Abstract
Chronic mood disorders have been associated with a shortened telomere, a marker of increased mortality rate and ageing, and impaired cellular immunity. However, treatment may confound these relationships. We examined the relationship of glucose tolerance, white blood cell count and telomere length to depression in newly diagnosed, antidepressant-naïve patients. Subjects with major depression (n=15), and matched healthy control subjects (n=70) underwent a two-hour oral glucose tolerance test and evaluation of blood cell count and telomere content. The depression group had significantly higher two-hour glucose concentrations and a lower lymphocyte count than control subjects (respective means [SD] for two-hour glucose were 125.0 mg/dL [67.9] vs 84.6 [25.6] (p<.001); for lymphocyte count 2.1 × 109/L [0.6] vs. 2.5 ×109/L [0.7] p=.028).Telomere content was significantly shortened in the depression group (87.9 [7.6]) compared to control subjects (101.0 [14.3]; p<0.01). Abnormal glucose tolerance, lymphopenia and a shortened telomere are present early in the course of depression independently of the confounding effect of antidepressant treatment, supporting the concept of major depression as an accelerated ageing disease.
Keywords: Major depressive disorder, Telomere, Diabetes mellitus, Immunosenescence, Lymphopenia, Glucose tolerance, Accelerated ageing, Drug-naïve
Introduction
Depression will be the second contributor to the global burden of disease by 2020, according to the World health organization (WHO). The burden of depression is due not only to psychiatric disability but also to the associated comorbidity with physical diseases. The pathophysiology underlying the comorbidity remains unclear although several mechanisms have been implicated. As an etiological explanation, the association between mood disorders, cardiovascular diseases and immune disturbances has received considerable attention (Blume et al., 2011; Brown et al., 2009; Marano et al., 2009) suggesting that both systems might contribute to the increased mortality associated with mood disorders (Lippi et al., 2009).
Diabetes mellitus has been studied as a key factor contributing to the relationship between depression and increased cardiovascular disease. Epidemiological studies show that patients with diabetes have an increased risk of developing major depression (Egede, 2005), an association first described in 1969 (Mueller et al., 1969). The prevalence of depression among patients with type 2 diabetes mellitus is twice that of the general population (Nichols and Brown, 2003), with an estimated prevalence of depression in diabetic patients of 11–15% (Anderson et al., 2001). A meta-analysis suggested that a depressed adults have a 37% increased risk of developing type 2 diabetes (Knol et al., 2006) while another study showed a 23% increased risk of developing type 2 diabetes mellitus in depressed young adults (Brown et al., 2005; Knol et al., 2007). The mechanism of the association between depression and diabetes is still unknown, although the physiological reaction to the psychological stress associated with diabetes cannot completely explain the association (Grandinetti et al., 2000). In addition, potential confounding factors that are associated with weight gain and impaired glycemic control, such as body mass index and antidepressant treatment, must be taken into account (Derijks et al., 2008).
Immune disturbances have been described in patients with depression. Both immune activation and immune suppression (Blume et al., 2011) through an impaired cellular immunity function (increased inflammatory markers, neutrophilia and lymphopenia) have been described to underlie a major depressive episode. As stated before with diabetes and depression, the mechanism of association is unknown, although lymphopenia and a reduced lymphocyte proliferative response to mitogen have been confirmed in a review in depression suggesting an immune suppression related with an increased mortality. On the same line of evidence depression is associated with decreases in the number and percentage of lymphocytes (Zorrilla et al., 2001).
Another possible marker of morbidity in depression that has received considerable attention in mood disorders is telomere length. Telomere length has been linked to increased senescence in patients with depressed mood (Wolkowitz et al., 2010). The telomere is a nucleoprotein complex at the end of mammalian chromosomes that maintains chromosomal integrity and consists of double-stranded 5′-TTAGGG-3′ repeats. A shortened telomere has been linked to post-stroke mortality, dementia, cognitive decline (Martin-Ruiz et al., 2006), all-cause mortality in people above age 60 (Cawthon et al., 2003), type 1 diabetes (Balasubramanyam et al., 2007), type 2 diabetes (Adaikalakoteswari et al., 2007), and cardiovascular disease (Obana et al., 2003). Patients with major depressive disorder have been found to have a shorter telomere length (Lung et al., 2007; Wolkowitz et al., 2011), a marker of increased mortality rate and ageing (Fitzpatrick et al., 2007) associated with oxidative and inflammatory stress. However, the subjects studied were not treatmentnaïve, raising the question of whether this association was confounded by antidepressant treatment.
