Abstract
Background
Individuals infected with both HIV and HCV have shown impaired performance on different neuropsychological (NP) tests; however, whether coinfected individuals with controlled HIV and minimal liver damage in the era of antiretroviral therapy have impairment is understudied.
Methods
Nineteen HCV monoinfected, 17 HIV/HCV coinfected and 17 control male participants were evaluated for depression, attention, executive function, information processing, fine motor speed, and verbal/visual learning/memory. Eleven controls and 14 HIV monoinfected participants with controlled viral load from a previous study were also included for comparison. At time of testing, participants were not using drugs or alcohol and did not have cirrhosis. A global deficit score (GDS) was calculated from 7 domains of NP tests and alterations in specific domains were determined.
Results
HIV/HCV subjects had a higher depression score (11.1±7.5) than controls (5.4±4.1, p=0.010) and a higher GDS score (0.77±0.47) than HCV (0.46±0.34, p=0.036), HIV (0.45±0.36, p=0.008) and controls (0.30±0.29, p=0.001). Coinfection was associated with worse scores in attention working memory (p=0.007), executive function (p=0.01), fine motor function (p=0.011), verbal learning/memory (p<0.001) and visual learning/memory (p<0.001) compared to controls. Within the HCV group, viral load was associated with lower attention, executive function and information processing speed and positively with GDS.
Conclusions
Coinfection significantly increased the risk of cognitive impairment in subjects with controlled HIV viral loads. In HCV monoinfected but not coinfected subjects, HCV viral load correlated with worsening GDS, suggesting different pathways for neuropsychological impairment.
INTRODUCTION
Hepatitis C virus (HCV) has become a major cause of morbidity and mortality in HIV-infected individuals1–3. It has been reported that 20–30% of HIV-infected individuals are infected with HCV in the United States and worldwide4, 5. Since the introduction of antiretroviral treatment (ART) and pegylated-interferon treatment, ART has led to a significant decrease in HIV-associated disease, but the development of cirrhosis in HIV+ patients with longstanding HCV infection continues to increase. Although ART has improved the life expectancy of persons with HIV, coinfected persons survive to at times develop neuropsychological (NP) impairment which can greatly impact their quality of life. Various studies have shown controlled chronic HIV infection can result in neurocognitive dysfunction. Chronic HCV infection has also been associated with neurocognitive dysfunction, even in the absence of advanced liver damage6–8. HIV/HCV coinfection has been found in some studies to be associated with impairment in executive9 and psychomotor function10, as well as overall cognition11, 12. While some reports did not demonstrate cognitive differences between coinfected and HCV monoinfected subjects, this could be due to the heterogeneous composition of some cohorts, including active HIV infection and various stages of AIDS, drug or alcohol abuse and the prevalence of cirrhosis. Some studies included HIV+ subjects with high viral load or advanced AIDS, who are fortunately now quite uncommon in the era of ART. AIDS, drug or alcohol abuse, alone or in combination, have been related to neurocognitive dysfunction13, 14. To minimize the interfering factors and comorbid conditions and in an effort to clarify the effect of HCV on cognitive dysfunction or combined with controlled HIV infection, the current cohort was enrolled that was: free of substance abuse, without current clinical depression, and with undetectable HIV viral load. We used an extensive neuropsychological battery of tests including a depression scale to obtain a better understanding of the impact of HCV among patients with well-controlled HIV who reflect the current use of effective ART treatment.
