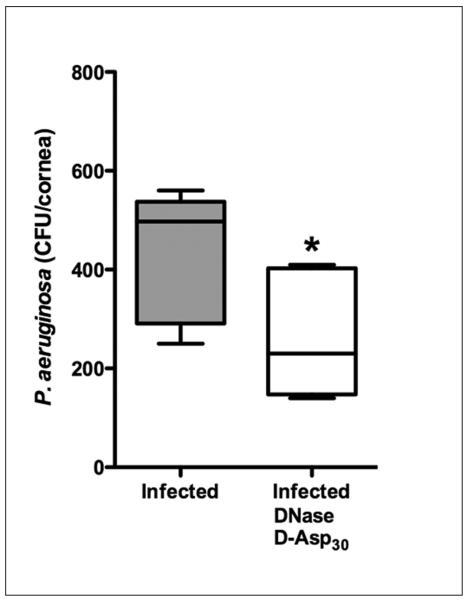
The concomitant use of DNase, which targets extracellular DNA, and a soluble polyanionic amino acid, which facilitates dissociation of DNA from F-actin and further functions to disaggregate actin bundles, has been shown to disrupt F-actin:DNA complex formation and inhibit PA adherence to contact lens surfaces.65, 70 When tested in vivo using intracellular invasion assays, the simultaneous use of these compounds in the lens wearing rabbit eye resulted in a significant reduction in internalized PA (Figure 3).65 While much work remains to be done to establish the mechanism by which dissolution of these complexes in the post lens microenvironment results in a reduction in intracellular PA, these findings provide evidence supporting a linkage between the persistent inflammatory response and risk for contact lens-related MK.
Figure 3.
Inflammation contributes to PA internalization in the rabbit corneal epithelium. Rabbits were fit with PMMA lenses for 24 hours in both eyes, followed by inoculation with PA and lens wear for an additional 24 hours. Treatment with DNase and D-Asp30 to dissolve scaffolds formed from necrotic neutrophil debris resulted in a significant reduction in internalized PA in the rabbit corneal epithelium in vivo; contralateral eye treated with vehicle only control (n=6, p=0.03). Figure adapted from Robertson et al., IOVS 2011;65 copyright IOVS.
Conclusion
The adverse effects on corneal physiology in response to wear of conventional hydrogel lenses appear to have been largely overcome with the introduction of silicone hydrogels; yet, overall MK rates remain unchanged and inflammatory events are on the rise. Lens-solution incompatibilities have now been identified as a contributing factor, as inherent differences in lens polymers and surface coatings differentially interact with the currently available chemically-preserved care solutions and the corneal epithelium. The full effects of these bio-incompatibilities on the epithelium are still uncharacterized and the reported inconsistencies between human clinical trials and in vitro cell culture data further clouds our current understanding of the interactive nature of the lens-solution-cornea relationship. Since animal models in contact lens wear are limited, the development of a standardized cell culture model that correlates with clinical outcomes is urgently needed.
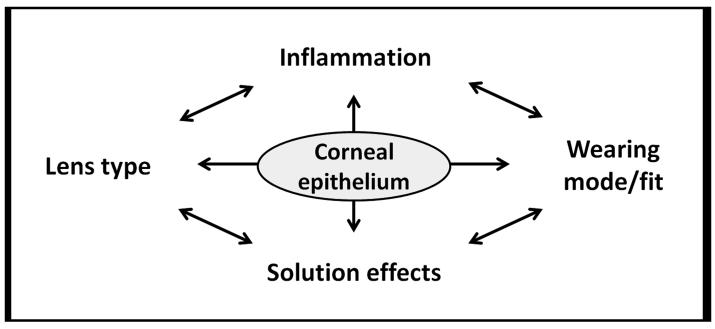
In summary, the overall strategy to reduce MK remains an interactive approach accomplished by an effective reduction in lens-associated bioburden with minimal epithelial surface damage and preservation of intact innate defense mechanisms. Even in the absence of frank lens-solution epithelial damage, the host inflammatory response may still contribute to bacterial colonization on the lens surface and subsequent epithelial internalization. Therefore, the prevention of silicone hydrogel lens-induced, solution-induced and inflammatory-mediated epithelial surface damage that results in epithelial compromise becomes a zero sum game in which lens type, wearing modality, solution, and inflammatory effects must all be optimized (Figure 4).
Figure 4.
