Abstract
Sigma-1 receptors (Sig-1Rs) have been implicated in many neurological and psychiatric conditions. The Sig-1R is an intracellular chaperone that resides specifically at the endoplasmic reticulum (ER)-mitochondrion interface referred to as the mitochondrion-associated ER membrane (MAM). Here, Sig-1Rs regulate ER-mitochondrion Ca2+ signaling. In this review, we discuss the current understanding of Sig-1R functions. Based on this, we suggest that the key cellular mechanism linking Sig-1Rs to neurological disorders involve the translocation of Sig-1Rs from the MAM to other parts of the cell, whereby Sig-1Rs bind and modulate the activities of various ion channels, receptors, or kinases. Thus, Sig-1Rs and their associated ligands may represent new avenues for treating some aspects of neurological and psychiatric diseases.
Keywords: neuronal excitability, voltage-gated ion channels, glutamate receptors, GABA receptors, mitochondrion-associated ER membrane, binding immunoglobulin protein
Introduction
The Sig-1R is an ER resident protein that has been implicated in many diseases, ranging from cocaine or alcohol addiction to the most recently reported familial adult or juvenile amyotrophic lateral sclerosis (ALS) [1–3]. The sequence of the Sig-1R gene does not resemble that of any other mammalian protein. So far, no other members have been found in this class of protein except for a short variant of the Sig-1R that has been recently reported [4]. The so-called sigma-2 receptor (Sig-2R) was identified by binding assays in which certain ligands show slightly different affinities from those at the Sig-1R. However, the Sig-2R has not been cloned yet. The Sig-1R contains two transmembrane regions (Box 1).
Box 1. Sigma receptors: topology and ligand binding sites.
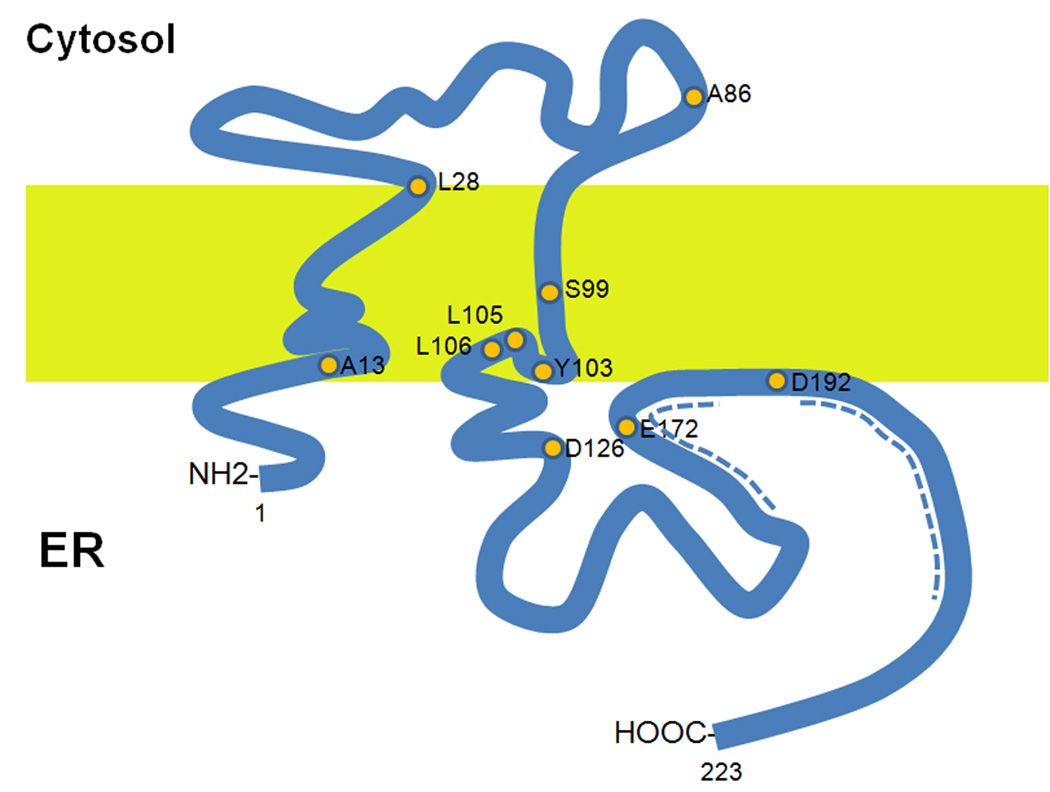
The Sig-1R contains two transmembrane domains with a short N-terminus and a long C-terminus facing the ER lumen (Figure I). The long C-terminus of Sig-1R is shown as attaching to the ER membrane. However, a study has suggested that the hydrophobic region at the C-terminus may associate with either one or both of the transmembrane domains [103]. The chaperone activity of the Sig-1R is at its long C-terminus from amino acid 116 to amino acid 223. Sig-1Rs exist not only in the brain but also in the peripheral organs including the lung, kidney, liver, pancreas, spleen, adrenal gland [20] and heart [104]. The function of Sig-1Rs in those organs has not been systematically explored except in the heart where Sig-1Rs are known to play an important role in cardioprotection [104].
The other subtype of Sigma receptors, the Sigma-2 receptor (Sig-2R), was identified by binding studies which showed that certain common ligands exhibit differential affinities at this subtype of binding site from that at Sig-1Rs. Thus, the term Sig-2R was coined. The Sig-2R has not been cloned yet. However, a complex of progesterone receptor membrane component 1 has been purported to contain the Sig-2R in a photoaffinity labeling study [105]. Sig-2Rs also exist in the CNS.