METHODS
Cohort
This is a cross sectional study of 53 participants recruited from the Gastroenterology clinics at the Veterans Affairs Medical Center, San Francisco (VAMCSF) and the University of California, San Francisco affiliated hospitals who signed written informed consent for protocols that had been approved by the University of California, San Francisco Committee on Human Research. Determining compatibility with the inclusion and exclusion criteria was based on medical record review for HIV and/or HCV-infected subjects and self-report for the controls. All participants were given minimal compensation for participation that was not disclosed on recruitment flyers. In North America, HCV transmission occurs primarily through shared needles during intravenous drug use, blood transfusions prior to 1992, body piercings or tattoos. Among these risk factors, intravenous drug use typically coincides with a history of drug abuse and there is evidence that the use of psychoactive substances can impair cognition, as such patients’ histories of past intravenous drug use and other psychoactive substance use were recorded in detail. Inclusion criteria for this study were: males between the ages of 45 and 65 with HCV infection, subjects with HIV/HCV coinfection (HCV genotype 1) or healthy controls. Coinfected subjects were compliant on ART for at least 2 years with undetectable HIV viral load (<50 copies/ml) for 6 months prior to enrollment. Exclusion criteria included: illicit drug use or prescribed opiate pain medications 6 months prior to enrollment; clinical evidence of hepatic cirrhosis (most patients had past liver biopsies without cirrhosis); evidence of any other chronic infectious processes; consumption of >20 grams alcohol per day for the 6 months prior to enrollment; IFNα-based therapy within the previous four years; clinical depression or other significant psychiatric disease; seizure disorders or history of head injury. Mood disorders and psychiatric exclusionary diagnosis were defined by a clinical interview by the study psychologist (L.A.), using the Structured Clinical Interview for DSM-IV 15.
In order to compare HCV mono- and coinfected subjects with HIV infection, we included 14 HIV monoinfected individuals with undetectable viral loads along with 11 controls from a previous study published in 2010 16. Subjects in the HIV cohort were recruited from the Infectious Disease clinic at the same VAMCSF, using the same inclusion/exclusion criteria with the exception of being HIV infected, on ART, with an undetectable (<50 copies/ml) viral load. All the neuropsychological testing was performed by the same investigator (LA).
Neuropsychological evaluation
Neuropsychological test measures covered 7 domains. The Structured Clinical Interview for DSM-IV 15 was used to establish a lifetime history of depressive illness and to diagnose exclusionary psychiatric disorders. Participants also completed a self-report depression measure, the Beck Depression Inventory-II (BDI) 17. General intellect (estimated IQ) was assessed using the Information subtest from the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) 18. Neuropsychological test results were demographically corrected according to educational level, gender, and age based on the normative scoring for each test. All individual test scores were converted to standard T scores.
Global Deficit Score (GDS)
The GDS is a single number representing overall NP test performances. The T scores from each test were converted to a deficit score based on Carey, et al19. Briefly, ≥40T=0; 39T-35T=1; 34T-30T=2; 29T-25T=3; 24T-20T=4; ≤19T=5. The mean of the deficit scores from each test was assigned to the participant as GDS. A GDS of 0.5–1 was considered mild impairment. As reported previously, we used a GDS of ≥ 0.5 as neuropsychologically impaired16.
Statistical analysis
Demographic data were analyzed using non-parametric statistical analyses (Pearson’s Chi-squared test) for categorical data and the rates of significant cognitive impairment across groups. One-way analyses of variance (ANOVA) were used for continuous data. Since education and IQ showed differences, they were included as covariates in the analyses of BDI, GDS and all NP tests in the multivariate analysis of variance (MANOVA). A Tukey’s posthoc test was used to compare group differences after MANOVA analysis. Pairwise comparisons of group means were achieved using Student’s two sample t test for unequal variances (Welch’s t test) with p values adjusted using Benjamini and Hochberg’s correction for multiple comparisons20 where necessary. Correlations of neuropsychological domains and GDS with plasma HCV RNA levels were analyzed using Spearman’s rank correlation coefficient due to the distribution nature of the data. The correlation between GDS and BDI was also analyzed using Spearman’s correlation. All tests were performed with R v2.13.121.
RESULTS
Study Population
We enrolled 19 HCV monoinfected individuals (HCV), treatment naïve except for 2 treatment failures; 17 HCV-infected individuals with controlled HIV-1 infection (Co) and 17 age, education and ethnicity-matched controls (C). Fourteen HIV-infected subjects with undetectable HIV viral load (HIV) and 11 controls from a previous study16, 22 were also included for comparison purposes (Table 1). There was no significant difference in HCV plasma viral load between HIV/HCV coinfection and HCV monoinfection (p=0.31). Also, there was no significant difference in the mean age of the different groups, while the mean education level of controls was about 2–2.5 years higher than participants with HCV (p=0.011), coinfection (p=0.013) and HIV (p=0.012) (Table 1). IQ was lower in HIV/HCV coinfected subjects than controls (p=0.004) and HIV monoinfected subjects (p=0.025) after adjusting education. HCV monoinfected subjects had lower IQ than controls and HIV monoinfected individuals, but this was not statistically significant (p=0.098 and 0.260, respectively) after adjusting for education. All HIV-infected participants were on ART16 for over 2 years. All HIV-infected and HIV/HCV coinfected participants had undetectable plasma HIV viral load at the time of participation (<50 copies/ml). Ethnicity was not a significant variable (χ2 =6.84, p value=0.654). No subjects with known cirrhosis were included.