Zero-sum model for silicone hydrogel lens wear. In the absence of lens-induced hypoxia, solution incompatibilities and inflammatory effects contribute to breakdown of the corneal epithelial defense mechanisms and a subsequent increase in infectious burden in the corneal epithelium.
Acknowledgments
Supported in Part by NIH Grant R01 EY018219 (DMR), NIH Grant P30 EY020799, OneSight Research Foundation, Dallas, Texas (DMR), and a Career Development Award (DMR) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York. CR: None.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41:60–75. doi: 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni M, Evans DJ, Hawgood S, et al. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 4.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci. 2000;41:4093–4100. [PubMed] [Google Scholar]
- 5.Ban Y, Dota A, Cooper LJ, et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003;76:663–669. doi: 10.1016/s0014-4835(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh HD, Robertson DM, Petroll WM, et al. Castroviejo Lecture 2009: 40 years in search of the perfect contact lens. Cornea. 2010;29:1075–1085. doi: 10.1097/ICO.0b013e3181d103bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam C, Mun JJ, Evans DJ, et al. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J Clin Invest. 2012;122:3665–3677. doi: 10.1172/JCI64416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in North Texas. Eye & Contact Lens. 2007;33:45–49. doi: 10.1097/01.icl.0000234002.88643.d0. [DOI] [PubMed] [Google Scholar]
- 9.Ormerod LD, Smith RE. Contact lens-associated microbial keratitis. Arch Ophthalmol. 1986;104:79–83. doi: 10.1001/archopht.1986.01050130089027. [DOI] [PubMed] [Google Scholar]
- 10.Mondino BJ, Weissman BA, Farb MD, et al. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am J Ophthalmol. 1986;102:58–65. doi: 10.1016/0002-9394(86)90210-2. [DOI] [PubMed] [Google Scholar]
- 11.Erie JC, Nevitt MP, Hodge DO, et al. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111:1665–1671. doi: 10.1001/archopht.1993.01090120087027. [DOI] [PubMed] [Google Scholar]
- 12.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–783. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- 13.Schein OD, Poggio EC. Ulcerative keratitis in contact lens wearers. Incidence and risk factors. Cornea. 1990;9:S55–S58. doi: 10.1097/00003226-199010001-00023. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- 15.Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338:650–653. doi: 10.1016/0140-6736(91)91231-i. [DOI] [PubMed] [Google Scholar]
- 16.Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. N Engl J Med. 1989;321:773–778. doi: 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007;84:247–256. doi: 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011;52:6690–6696. doi: 10.1167/iovs.10-7018. [DOI] [PubMed] [Google Scholar]
- 20.Radford CF, Minassian D, Dart JK, et al. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009;116:385–392. doi: 10.1016/j.ophtha.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight lens wear. Contact Lens Anterior Eye. 2002;25:11–21. doi: 10.1016/s1367-0484(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Ladage PM, Jester JV, Petroll WM, et al. Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Invest Ophthalmol Vis Sci. 2003;44:1056–1063. doi: 10.1167/iovs.02-0725. [DOI] [PubMed] [Google Scholar]
- 23.Ladage PM, Ren DH, Petroll WM, et al. Effects of eyelid closure and disposable and silicone hydrogel extended contact lens wear on rabbit corneal epithelial cell proliferation. Invest Ophthalmol Vis Sci. 2003;44:1843–1849. doi: 10.1167/iovs.02-0897. [DOI] [PubMed] [Google Scholar]
- 24.Ladage PM, Yamamoto K, Ren DH, et al. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Invest Ophthalmol Vis Sci. 2001;42:2804–2812. [PubMed] [Google Scholar]
- 25.Yamamoto K, Ladage PM, Ren DH, et al. Effects of low and hyper Dk rigid gas permeable contact lenses on Bcl-2 expression and apoptosis in the rabbit corneal epithelium. CLAO J. 2001b;27:137–143. [PubMed] [Google Scholar]
- 26.Yamamoto K, Ladage PM, Ren DH, et al. Effect of eyelid closure and overnight contact lens wear on viability of surface epithelial cells in rabbit cornea. Cornea. 2002;21:85–90. doi: 10.1097/00003226-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Robertson DM, Petroll WM, Jester JV, et al. The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview. Eye Contact Lens. 2007;33:394–398. doi: 10.1097/ICL.0b013e318157e609. [DOI] [PubMed] [Google Scholar]