There are a number of selective ligands for Sig-1Rs and Sig-2Rs [106], as exemplified in the following list (ordered by theirrelative affinities). Sig-1R: haloperidol > (+)pentazocine > DTG > progesterone > fluvoxamine > dextromethorphan > cocaine > N,N-dimethyltryptamine. Sig-2R: DTG > (−)pentazocine > haloperidol > progesterone > dextromethorphan.
Sig-1Rs resides at the specialized ER membrane directly apposing mitochondria. The specialized ER membrane that Sig-1Rs reside in is called the mitochondrion-associated ER membrane (MAM) [5, 6]. At the MAM, Sig-1Rs have been demonstrated to regulate dendritic spine formation and dendrite arborization [7]. Interestingly, the localization of Sig-1Rs is dynamic in nature. Specially, Sig-1Rs have been shown to translocate from the MAM to other areas of cells [8, 9] where they can interact with a plethora of membrane targets including voltage-gated ion channels, glutamate and GABA ionotropic receptors, the dopamine (DA) D1 receptor (D1R), muscarinic and nicotinic acetylcholine receptors, neurotrophic tyrosine kinase receptor type 2 (TrkB), and intracellular targets such as kinases (e.g. Src kinase) and inositol triphosphate (IP3) receptors [9, 10]. For brevity, this review focuses on the interaction between Sig-1Rs and ion channels and receptors known to be relevant in neuronal excitability or synaptic strength, and analyzes how these interactions reveal the role of the Sig-1R in neuronal functions and dysfunctions.
Mechanistic considerations
Information transmission within the brain involves complex and subtle variations in neuronal activity. In particular, electrical signals in the brain are constantly modulated and are heavily influenced by excitatory (glutamate) and inhibitory (GABA) inputs. These, in turn, are translated into excitatory and inhibitory post-synaptic potentials (EPSPs and IPSPs, respectively), which eventually give rise to action potentials (APs). An AP travels from the somato-dendritic compartment along an axon to its presynaptic terminal where it triggers the release of neurotransmitters, which are molecules that transmit information chemically by binding to their specific postsynaptic receptors on adjacent neurons. Over the last two decades, Sig-Rs (Sig-1R when possible) have been shown to affect each stage of this process, in both the central nervous system (CNS) and peripheral nervous system (PNS) [3, 11]. A persistent change in any of these events will affect information coding and impact underlying cognitive or sensory processes, which may result in adaptive or maladaptive neuronal functions [12, 13]. Voltage-gated ion channels (VGICs) are critical for shaping APs[14]. The voltage-gated sodium (Na+), calcium (Ca2+), and potassium (K+) channels are subdivided in many subtypes, with each family being composed of subfamilies (for a comprehensive review of these channels, see [14, 15]).