Table 1.
Participant demographics, clinical and laboratory characteristics
| C | HCV | Co | HIV | P values | |
|---|---|---|---|---|---|
| N | 28 | 19 | 17 | 14 | |
| Age (yr) | 53.2 (6.0) | 56.6 (4.5) | 54.5 (5.2) | 51.6 (7.2) | 0.081 |
| Education (yr) | 15.4 (2.3) | 13.1 (2.2) | 13.4 2.1) | 13.6 (1.2) | 0.001 a |
| Ethnicity | 0.868 | ||||
| Asian | 2 | 0 | 0 | 0 | |
| African.American | 5 | 6 | 6 | 2 | |
| Caucasian | 19 | 11 | 10 | 11 | |
| Hispanic | 2 | 2 | 1 | 1 | |
| Estimated IQ (T score) | 57.2 (9.1) | 51.7 (8.6) | 49.0 (8.6) | 56.9 (8.1) | 0.010 b |
| Global deficit score | 0.30 (0.29) | 0.46 (0.34) | 0.77 (0.47) | 0.45 (0.36) | <0.001c |
| Beck Depression Inventory Score | 5.4 (4.1) | 6.7 (6.0) | 11.1 (7.5) | NA | 0.010 d |
| HCV RNA (log10 IU/mL) | NA | 5.9 (0.7) | 6.2 (0.5) | NA | 0.305 |
| HIV RNA (log10 RNA/mL) | NA | NA | UD | UD | NA |
| CD4 count (cells/ul) | NA | NA | 501 (243) | 516 (261) | 0.890 |
Note: Values are presented as mean (SD) except for N and ethnicity which were the number of individuals and drug abuse history which is percent. All participants were male. NA: not applicable or not available. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection, HIV=HIV monoinfection. UD=undetectable. P values stand for one-way ANOVA test except ethnicity (Chi-squared test). MANOVA was used in GDS and BDI with education and IQ as covariates. P values for HCV RNA and CD4 count were from Student’s t tests.
mean years of education of HCV, Co and HIV were lower than controls, no difference between them.
mean IQ of HCV and Co were lower than C, mean IQ of Co was lower than HIV.
mean GDS score of Co was higher than C after adjusting for education and IQ, Tukey HSD posthoc test p<0.001.
mean BDI of Co was higher than C and HCV after adjusting for education and IQ, Tukey HSD posthoc test p=0.010 and 0.049, respectively.
We evaluated participants in the HCV cohort to determine whether exposure to drugs of abuse coincided with lower NP test results. In the HCV group, 63% (12/19) of the participants had a history of drug abuse, while 71% (12/17) of the coinfected also had abused drugs. A possible relationship between drug abuse and cognition in either of the mono- or coinfected groups in this study was examined using a Fisher’s exact test and was found not to be significant among either HCV monoinfected (p=0.4) or coinfected (p=1) subjects. The controls in the HCV cohort did not report any history of drug abuse. For the HIV cohort, a separate drug abuse history, apart from that collected for compliance with the exclusion criteria, was not obtained and is a limitation of this study.
Neuropsychological Findings
Since depressive symptoms may still exist in the HCV-infected patients, we used the Beck Depression Index (BDI), a self-report questionnaire, as a screening tool for perceived depression not diagnosed clinically. Coinfected subjects were found to have more depressive symptoms by BDI (11.1±7.5) than controls (5.4±4.1, p=0.011) (Fig. 1A) and HCV monoinfected subjects (6.7±6.0, p=0.049), even though no subject carried a clinical diagnosis of depression.
Figure 1.