In an effort to clarify the pathophysiology of major depressive episodes, we tested the hypothesis that patients with major depression have cardiovascular and immune disturbances, and a shortened telomere. In order to avoid confounding by antidepressant medication side effects, we examined this relationship in newly diagnosed, antidepressant-naïve patients.
Materials and methods
Subjects
Patients with a major depressive disorder without psychotic features were recruited at the time of their first lifetime contact with psychiatric services for a depression. They came to clinical contact in a general academic hospital (Hospital Clinic of Barcelona). The catchment area for the hospital, Esquerra Eixample, is a relatively homogeneous middle class/upper middle class neighbourhood in the center of Barcelona. These patients were enrolled if they had never previously received antidepressant or mood-stabilizing medications. They were allowed to receive anti-anxiety medication (lorazepam) the night before blood was drawn, to a maximum of 3 mg, but not on the day of the blood sampling and oral glucose tolerance test (GTT). Healthy control subjects were recruited using advertisements. All subjects came from a larger study of diabetes in neuropsychiatric disorders (Kirkpatrick et al., 2009).
Additional inclusion and exclusion criteria for all subjects were (1) age from 18 to 64 years, (2) no history of diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance (eg, Cushing disease), (3) not taking a medication associated with insulin resistance (hydrochlorothiazide, furosemide, ethacrynic acid, metolazone, chlorthalidone, beta blockers, glucocorticoids, phenytoin, nicotinic acid, cyclosporine, pentamidine, or narcotics) and (4) no history of cocaine use in the previous 30 days. For control subjects, additional exclusion criteria were (1) no lifetime diagnosis of psychosis (associated with glucose intolerance; (Fernandez-Egea et al., 2009a) or major depressive disorder, 2) no current diagnosis of adjustment disorder, and (3) had not previously received psychotropic medication.
All subjects gave written informed consent for participation in the study, which was conducted with the oversight of the investigators’ institutional review boards.
Assessments
All subjects were interviewed using the Spanish translation of the Structured Clinical Interview (SCID). The patients had no other history of Axis I disorder besides major depression. They were antidepressant-naïve, but no further information regarding past depressive symptoms was obtained. No subject with acute medical illness was included. For control subjects, additional exclusion criteria were (1) no lifetime diagnosis of psychosis (associated with glucose intolerance(Fernandez-Egea et al., 2009a); or major depressive disorder, 2) no current diagnosis of adjustment disorder, and (3) had not previously received psychotropic medication.
They were also administered the Dartmouth Assessment of Lifestyle Inventory (DALI), which quantifies substance abuse (Rosenberg et al., 1998), including a measure of smoking status (mean number of cigarettes per day). All subjects were given a 2-hour 75 g oral glucose tolerance test which began between 8 and 9 AM after an overnight fast. Cortisol was sampled between 8 and 9 AM prior to ingesting glucose for the GTT. Height and weight, while wearing underwear and without shoes, were recorded between the blood samplings. Body mass index (BMI) was calculated using the formula (weight [kg]/height [m]2). Further details on the GTT have been provided previously (Fernandez-Egea et al., 2009a).
Telomere DNA content (TC) was measured in blood leukocytes as previously described (Fernandez-Egea et al., 2009b). TC is directly proportional to telomere length measured by Southern blot (r = 0.904), can be measured with as little as 5 ng of genomic DNA, is insensitive to fragmentation of the DNA to <1 kb in length, and can be performed with DNA isolated from fresh, frozen, and paraffin-embedded tissues (Fordyce et al., 2006). Briefly, known DNA masses were UV cross-linked to a membrane, hybridized with telomere-specific oligonucleotides, end-labeled with fluorescein, and then detected with an alkaline phosphatase–conjugated antifluorescein antibody that produces light when incubated with CDP-Star substrate. Blots were exposed to Hyper film, digitized by scanning, and the telomere hybridization signals were measured. TC for each sample is reported as a percentage of the median chemiluminescent signal from 6 replicate determinations of each patient DNA relative to the chemiluminescent signal in the same amount of a reference DNA standard (placental DNA) measured in parallel. The laboratory determining TC was blind to the subject’s clinical diagnosis. Cortisol was measured using an immunoassay (ADVIA Centaur; Siemens Healthcare). The coefficient of variation was 6.5%. Glucose was measured by an Advia 2400 Analyzer (Siemens, Barcelona Spain), using glucose oxidase reagents from the same manufacturer. Intra assay reliability expressed as coefficient of variation were lower than 3.2 % for low values and lower than 2.8 % for higher values.