The following sections will focus on the principal ion channels that shape global neuronal excitability, i.e. VGICs (e.g. Na+, Ca2+ and K+) for intrinsic excitability and ligand-gated ion channels (LGICS) (i.e. glutamate and GABAA receptors) for synaptic excitability.
VGICs
Voltage-gated Ca2+ channels
Calcium is probably the ion that controls most of neuronal functions, both directly and indirectly. For example, calcium channels control the flux of calcium from extracellular to intracellular compartments, and this may regulate neurotransmitter release at the synaptic level. Calcium can also act as a second messenger to trigger specific intracellular signaling pathways. Overall, while Na+ and K+ channels are involved in processes requiring fast transduction signal, calcium plays a role in both fast synaptic transmission and slow changes in neuronal function through its action on intracellular signaling pathways [16, 17].
Through a variety of ways, Sig-Rs strongly modulate intracellular calcium concentration in both neuronal and non-neuronal cells (for reviews see [3, 9, 11]). However, it is not clear if this calcium regulation is mediated by Sig-1R, Sig-2R, or both. On the other hand, most of these effects seem to be mediated through indirect pathways.
To date, few studies have demonstrated that the direct action of Sig-1Rs on Ca2+ channels can occur in the nervous system (Table 1) (Figure 1a). For example, in cultured retinal ganglion cells, the Sig-1R agonist (+)-SKF10047 directly inhibited Ca2+ currents —an effect prevented by the Sig-1R antagonist BD 1047. Direct association between the Sig-1R and L-type Ca2+ channel was supported by co-immunoprecipitation [18].
Table 1.
Summary of direct effects of Sig-1R activation on voltage-gated ion channels (VGICs).
| Effects on VGICs | Experimental system | Refs |
|---|---|---|
| Ca2+ channels | ||
| ↓ L-type | Retinal ganglion cells (cell culture) | [18] |
| ↑ L-type | CA1 field of hippocampus (brain slices) | [19] |
| Na+ channels | ||
| ↓ INaa (Nav1.5) | Cardiac myocytes and cell lines (cell culture) | [24, 25] |
| ↓ INaa | Intracardiac ganglion neurons (cell culture) | [53] |
| K+ channels | ||
| ↓ IAa (Kv1.4) | Neurohypophysial terminals (pituitary gland slices) | [28] |
| ↓ IK(DR)a, IBKa | Parasympathic intracardiac neurons (cell culture) | [30] |
| ↓ IAa (Kv1.4) | Xenopus oocytes | [31] |
| ↓ Kv1.3 | Xenopus oocytes | [33] |
| ↓ ISKa | CA1 field of hippocampus (brain slices) | [46] |
| ↑ IhERGa | Xenopus oocytes | [32] |
Abbreviations: INa, voltage-gated Na+ current; IA, A-type K+ current; IK(DR), delayed outwardly rectifying K+ current; IBK, large-conductance Ca2+-activated K+ current; ISK, small conductance Ca2+-activated K+ current; IhERG, human ether-à-gogo K+ current.
Figure 1.