Group comparisons of depression and global deficit score. A) Comparisons of BDI scores for controls HCV and HIV/HCV infections. Controls were recruited in the HCV cohort. B) Comparisons of controls, HCV monoinfection, HIV/HCV coinfection and HIV monoinfection of GDS scores. Controls were used from both HCV and HIV cohorts. *p<0.05, ** p<0.01, ***p<0.001 using MANOVA with education and IQ adjusted. BDI=Beck depression inventory–II score. GDS=global deficit score. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection.
A summary of all NP tests measured in the study was represented by a global deficit score which reflects the overall cognitive status of the participants. HIV/HCV coinfection had a worse GDS score (0.77±0.74) than HCV monoinfection (0.46±0.34, p=0.015), HIV monoinfection (0.45±0.36, p=0.008) or controls (0.30±0.29, p<0.001) (Fig. 1B). The mean GDS of 0.77 for HIV/HCV puts the coinfected individuals in the mild cognitive impairment range (0.5–1.0)19. The rates of impairment based on GDS were higher in coinfection (65%) than HCV monoinfection (42%), HIV monoinfection (29%) and controls (18%). While this difference was not statistically significant among coinfection, HIV monoinfection or HCV monoinfection (χ2 =4.2, p=0.12), coinfection did have a higher rate than controls (χ2 =8.2, p=0.004). There was no correlation found between GDS and BDI in HCV-infected individuals (Spearman’s correlation, rho=0.13, p=0.45) suggesting that depression did not influence GDS. Controls from the HIV and HCV cohorts were used in GDS analysis, while for depression analysis only controls from HCV cohort were used since depression analysis was not done on the HIV cohort.
Although the tests were grouped into domains, individual NP tests showed significant differences among groups after adjusting for education and IQ (Table 2). Lower scores were found in Digit Span and Brown-Peterson tests in HIV/HCV coinfected subjects indicating impairment in attention and short-term memory. When considering the domain, subjects with HIV/HCV coinfection demonstrated lower performance in attention working memory compared to controls (p=0.006) (Fig. 2). Impairment in Symbol Digit Written test in HIV/HCV coinfection and not in the Symbol Digit Oral test indicates malfunction of psychomotor ability though the Information processing speed domain did not show significant difference (Table 2). Although the Stroop Color and Word test showed higher performance in HIV than C, HCV and coinfection, when considering all 4 tests in the executive function domain, coinfection had lower performance than controls in general (p=0.026) (Fig. 2). In the Grooved pegboard test, the non-dominant hand performed poorer in coinfection than controls (p=0.035). Fine motor function as a domain was impaired in coinfected subjects than controls (p=0.013). In Brief Visuospatial Memory Test (BVMT) and California Verbal Learning Tests (CVLT), coinfection showed significant impairment in visual learning and memory (p<0.001) and verbal learning and memory (p<0.001) than controls (Fig. 2 and Table 2). Despite undetectable HIV viral loads in both, HIV/HCV coinfected individuals showed lower ability on fine motor function (p<0.001), visual learning/memory (p<0.001) and verbal learning/memory (p=0.001) than HIV monoinfected individuals (Fig. 2). HIV/HCV coinfection showed an overall decrease in most of the domains tested (Fig. S1). Subtle deficits can be seen in verbal learning/memory in coinfected patients with T scores nearly 1 SD lower than the population mean (Fig. S1). The visual learning/memory domain showed a moderate deficit in coinfection of almost 2SDs below the mean (mean T score=30.9±10.0).
Table 2.