Statistical Analysis
The two matched groups were compared using the non-paired Student’s t-test, or the χ2 test for comparisons of proportions. Significance was defined as p<0.05 for all statistical tests, and these were performed using SPSS version 17.0 for Windows.
As noted above, data came from a larger study of glucose tolerance in psychiatric disorders (Fernandez-Egea et al., 2009a). Blind to glucose tolerance, white blood cell count and telomere length, subjects with major depressive disorder and healthy controls were matched (by group means, not individual matching) on age, gender, BMI, cortisol, catchment area and smoking (number of cigarettes per day). Smoking (measured as cigarettes/day) and two hour glucose showed an abnormal distribution; however, log transformation did yield a normal distribution for the latter variable. Age and fasting glucose were analyzed with non parametric tests (Mann Whitney U test) due to non normal distribution. A t-test comparison was conducted for two hour glucose load and lymphocyte count in 15 patients with MDD and 70 controls.
Data on telomere length was available for 9 subjects with depression and 48 control subjects. These two groups were also very similar with regard to age, gender, BMI, catchment area and smoking (data not shown). Using a t-test, we compared the mean telomere length in the depression and control groups.
Results
The two groups were very similar with regard to demographic and clinical variables (see Table). The depression group had one subject of Latin American origin; the control group had three subjects of Latin American origin, another of Indian origin and another of Asian origin; all other subjects were European Caucasians.
Table.
| Depression (N=15) | Control (N=70) | ||
|---|---|---|---|
| Mean Age (years) | 30.7 ± 10.0 | 27.8± 6.8 | p=.549 |
| Gender: % Male | 60.0 | 62.2 | p=.836 |
| % living in catchment area | 80.0 | 68.6 | p=.378 |
| Mean BMI | 23.4 ± 4.1 | 23.7 ± 2.9 | p=.766 |
| Mean number of cigarettes/day | 9.9± 12.7 | 6.2 ± 8.3 | p=.219 |
| Cortisol (µg/dL) | 19.2 ± 8.6 | 22.1± 6.3 | p=.130 |
| Mean fasting glucose (mg/dL) | 84.9.4 ± 11.2 | 83.4 ± 6.8 | p=.632 |
| Impaired fasting glucose % | 13 | 0 | p=.002 |
| Mean two hour glucose (mg/dL) | 125.0± 67.9 | 84.6± 25.6 | p<.001 |
| Impaired glucose tolerance/Diabetes % | 20 | 4 | p=.035 |