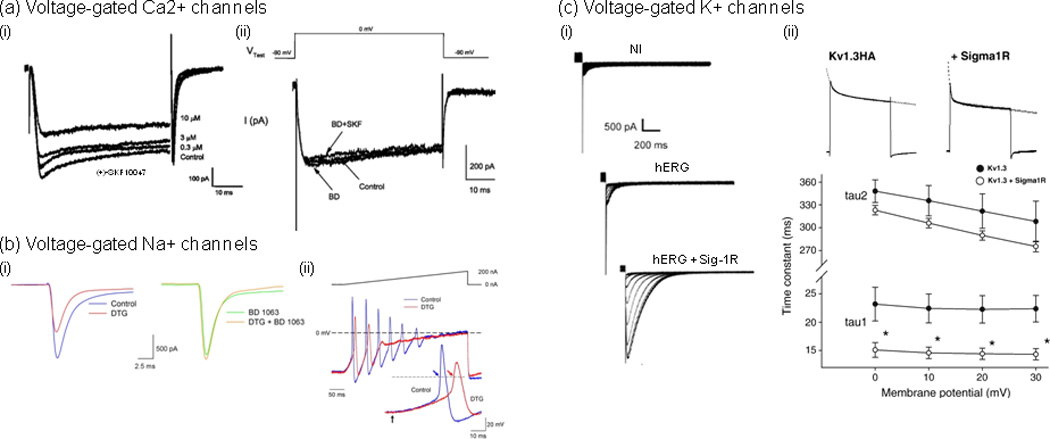
Examples of direct modulation of Ca2+, Na+ and K+ voltage-gated channels by Sig1Rs. (a) (i) The Sig1R agonist, (+)-SKF10047, inhibited voltage-gated Ca2+ currents in a concentration-dependent manner [18]. Whole-cell Ca2+ currents were recorded using patch clamp on cultured retinal ganglion cells (2 DIV) prepared from adult rats. Currents were evoked with a depolarization voltage step from −90 to 0 mV for 55 ms. (ii) (+)-SKF10047 inhibitory effect was prevented by the Sig-1R antagonist BD 1047. Treatment with BD 1047 alone had little effect on whole-cell Ca2+ currents. (b) (i), In rat intracardiac ganglion neurons, the Sig-1R antagonist BD 1063 blocked the effects of Sig-Rs pan-selective agonist 1,3-di(2-tolyl)guanidine (DTG) on peak Na+ currents [26]. Na+ currents were evoked by voltage steps from −90 to −10 mV in a single neuron in the absence (Control; left traces) and presence of 30 µM DTG (DTG; left traces), in the presence of 100 nM BD 1063 alone (BD 1063; right traces) and following co-application of both drugs (DTG+BD 1063; right traces). (ii) DTG-induced inhibition of Na+ currents resulted in delayed action potential latency and decreased firing rate. Action potentials generated by 400 ms depolarizing current ramps (0 to 200 nA) from a neuron in the absence (blue line) and presence of DTG (30 µM) (red line). Inset shows first action potentials generated by the ramps on an expanded time scale. Arrows in inset indicate start point of the injected current ramp and points at which latency times were measured. Dashed lines represent 0 mV, and the solid line above voltage traces represents the current ramp protocol used. (c) Sig-1Rs bidirectionally modulate K+ currents, which occurs through direct protein-protein interaction and results in either the modulation of K+ channels function [31, 33] or the regulation of subunits trafficking [32]. (i) Sig1R expression stimulates human ether-à-gogo K+ currents (hERG) in Xenopus oocytes [32]. Traces represent families of tail currents recorded in noninjected (NI), hERG cRNA-injected (hERG; 25 pg/oocyte), and hERG + Sig1R cRNA-injected (hERG + Sig1R; 25 pg and 5 ng/oocyte, respectively) oocytes (representative experiment). Tail currents were recorded following prepulses from −70 to 40 mV. (ii) Sig-1R co-expression accelerates Kv1.3 inactivation kinetics [33]. (i) Current decay of Kv1.3 alone and co-expressed with the Sig-1R were fitted with a double exponential function (dashed lines). (ii) A double exponential fit to the current decay showed a voltage-independent fast (A1*exp(−t/tau1)) and a voltage-dependent slow components (A2*exp(−t/tau2)) of inactivation time constants. Both time constants were smaller in the co-injected oocytes (open circles) than in those injected with Kv1.3 cRNA only (closed circles). The fast component demonstrated significant difference at all voltages (*p<0.05). Adapted, with permission, from [18] (a), [26] (b), [32] (c) (i) and [33] (c) (ii).