Neuropsychological test results
| Domain / Test Name | Mean (SD) |
P values | |||
|---|---|---|---|---|---|
| C | HCV | Co | HIV | ||
| Attention working memory | |||||
| WAIS-III Digit Span18 | 53.2 (10.4) | 46.2 (7.9) | 45.6 (9.3) | 50.4 (9.1) | 0.005 |
| Brown Peterson 18 23 | 53.7 (10.0) | 45.3 (11.3) | 45.2 (12.2) | 39.1 (17.3) | 0.004 |
| Brown Peterson 36 23 | 55.1 ( 9.1) | 44.8 (13.1) | 49.0 (6.8) | 43.9 (11.2) | 0.001 |
| Information processing speed | |||||
| Symbol Digit Oral 24 | 48.8 (11.6) | 45.6 (10.0) | 43.2 (10.8) | 48.6 (11.5) | 0.126 |
| Symbol Digit Written 24 | 50.2 (11.5) | 43.8 (10.3) | 43.2 (9.7) | 46.9 (8.3) | 0.026 |
| Stroop Word 25 | 47.3 (7.7) | 45.9 (7.7) | 46.5 (8.1) | NA | 0.880 |
| Stroop Color 25 | 43.2 ( 9.9) | 42.9 (8.3) | 43.0 (5.3) | 45.4 (7.1) | 0.717 |
| Executive function | |||||
| Stroop Color and Word 25 | 46.4 (6.9) | 43.3 (7.3) | 43.1 (8.3) | 52.6 (7.4) | <0.001 |
| Stroop Interference 25 | 49.9 (5.9) | 48.4 (5.3) | 47.5 (6.0) | NA | 0.136 |
| WCST total errors 26 | 43.2 (9.1) | 41.1 (10.5) | 35.1 (8.3) | 39.2 (12.8) | 0.075 |
| WCST perseveration 26 | 46.0 (12.6) | 47.7 (8.3) | 39.9 (7.6) | 42.0 (11.2) | 0.123 |
| Fine motor skills | |||||
| Grooved Pegboard Dominant Hand 27 | 50.3 (14.5) | 50.2 (11.8) | 45.2 (8.6) | 56.3 (12.8) | 0.069 |
| Grooved Pegboard Non-Dominant Hand 27 | 51.2 (11.9) | 49.2 (13.7) | 43.1 (7.8) | 57.8 (10.5) | 0.002 |
| Finger Tapping Dominant Hand 28 | 44.3 (9.0) | 45.6 (8.4) | 39.4 (9.4) | NA | 0.115 |
| Finger Tapping Non-Dominant Hand 28 | 42.0 (9.5) | 45.3 (7.6) | 41.2 (7.1) | NA | 0.107 |
| Verbal fluency | |||||
| COWA Test 29 | 47.4 (9.2) | 47.6 (8.1) | 46.1 (8.6) | 49.9 (8.4) | 0.663 |
| Visual learning/memory | |||||
| BVMT trials 1–3 30 | 44.9 (13.5) | 36.8 (12.0) | 29.8 (9.3) | 44.1 (14.0) | <0.001 |
| BVMT delay 30 | 46.0 (12.8) | 42.5 (14.4) | 31.9 (11.4) | 47.6 (11.7) | <0.001 |
| Verbal learning/ memory | |||||
| CVLT total 1–5 31 | 55.0 (10.9) | 47.1 (9.2) | 39.8 (11.6) | 53.9 (8.8) | <0.001 |
| CVLT Long Delay Free Recall 31 | 52.3 (10.6) | 47.4 (8.2) | 43.2 (6.6) | 52.5 (6.4) | 0.001 |
Note: Values are presented as mean (SD) of T scores for all tests. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection, HIV=HIV monoinfection. NA=not available. WAIS=Wechsler Adult Intelligence Scale; WCST= Wisconsin Card Sorting Test); COWA= Controlled Oral Word Association; BVMT=Brief Visuospatial Memory Test; CVLT= California Verbal Learning Test. P values are of MANOVA with education and IQ as covariate.
Figure 2.
Group comparisons of significant neuropsychological domains. MANOVA analyses with education and IQ adjusted were used to compare group means. * p<0.05, ** p<0.01, *** p<0.001. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection, HIV=HIV monoinfection.