| Mean telomere content** | 87.9±7.6 | 101.2±14.3 | p=.009 |
| Mean White Blood Cell count (109/L) | 6.4±1.3 | 7.1±1.8 | p=.182 |
| Mean lymphocyte count (109/L) | 2.1±0.6 | 2.5±0.7 | p=.028 |
For TC (Depression N=9 and Control N=48)
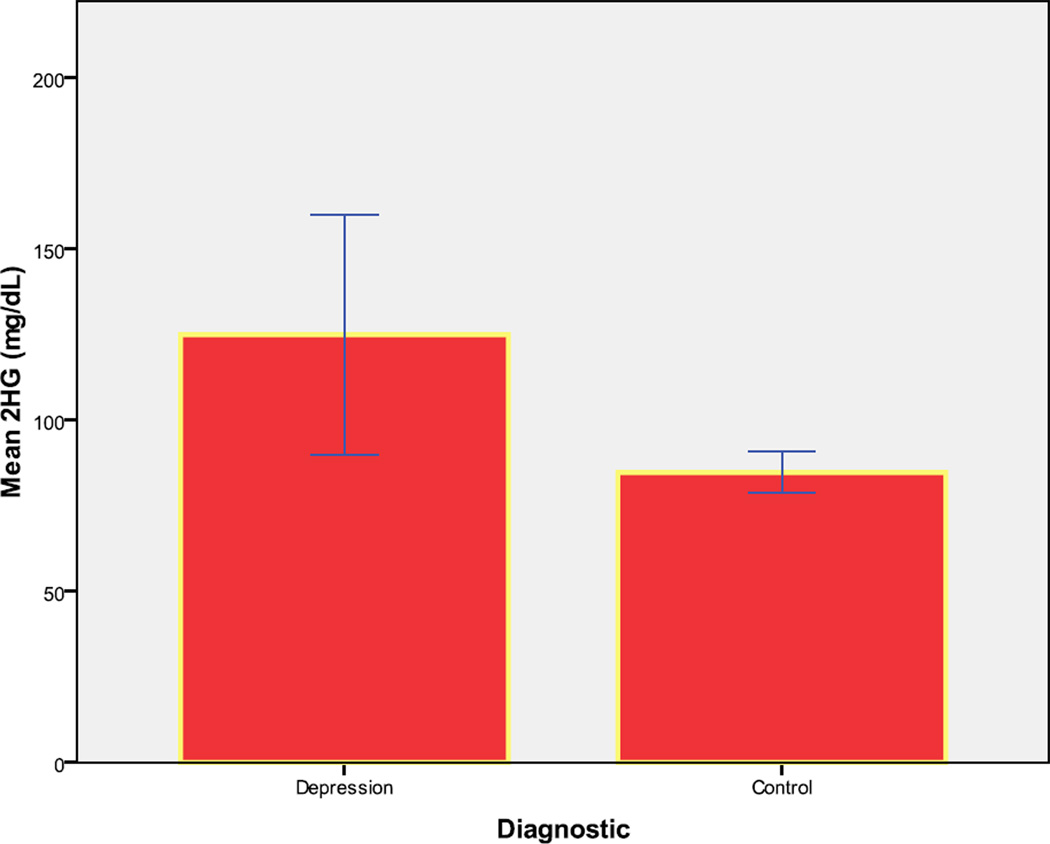
Mean fasting glucose concentration was similar in the depression group compared with controls (84.9 mg/dL[11.2] vs 83.4 mg/dL [6.7]; (p=0.63)). Cortisol values did not differ statistically between groups, with a value of 19.2 µg/dL [8.6] for major depression group compared with 22.1 µg/dL [6.3] for matched controls (p=.130). Mean two-hour glucose (2HG) concentration was significantly greater in patients with depression (125.0 mg/dL [67.9]) compared to control subjects (84.6 mg/dL [25.6]); (p <.001; as noted above, this analysis used log-transformed glucose values)(Figure 1). Two subjects (13%) with depression had fasting glucose values that met criteria for impaired fasting glucose (>100mg/dL and <126mg/dL) while no control subject met the criteria (p=.002);(none of the subjects met fasting glucose criteria for diabetes mellitus (≥126mg/dL)).Three patients (20%) met criteria for impaired glucose tolerance (N=2) (a two-hour glucose concentration >140mg/dL and <200mg/dL) or diabetes (N=1) (two-hour glucose concentration ≥200 mg/dL) while 3 control subjects ( 4%) met criteria for impaired glucose tolerance (p=.035). Three patients had taken lorazepam the previous night; their values for glucose tolerance, telomere length, and white blood cell count did not differ statistically to those of the other patients.
Figure 1.
Mean 2 hour glucose load (2HG) (mg/dL) (Error Bar: ± 2 Standard Error)
White Blood Cell (WBC) count [SD] was similar between both groups; major depression 6.4 × 109/ L [1.3] vs control group 7.1 × 109/ L [1.8] (p=.182). Granulocytes did not differ between both groups. Neutrophil count in major depression was 3.9 × 109/ L [0.8] and in control group 4.1 × 109/ L [1.8] (p=.587); Eosinophil count in major depression was 0.26 × 109/ L[0.2] and in control group 0.23 × 109/ L [0.2] (p=.591); Basophil count in major depression was 0.07 × 109/ L [0.01] and control group 0.02 × 109/ L [0.01] (p=.157). In the agranulocytes we did find differences only in the lymphocyte count. Monocyte count in major depression group was 0.38 × 109/ L [0.2] and in control group 0.41 × 109/ L [0.2] (p=.482).