In another study, activation of Sig-1Rs by the endogenous neuroactive steroid pregnenolone sulfate (PREGS) facilitated an NMDAR-independent form of long-term potentiation (LTP) in acute slices from the CA1 region of the hippocampus [19]. However, the Sig-1R antagonist BD 1047 prevented PREGS facilitation of L-type Ca2+ channel-dependent LTP and mimicked the effect of nimodipine, a specific L-type Ca2+ channel blocker [19]. Although it is unclear whether in this model Sig-1Rs act directly via L-type Ca2+ channels to induce this form of LTP, data suggest that current flow is facilitated, which is in contrast with Sig-1R-induced inhibition of L-type Ca2+ channel observed in retinal ganglion cells [18] (Figure 2a).
Figure 2.
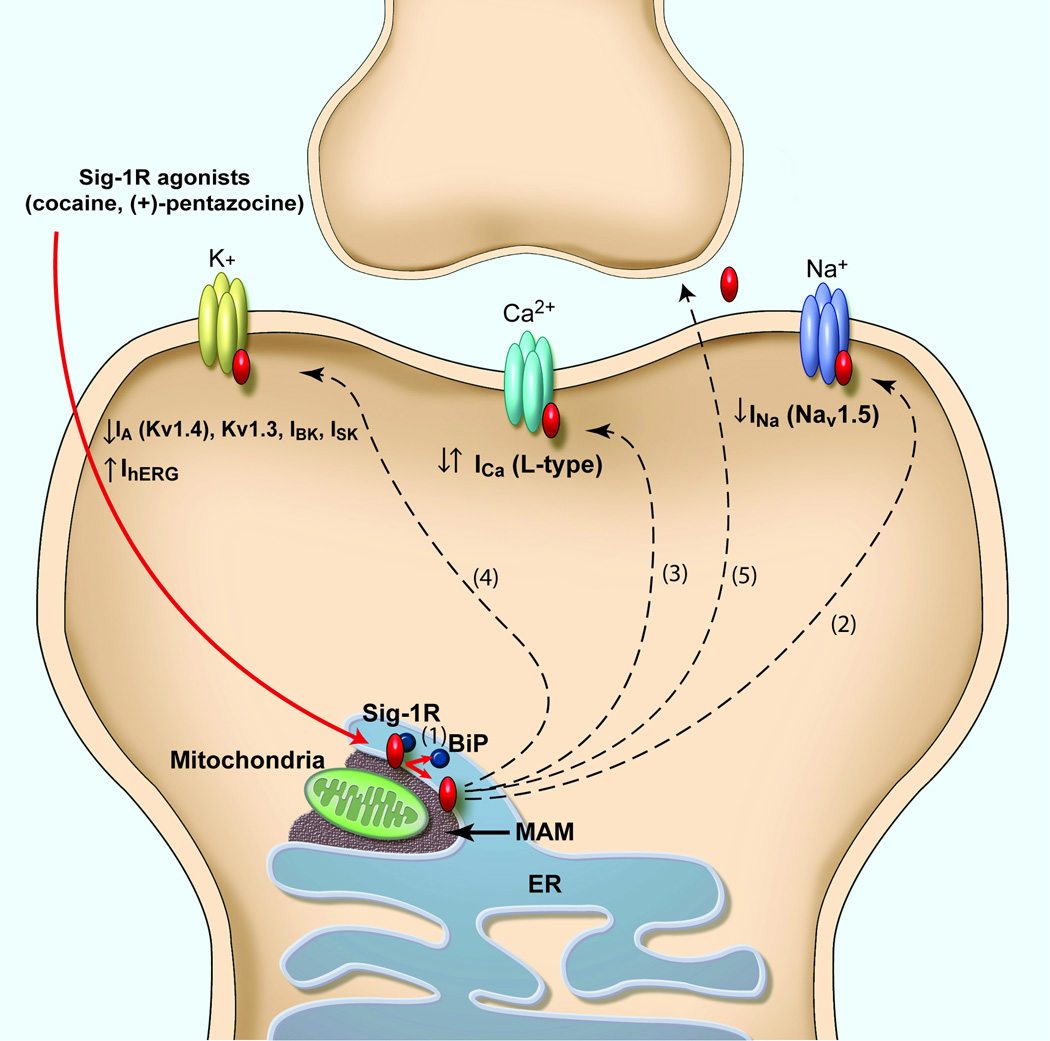
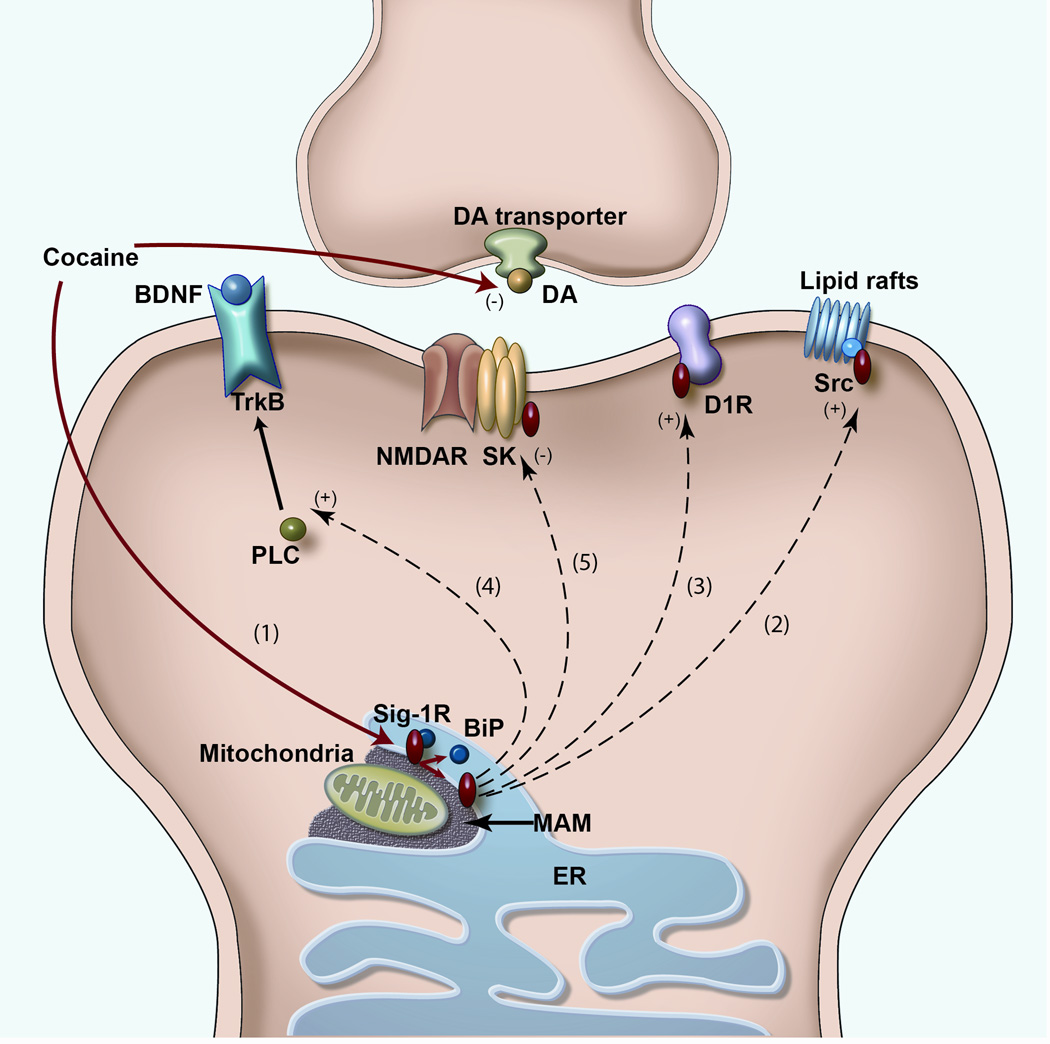
A schematic illustrating various targets of the sigma-1 receptor (Sig-1R). Upon ligand stimulation (e.g. cocaine), Sig-1Rs dissociate from BiP (Binding immunoglobulin Protein) (1), another endoplasmic reticulum (ER) chaperone protein, then translocate from the MAM (mitochondrion-associated ER membrane) to the ER and plasmalemma. Acting as inter-organelle signaling modulatord, Sig-1Rd regulate a variety of functional proteins, both directly and indirectly. (a) Schematic diagram