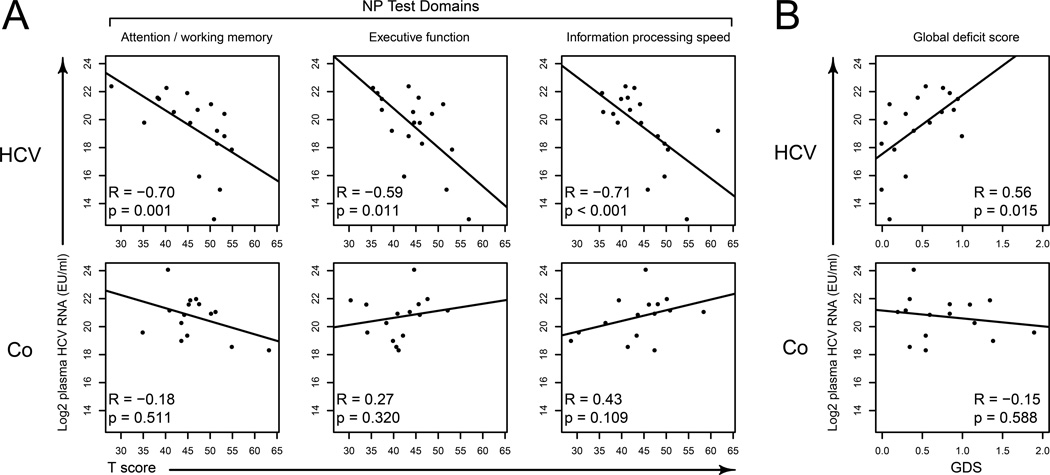
While the HCV group did not cross the threshold of cognitive impairment (GDS ≥ 0.5), HCV plasma viral load was negatively correlated with attention, executive function, and information processing speed in HCV monoinfection (Fig. 3A). These relationships were not observed in coinfected subjects where HCV viral load did not correlate with cognitive performance. The same respective outcomes for HCV mono- and coinfected subjects were found when T scores were switched with GDS as a measure of overall cognition (Fig. 3B). Again, only HCV viral load in the context of monoinfection was related to poorer cognitive performance.
Figure 3.
Correlations of plasma HCV viral load with neuropsychological data. Spearman's rank correlation was used to compute the correlations. HCV RNA was shown as logarithm base 2 transformed. HCV=HCV monoinfection, Co=HIV/HCV coinfection. GDS=global deficit score. A) HCV viral load negatively correlated with attention/working memory, executive function and information processing speed. Lower T scores are evidence of poorer performance. As HCV plasma load increases, T scores decrease in monoinfection only. B) In monoinfection, HCV viral load significantly correlated with global deficit scores. Higher GDS indicates poorer cognition.
DISCUSSION
Over the last decade, numerous studies have assessed the impact of HCV monoinfection and HIV/HCV coinfection on cognition. Overall, results from these studies have been inconclusive with some indicating greater cognitive impairment in coinfected individuals while other studies fail to detect significant difference between mono- and coinfection11. Potentially there are multiple reasons for this disparity including cohort composition, utilization of different neuropsychological assessments and subtlety of the cognitive dysfunction related to HCV infection. For this cross-sectional study, males under stable medical care who were not abusing drugs were assessed for neuropsychological deficits in seven domains and evaluated for subclinical depression. The study was designed to test for cognitive impairment in coinfected subjects whose HIV was controlled as well as to measure cognitive dysfunction in HCV-infected subjects with no current drug or alcohol abuse. In fact, this study represents many American HIV-infected individuals today, who are compliant with their ART and live their lives with undetectable HIV viral loads, as did both coinfected and HIV monoinfected subjects in this study. Moreover, to remove liver dysfunction as a factor influencing cognition, subjects with cirrhosis were excluded from the study. So while this study comprises a limited number of individuals, its strength lies in a well-characterized cohort with minimal comorbid conditions.
Indications are that coinfection in subjects with undetectable HIV viral loads has at most a subtle impact on cognition. By examining a relatively homogeneous cohort of men all receiving a similar level of medical care, we were able to detect a mild, yet significant impairment in cognition among the coinfected group. Coinfected subjects performed poorly on the attention, executive function, fine motor function and visual and verbal learning/memory tests, with significantly lower T scores than either controls or monoinfected subjects. Our results from the male cohort are in contrast to a recent study of coinfected women, which found no association between viral infection and cognition32. Importantly, in the women’s study only a limited number of NP tests were performed to assess two domains: information processing (symbol-digit modalities, Trails part A and B) and executive function (Comalli-Kaplan Stroop test) domains; we also found no differences in these two domains. Our more comprehensive evaluation utilized 20 tests which yielded information for seven domains. Historically, studies on the impact of HIV on neuropsychological function have indicated deficits most consistently in the domain of motor skills, although more recent studies have shown deficits also in attention, executive function and information processing speed16. Overall, neuropsychological impairment has been reported in a coinfected cohort with deficits in visual and verbal learning/memory domains, which can interfere with daily life12. HIV-infected individuals subsequently exposed to HCV also have neurocognitive impairment with abnormal brain metabolites ratios33. We found both HIV and HCV monoinfected subjects had test results similar to controls in most domains suggesting a synergistic effect of HIV and HCV in coinfection that is responsible for neuropsychological deficits in the coinfected population. Previous substance abuse did not correlate with cognitive impairment in the coinfected group. The majority of these individuals were in the military, and by self-report their substance abuse was related to their youth and since these subjects were all over 45 years old, the impact of previous substance abuse is probably minimal. However, verification of self-report of participants was not done and therefore a limitation of the study.