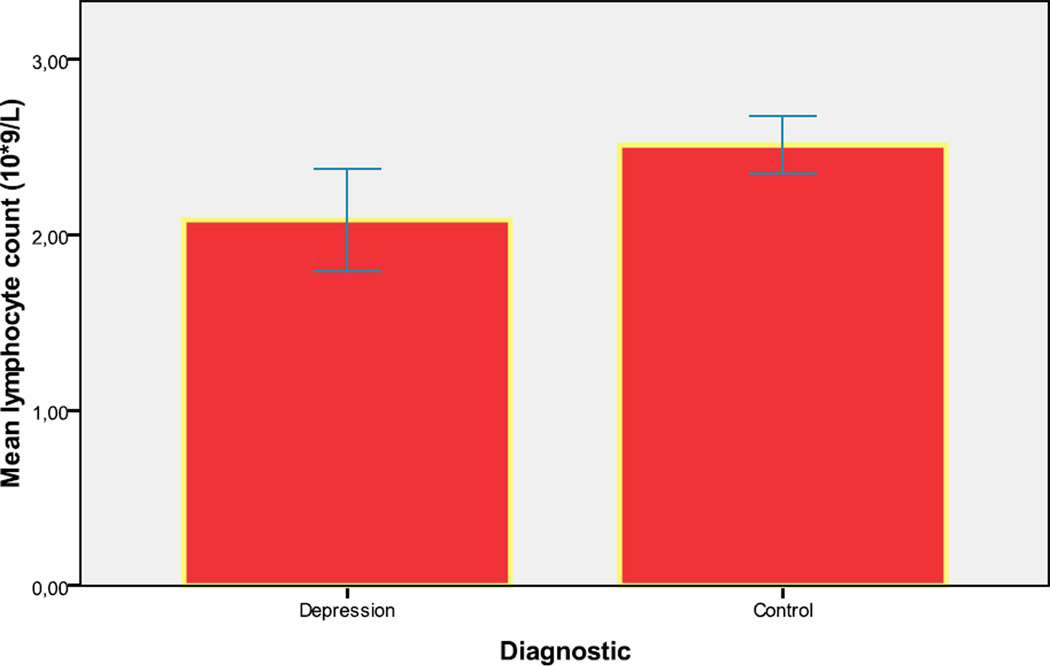
Lymphocyte count [SD] was significantly lower in the depression group 2.1× 109/ L [0.6] compared with control subjects 2.5 × 109/ L [0.7] (p=.028). (Figure 2).
Figure 2.
Mean lymphocyte count (109/L) (Error Bar: ± 2 Standard Error)
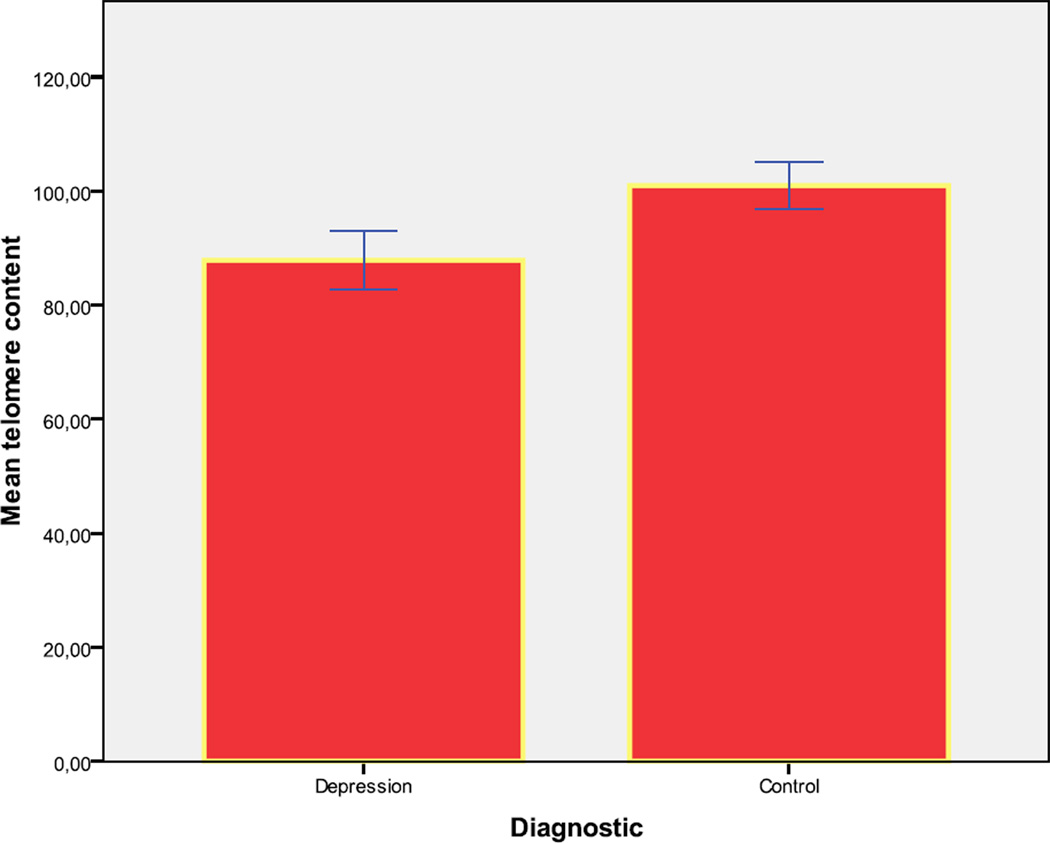
Data on telomere content was available for 9 of these same subjects with depression, and for 48 control subjects. These groups were also similar with regard to age, gender, body mass index, cortisol, catchment area and smoking (data not shown). The depression disorder group had a significantly decreased mean [SD] telomere content (89.0 [7.4]) compared with control subjects (103.7 [11.3]; p=.002). (Figure 3)
Figure 3.