- 28.Ren DH, Yamamoto K, Ladage PM, et al. Adaptive effects of 30-night wear of hyper-O2 transmissible contact lenses on bacterial binding and corneal epithelium. Ophthalmology. 2002;109:27–39. doi: 10.1016/s0161-6420(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 29.Cavanagh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium. Ophthalmology. 2002;109:1957–1969. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- 30.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 31.Ren DH, Petroll WM, Jester JV, et al. The effect of rigid gas permeable contact lens wear on proliferation of rabbit corneal and conjunctival epithelial cells. CLAO J. 1999;25:136–141. [PubMed] [Google Scholar]
- 32.Robertson DM, Petroll WM, Cavanagh HD. The effect of nonpreserved care solutions on 12 months of daily and extended silicone hydrogel contact lens wear. Invest Ophthalmol Vis Sci. 2008;49:7–15. doi: 10.1167/iovs.07-0940. [DOI] [PubMed] [Google Scholar]
- 33.Dua HS, Saini JS, Azuara-Blanco A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83–92. [PubMed] [Google Scholar]
- 34.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 35.Bloomfield SE, Jakobiec FA, Theodore FH. Contact lens induced keratopathy: a severe complication extending the spectrum of keratoconjunctivitis in contact lens wearers. Ophthalmology. 1984;93:290–294. doi: 10.1016/s0161-6420(84)34308-1. [DOI] [PubMed] [Google Scholar]
- 36.Jeng BH, Halfpenny CP, Meisler DM, et al. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30:18–23. doi: 10.1097/ICO.0b013e3181e2d0f5. [DOI] [PubMed] [Google Scholar]
- 37.Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007;90:26–30. doi: 10.1111/j.1444-0938.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 38.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Gurdal C, Takmaz T, Sargon MF, et al. Electron microscopic evaluation of the effect of therapeutic silicone hydrogel lenses on the limbal area. Eye Contact Lens. 2006;32:133–137. doi: 10.1097/01.icl.0000182874.27371.2a. [DOI] [PubMed] [Google Scholar]
- 40.Imayasu M, Petroll WM, Jester JV, et al. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–388. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto N, Yamamoto N, Petroll WM, et al. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1348–1355. doi: 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto N, Yamamoto N, Jester JV, et al. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye & Contact Lens. 2006;32:114–120. doi: 10.1097/01.icl.0000177384.27778.4c. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto N, Yamamoto N, Petroll WM, et al. Regulation of Pseudomonas aeruginosa internalization after contact lens wear in vivo and in serum-free culture by ocular surface cells. Invest Ophthalmol Vis Sci. 2006;47:3430–3440. doi: 10.1167/iovs.05-1332. [DOI] [PubMed] [Google Scholar]
- 44.Zaidi T, Mowrey-Mckee M, Pier GB. Hypoxia increases corneal cell expression of CFTR leading to increased Psuedomonas aeruginosa binding, internalization, and initiation of inflammation. Invest Ophthalmol Vis Sci. 2004;45:4066–4074. doi: 10.1167/iovs.04-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleiszig SMJ, Efron N, Pier GB. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci. 1992;33:2908–2916. [PubMed] [Google Scholar]
- 46.Santodomingo-Rubido J, Mori O, Kawaminami S. Cytotoxicity and antimicrobial activity of six multipurpose soft contact lens disinfecting solutions. Ophthal Physiol Opt. 2006;26:476–482. doi: 10.1111/j.1475-1313.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 47.Gorbet MB, Tanti NC, Crockett B, et al. Effect of contact lens material on cytotoxicity potential of multipurpose solutions using human corneal epithelial cells. Mol Vis. 2011;17:3458–3467. [PMC free article] [PubMed] [Google Scholar]
- 48.Gorbet MB, Tanti NC, Jones L, et al. Corneal epithelial cell biocompatibility to silicone hydrogel and conventional hydrogel contact lens packaging solutions. Mol Vis. 2010;16:272–282. [PMC free article] [PubMed] [Google Scholar]
- 49.Choy CKM, Cho P, Boost MV, et al. Do multipurpose solutions damage porcine corneal epithelial cells? Optom Vis Sci. 2009;86:E447–E453. doi: 10.1097/OPX.0b013e31819fa422. [DOI] [PubMed] [Google Scholar]
- 50.McCanna DJ, Karrington KL, Driot J-Y, et al. Use of a human corneal epithelial cell line for screening the safety of contact lens care solutions in vitro. Eye Contact Lens. 2008;34:6–12. doi: 10.1097/ICL.0b013e31804fa141. [DOI] [PubMed] [Google Scholar]