Our expectation was that lower cognitive scores in HCV monoinfection would be similar to coinfection, likely affecting the same domains but perhaps less severe. Instead, there was a distinctly different pattern in the NP tests which varied with HCV viral load in monoinfection inversely correlating with T scores in three separate domains. While as a group, cognitive scores did not cross the threshold of impairment, these results suggest that greater HCV viral load negatively impacts cognition. HCV viral load has been investigated as an independent factor influencing cognition in HCV monoinfected individuals, but the results have been equivocal. No association between cognition and viral load was detected when HCV-monoinfected subjects were grouped based on their level of fatigue and secondarily evaluated for viral load34. However, when evaluated by Spearman rank coefficient analysis, lower T scores in the attention / working memory domain correlated with higher HCV viral load12. This was one of the same domains affected in our HCV monoinfected subjects, which also showed a significant correlation with lower T scores. Together these findings imply that HCV viral load may play an important but subtle negative role in cognition, a role which could be better elucidated by experiments specifically designed to assess its impact. The relationship of HCV viral load on NP tests was not detected in coinfected subjects in this study, suggesting that cognitive impairment observed with coinfection could have a different pathogenesis from that in monoinfection. Some studies showed impairment in attention, concentration, psychomotor speed in HCV infection independent of coinfection11. The mechanisms for NP impairment in HCV infection are still not well elucidated and may be related to direct HCV viral toxicity, as replicable forms of HCV virus have been detected in autopsy brains35, 36. Brain MRS studies in HCV infection have also demonstrated inflammation in the brain37. HCV has been shown to induce microglia activation38. Brain levels of MCP-1, TNFα, and soluble TNF receptor were found higher in HCV-infected individuals12. These observations suggest that HCV is neurotoxic although whether the mechanism is direct versus indirect is unknown.
Depression has also been reported to be more severe in coinfection than monoinfection6. We found the depression scores in coinfection were slightly higher than controls and HCV monoinfection. Elevated BDI scores indicated that coinfected individuals experienced more symptoms of depression but did not meet a clinical depression diagnosis. Importantly, BDI scores did not correlate with neuropsychological measures or GDS indicating that depression was not responsible for neurocognitive deficits.
Whether coinfection constitutes a legitimate risk factor for cognition remains to be demonstrated unambiguously. This targeted study indicates that coinfection in males is sufficient to push this group over the threshold into mild impairment and high viral load in HCV monoinfection may impact cognition.
Supplementary Material
Figure S1. Mean T scores of neuropsychological domains. Mean denotes the population mean T score level is 50 and 1 SD denotes 1 standard deviation from the mean. Although most of the T scores for the domains were within 1 SD range, the mean T scores for coinfection were on the lower end for almost all of the domains. Global was the average of all domains. Connected lines only demonstrate groups, no relationship between domains was implicated. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection, HIV=HIV monoinfection.
ACKNOWLEDGEMENTS
We thank our patients for their participation and Dorthe Welch RN for recruitment.