Mean telomere content (Error Bar: ± 2 Standard Error)
Using a Pearson correlation analysis, 2HG, lymphocyte count and telomere length were evaluated and no significant correlation was found (data not shown)
Discussion
In this study, drug-naïve patients with depression had a higher two-hour glucose concentration than control subjects, matched for age, gender, body mass index, cortisol, catchment area and smoking.. Cortisol concentrations, a measure of stress and a risk factor for insulin resistance, were not significantly associated with two-hour glucose concentrations and did not differ significantly between groups, although there was a trend towards higher levels in controls compared with patients. Another study in first episode naïve patients showed no difference in cortisol levels between patients and controls. (Wang et al., 2012). The same article also states that more recent studies suggested that hypercortisolemia is almost exclusively a feature of very severe depressive episodes, such as are observed when depression is accompanied by psychotic symptoms.
Decreased insulin sensitivity in younger patients with major depression has previously been reported using an oral glucose tolerance test, although treatment may have confounded the results (Hung et al., 2007). Insulin resistance was also found in people with depressive symptoms in a middle-aged and elderly population (Pan et al., 2008). Insulin resistance has also been associated with moderate to severe depressive symptoms in both a follow-up survey and a cross-sectional study (Timonen et al., 2007) in which depressive symptoms were rated by self-report. One study using GTT did not find glucose intolerance in subjects with depressive symptoms. However, the fact of considering depressive symptoms and not a diagnosis of major depressive disorder, antidepressant treatment (previous and current), and a low prevalence of depressive symptoms in the study population, may have affected the results (Rhee et al., 2008).
Depressed patients also had significantly shortened telomere length and a reduced lymphocyte count, compared with matched control subjects.
Our results also extend previous findings of a shortened telomere in patients with major depressive disorder with previous antidepressant treatment (Lung et al., 2007; Simon et al., 2006; Wolkowitz et al., 2011). Pre-diabetic states such as impaired glucose tolerance have been also been associated with a shortened telomere (Adaikalakoteswari et al., 2007).
Decreased lymphocyte blood count has been previously described in major depression disorder (Darko et al., 1988) implying a possible mechanism characterized by immune suppression (Zorrilla et al., 2001). Total lymphocyte count, a crude marker of immune status, has been inversely correlated with age-related frailty (Collerton et al., 2012) and subsequent mortality of elderly men (Bender et al., 1986).
All those previous findings support the concept of major depression as an accelerated ageing disease, as others have previously stated (Heuser, 2002). Decreased lymphocyte count is a maker of normal immunosenescence (Ferrando-Martinez et al., 2011), as a shortened telomere length and abnormal glycemic homeostasis (Kassi and Papavassiliou, 2008). Several authors have shown diverse abnormalities in naïve patients with a first episode of major depression; decreased adiponectin (Leo et al., 2006a), a pro inflammatory and procoagulant state (Leo et al., 2006b), decreased plasma amino acid levels (Zheng et al., 2012), and immune dysregulation (Gabbay et al., 2009) (although some patients were treatment free and not naïve). However, no study has reported in naïve patients our results, suggesting an accelerated aging state.
As other authors have described (Wolkowitz et al., 2011) as a partial explanation of the previous findings, depressed individuals may be more sensitive to the telomere-shortening effects of oxidation and inflammation due to some biochemical “‘co-factor” (hyperglycaemia) or due to a lack of counter-regulatory anti-oxidant and anti-inflammatory activities (immune suppression through lympophenia). Our results suggest that those disturbances are present at the onset of the disease, allowing another causal explanation for those findings (Wolkowitz et al., 2011), that the diagnosis of major depression has several intrinsic features related with accelerated ageing.