- 51.Chuang EY, Li DQ, Bian F, et al. Effects of contact lens multipurpose solutions on human corneal epithelial survival and barrier function. Eye Contact Lens. 2008;34:281–286. doi: 10.1097/ICL.0b013e3181842518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imayasu M, Shiraishi A, Ohashi Y, et al. Effects of multipurpose solutions on corneal epithelial tight junctions. Eye & Contact Lens. 2008;34:50–55. doi: 10.1097/ICL.0b013e318073cbdb. [DOI] [PubMed] [Google Scholar]
- 53.Li SL, Ladage PM, Yamamoto T, et al. Effects of contact lens care solutions on surface exfoliation and bacterial binding to corneal epithelial cells. Eye & Contact Lens. 2003;29:27–30. doi: 10.1097/00140068-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Chuang EY, Li D-Q, Bian F, et al. Effects of contact lens multipurpose solutions on human corneal epithelial survival and barrier function. Eye Contact Lens. 2008;34:281–286. doi: 10.1097/ICL.0b013e3181842518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imayasu M, Shiraishi A, Ohashi Y, et al. Effects of multipurpose solutions on corneal epithelial tight junctions. Eye Contact Lens. 2008;34:50–55. doi: 10.1097/ICL.0b013e318073cbdb. [DOI] [PubMed] [Google Scholar]
- 56.Gorbet M, Tanti N, Crockett B, et al. Impact of lens material on in vitro cytotoxicity potential of multipurpose solutions on corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3415. [PMC free article] [PubMed] [Google Scholar]
- 57.Imayasu M, Hori Y, Cavanagh HD. Effects of multipurpose contact lens care solutions and their ingredients on membrane-associated mucins at the corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:1525. doi: 10.1097/ICL.0b013e3181faa43e. [DOI] [PubMed] [Google Scholar]
- 58.Lim MJ, Hurst RK, Konynenbelt BJ, et al. Cytotoxicity testing of multipurpose contact lens solutions using monolayer and stratified cultures of human corneal epithelial cells. Eye Contact Lens. 2009;35:287–296. doi: 10.1097/ICL.0b013e3181b9e92c. [DOI] [PubMed] [Google Scholar]
- 59.Imayasu M, Shimizu H, Shimada S, et al. Effects of multipurpose contact-lens care solutions on adhesion of Pseudomonas aeruginosa to corneal epithelial cells. Eye Contact Lens. 2009;35:98–104. doi: 10.1097/ICL.0b013e31819a67fd. [DOI] [PubMed] [Google Scholar]
- 60.Carnt N, Jalbert I, Stretton S, et al. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci. 2007;84:309–315. doi: 10.1097/OPX.0b013e318046551b. [DOI] [PubMed] [Google Scholar]
- 61.Ren DH, Petroll WM, Jester JV, et al. The Relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended Wear. CLAO J. 1999;25:80–100. [PubMed] [Google Scholar]
- 62.Hofman P. Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Curr Drug Targets Inflamm Allergy. 2004;3:1–9. doi: 10.2174/1568010043483935. [DOI] [PubMed] [Google Scholar]
- 63.Szliter EA, Barrett RP, Gabriel MM, et al. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye & Contact Lens. 2006;32:12–18. doi: 10.1097/01.icl.0000167611.03883.58. [DOI] [PubMed] [Google Scholar]
- 64.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: A review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 65.Robertson DM, Parks QM, Young RL, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci. 2011 Jan 18; doi: 10.1167/iovs.10-6469. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burnham GW, Cavanagh HD, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens. 2012;38:7–15. doi: 10.1097/ICL.0b013e31823bad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang JX, Janmey PA. The polyelectrolyte nature of f-actin and the mechanism of actin bundle formation. J Biol Chem. 1996;271:8556–8563. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 68.Walker TS, Tomlin KL, Worthen GS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73:3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parks QM, Young RL, Poch KR, et al. Neutrophil enhancement of Pseudomonas aerugninosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol. 2009;58:492–502. doi: 10.1099/jmm.0.005728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang JX, Wen Q, Bennett A, et al. Anionic poly(amino acid)s dissolve f-actin and DNA bundles, enhance DNase activity, and reduce the viscosity of cystic fibrosis sputum. Am J Physiol Lung Cell Mol Physiol. 2005;259:L599–L605. doi: 10.1152/ajplung.00061.2005. [DOI] [PubMed] [Google Scholar]