Source of Funding:
This study was supported by NIMH RO1MH085538 (LP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
No authors have any conflicts of interest to declare
REFERENCES
- 1.Monto A, Schooley RT, Lai JC, et al. Lessons from HIV therapy applied to viral hepatitis therapy: summary of a workshop. Am J Gastroenterol. 2010;105:989–1004. doi: 10.1038/ajg.2009.726. quiz 1988-1005. [DOI] [PubMed] [Google Scholar]
- 2.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 5.Cargill VA. HIV/hepatitis C virus co-infection: its human face. AIDS. 2005;19(Suppl 3):S1–S2. doi: 10.1097/01.aids.0000192062.63539.98. [DOI] [PubMed] [Google Scholar]
- 6.Clifford DB, Evans SR, Yang Y, et al. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005;19(Suppl 3):S64–S71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- 7.Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 8.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- 9.Ryan EL, Morgello S, Isaacs K, et al. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62:957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Giesen HJ, Heintges T, Abbasi-Boroudjeni N, et al. Psychomotor slowing in hepatitis C and HIV infection. J Acquir Immune Defic Syndr. 2004;35:131–137. doi: 10.1097/00126334-200402010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Perry W, Carlson MD, Barakat F, et al. Neuropsychological test performance in patients co-infected with hepatitis C virus and HIV. AIDS. 2005;19(Suppl 3):S79–S84. doi: 10.1097/01.aids.0000192074.18691.31. [DOI] [PubMed] [Google Scholar]
- 12.Letendre SL, Cherner M, Ellis RJ, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19(Suppl 3):S72–S78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- 13.Devlin KN, Gongvatana A, Clark US, et al. Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc. 2012;18:68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persidsky Y, Ho W, Ramirez SH, et al. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav Immun. 2011;25(Suppl 1):S61–S70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First M, Spitzer R, Williams J, et al. Structured Clinical Interview for DSM-IV (SCID-I; Patient Version ed.) New York: New York State Psychiatric Institute, Biometrics Research; 1996. [Google Scholar]
- 16.Sun B, Abadjian L, Rempel H, et al. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010;16:115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 18.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 19.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:125–133. [Google Scholar]
- 21.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- 22.Rempel H, Sun B, Calosing C, et al. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS. 2010;24:1415–1423. doi: 10.1097/QAD.0b013e32833ac623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struss DT, Stethem LL, Poirier CA. Comparison of three tests of attention and rapid information processing across six age groups. The Clinical Neuropsychologist. 1987;1:139–152. [Google Scholar]
- 24.Smith A. Symbol Digit Modalities Test (SDMT) Manual. Revised ed. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 25.Golden CJ. Stroop Color and Work Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 26.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Cart Sorting Test (WCST) manual. Revised and expanded ed. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 27.Klove H. Clinical neuropsychology. In: FM F, editor. The medical clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- 28.Halstead W. Brain and Intelligence. Chicago: University of Chicago Press; 1947. [Google Scholar]
- 29.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd ed. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- 30.Benedict RH. Brief Visuospatial Memory Test- Revised (BVMT-R) Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 31.Delis DC, Kaplan E, Kramer JH, et al. California Verbal Learning Test – Second Edition (CVLT-II) San Antonio, TX: The Psychological Corporatino; 1987. [Google Scholar]
- 32.Crystal H, Kleyman I, Anastos K, et al. Effects of hepatitis C and HIV on cognition in women: data from the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2012;59:149–154. doi: 10.1097/QAI.0b013e318240566b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winston A, Garvey L, Scotney E, et al. Does acute hepatitis C infection affect the central nervous system in HIV-1 infected individuals? J Viral Hepat. 2010;17:419–426. doi: 10.1111/j.1365-2893.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 34.McAndrews MP, Farcnik K, Carlen P, et al. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson J, Radkowski M, Laskus T. Hepatitis C virus neuroinvasion: identification of infected cells. J Virol. 2009;83:1312–1319. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letendre S, Paulino AD, Rockenstein E, et al. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- 37.Forton DM, Hamilton G, Allsop JM, et al. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. J Hepatol. 2008;49:316–322. doi: 10.1016/j.jhep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Grover VP, Pavese N, Koh SB, et al. Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J Viral Hepat. 2012;19:e89–e96. doi: 10.1111/j.1365-2893.2011.01510.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean T scores of neuropsychological domains. Mean denotes the population mean T score level is 50 and 1 SD denotes 1 standard deviation from the mean. Although most of the T scores for the domains were within 1 SD range, the mean T scores for coinfection were on the lower end for almost all of the domains. Global was the average of all domains. Connected lines only demonstrate groups, no relationship between domains was implicated. C=controls, HCV=HCV monoinfection, Co=HIV/HCV coinfection, HIV=HIV monoinfection.