Our study was limited by the relatively small sample sizes and a cross-sectional design. Our measure of the stress response was a single, fasting, morning cortisol sample; specific data from a twenty-four hour curve would have provided a more complete assessment. We also did not record the duration of depressive symptoms. Strengths of our study included a drug-naive population, and a relative ethnic homogeneity of the sample. In order to evaluate the directionality of the relationship between abnormal glucose tolerance, telomere length, lymphocyte count and depression, longitudinal studies are needed.
Our results confirm that patients with major depressive disorder show subtle disturbances prior to exposure to pharmacological treatment that may contribute to their increased morbidity and mortality rate. Those disturbances behave as markers of cell ageing, which taken all together with the diagnosis of major depression, might reflect that psychiatric and physical illness represent the same pathology. Our findings are consistent with the concept of depression as a systemic disease (Wolkowitz et al., 2010)
Acknowledgements
Supported in part by Grant RO1 DK069265 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Kirkpatrick), NARSAD (Dr. Fernandez-Egea) and by the Instituto de Salud Carlos III, FEDER, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM, Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2009SGR1295) and by Esther Koplowitz Center-Barcelona (Dr. Bernardo). The views stated in this article represent those of the authors and are not official statements of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195:83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening & metabolic/vascular diseases. Indian J Med Res. 2007;125:441–450. [PubMed] [Google Scholar]
- Bender BS, Nagel JE, Adler WH, Andres R. Absolute peripheral blood lymphocyte count and subsequent mortality of elderly men. The Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 1986;34:649–654. doi: 10.1111/j.1532-5415.1986.tb04906.x. [DOI] [PubMed] [Google Scholar]
- Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, Barton DA, Lambert GW. Cardiovascular abnormalities in patients with major depressive disorder: autonomic mechanisms and implications for treatment. CNS Drugs. 2009;23:583–602. doi: 10.2165/00023210-200923070-00004. [DOI] [PubMed] [Google Scholar]
- Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28:1063–1067. doi: 10.2337/diacare.28.5.1063. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Darko DF, Rose J, Gillin JC, Golshan S, Baird SM. Neutrophilia and lymphopenia in major mood disorders. Psychiatry Res. 1988;25:243–251. doi: 10.1016/0165-1781(88)90095-9. [DOI] [PubMed] [Google Scholar]
- Derijks HJ, Meyboom RH, Heerdink ER, De Koning FH, Janknegt R, Lindquist M, Egberts AC. The association between antidepressant use and disturbances in glucose homeostasis: evidence from spontaneous reports. Eur J Clin Pharmacol. 2008;64:531–538. doi: 10.1007/s00228-007-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede LE. Effect of comorbid chronic diseases on prevalence and odds of depression in adults with diabetes. Psychosom Med. 2005;67:46–51. doi: 10.1097/01.psy.0000149260.82006.fb. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, Esmatjes E, Garcia-Rizo C, Kirkpatrick B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009a;194:434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, Conget I, Nguyen L, George V, Stoppler H, Kirkpatrick B. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009b;35:437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Ruiz-Mateos E, Hernandez A, Gutierrez E, Rodriguez-Mendez Mdel M, Ordonez A, Leal M. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr) 2011;33:197–207. doi: 10.1007/s11357-010-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Fordyce CA, Heaphy CM, Bisoffi M, Wyaco JL, Joste NE, Mangalik A, Baumgartner KB, Baumgartner RN, Hunt WC, Griffith JK. Telomere content correlates with stage and prognosis in breast cancer. Breast Cancer Res Treat. 2006;99:193–202. doi: 10.1007/s10549-006-9204-1. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandinetti A, Kaholokula JK, Chang HK. Delineating the relationship between stress, depressive symptoms, and glucose intolerance. Diabetes Care. 2000;23:1443–1444. doi: 10.2337/diacare.23.9.1443. [DOI] [PubMed] [Google Scholar]
- Heuser I. Depression, endocrinologically a syndrome of premature aging? Maturitas. 2002;41(Suppl 1):S19–S23. doi: 10.1016/s0378-5122(02)00012-9. [DOI] [PubMed] [Google Scholar]
- Hung YJ, Hsieh CH, Chen YJ, Pei D, Kuo SW, Shen DC, Sheu WH, Chen YC. Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clin Endocrinol (Oxf) 2007;67:784–789. doi: 10.1111/j.1365-2265.2007.02963.x. [DOI] [PubMed] [Google Scholar]
- Kassi E, Papavassiliou AG. Could glucose be a proaging factor? J Cell Mol Med. 2008;12:1194–1198. doi: 10.1111/j.1582-4934.2008.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fernandez-Egea E, Garcia-Rizo C, Bernardo M. Differences in glucose tolerance between deficit and nondeficit schizophrenia. Schizophr Res. 2009;107:122–127. doi: 10.1016/j.schres.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Heerdink ER, Egberts AC, Geerlings MI, Gorter KJ, Numans ME, Grobbee DE, Klungel OH, Burger H. Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosom Med. 2007;69:300–305. doi: 10.1097/PSY.0b013e31805f48b9. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Decreased plasma adiponectin concentration in major depression. Neurosci Lett. 2006a;407:211–213. doi: 10.1016/j.neulet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, Cola C, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006b;67:1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Favaloro EJ, Franchini M. Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Semin Thromb Hemost. 2009;35:325–336. doi: 10.1055/s-0029-1222611. [DOI] [PubMed] [Google Scholar]
- Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Marano G, Harnic D, Lotrionte M, Biondi-Zoccai G, Abbate A, Romagnoli E, Mazza M. Depression and the cardiovascular system: increasing evidence of a link and therapeutic implications. Expert Rev Cardiovasc Ther. 2009;7:1123–1147. doi: 10.1586/erc.09.78. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mueller PS, Heninger GR, McDonald RK. Insulin tolerance test in depression. Arch Gen Psychiatry. 1969;21:587–594. doi: 10.1001/archpsyc.1969.01740230075011. [DOI] [PubMed] [Google Scholar]
- Nichols GA, Brown JB. Unadjusted and adjusted prevalence of diagnosed depression in type 2 diabetes. Diabetes Care. 2003;26:744–749. doi: 10.2337/diacare.26.3.744. [DOI] [PubMed] [Google Scholar]
- Obana N, Takagi S, Kinouchi Y, Tokita Y, Sekikawa A, Takahashi S, Hiwatashi N, Oikawa S, Shimosegawa T. Telomere shortening of peripheral blood mononuclear cells in coronary disease patients with metabolic disorders. Intern Med. 2003;42:150–153. doi: 10.2169/internalmedicine.42.150. [DOI] [PubMed] [Google Scholar]
- Pan A, Ye X, Franco OH, Li H, Yu Z, Zou S, Zhang Z, Jiao S, Lin X. Insulin resistance and depressive symptoms in middle-aged and elderly Chinese: findings from the Nutrition and Health of Aging Population in China Study. J Affect Disord. 2008;109:75–82. doi: 10.1016/j.jad.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Rhee MK, Musselman D, Ziemer DC, Vaccarino V, Kolm P, Weintraub WS, Caudle JM, Varughese RM, Irving JM, Phillips LS. Unrecognized glucose intolerance is not associated with depression. Screening for Impaired Glucose Tolerance study 3 (SIGT 3) Diabet Med. 2008;25:1361–1365. doi: 10.1111/j.1464-5491.2008.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SD, Drake RE, Wolford GL, Mueser KT, Oxman TE, Vidaver RM, Carrieri KL, Luckoor R. Dartmouth Assessment of Lifestyle Instrument (DALI): a substance use disorder screen for people with severe mental illness. Am J Psychiatry. 1998;155:232–238. doi: 10.1176/ajp.155.2.232. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Timonen M, Salmenkaita I, Jokelainen J, Laakso M, Harkonen P, Koskela P, Meyer-Rochow VB, Peitso A, Keinanen-Kiukaanniemi S. Insulin resistance and depressive symptoms in young adult males: findings from Finnish military conscripts. Psychosom Med. 2007;69:723–728. doi: 10.1097/PSY.0b013e318157ad2e. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Chen X, Ling X, Liu S, Xu G, Huang L. Hippocampal N-acetylaspartate and morning cortisol levels in drug-naive, first-episode patients with major depressive disorder: effects of treatment. J Psychopharmacol. 2012 doi: 10.1177/0269881112450781. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Gao HC, Li Q, Shao WH, Zhang ML, Cheng K, Yang de Y, Fan SH, Chen L, Fang L, Xie P. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J Proteome Res. 2012;11:1741–1748. doi: 10.1021/pr2010082. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]