illustrating the direct regulation of Na+, Ca2+ and K+ voltage-gated ion channels by Sig-1Rs. To date, the effect of Sig-1R function on Na+ current seems inhibitory (2), but bidirectional on Ca2+ (3) and K+ currents (4) (see Table 1). Sig-1Rs have also been found in the extracellular space in NG108-15 cultured cells (5) [8]. However, its role at this location and whether this phenomenon is relevant in vivo is still unknown. (b) Effects of Sig-1R translocation and subsequent activation of Src kinase, dopamine D1 receptors (D1R), neurotrophic tyrosine kinase receptor type 2 (TrkB), and NMDA receptors (NMDAR). The targeting of multiple functional proteins on the plasma membrane by Sig-1Rs may explain the diverse actions or disorders that Sig-1Rs have been implicated in. (1) Cocaine as a Sig-1R agonist causes Sig-1Rs to translocate from the ER to the plasma membrane and (2) to interact with and activate Src kinase. Src kinase activation by Sig-1R has been shown to prevent the ischemic LTP impairment through regulating the tyrosine phosphorylation of the NMDAR GluN2B subunit [3]. (3) The Sig-1R-D1R interaction relates to cocaine addiction, as Sig-1Rs were found to enhance the D1R downstream signaling [70]. (4) Sig-1Rs have also been shown to enhance BDNF-induced phospholipase C (PLC)-gamma activation and glutamate release [71]. (5) The Sig-1R-NMDAR interaction relates to learning and memory. However, the effect on NMDAR function seems to occur through direct modulation of small conductance Ca2+-activated K+ channels (SK) [46].
For brevity, this section focuses on Ca2+ currents mediated by voltage-gated Ca2+ channels; however, one might mention that the Sig-1R, though physical interaction, can also regulate Ca2+ influx through non-voltage-gated Ca2+-permeable channels. This includes regulation of IP3 receptors (reviewed in Refs [3, 9, 11]), resulting in proper ER to mitochondria Ca2+ signaling and thought to regulate mitochondrial bioenergetics [20] (reviewed in Refs. [9, 11]), and plasma membrane acid-sensing ion channels 1a (ASIC1a) [21, 22], which results in ASIC1a-mediated Ca2+ currents inhibition and consequently intracellular Ca2+ accumulation [22].
Taken together, while it is clear that the Sig-1R modulates Ca2+ channels, the modulation can be direct or indirect, and facilitatory or inhibitory, depending on the physiological context. This highlights the complexity and diversity in Sig-1R actions and even suggests that the Sig-1R can exert opposite effects depending on the brain regions or neuronal types.
Voltage-gated Na+ channels
It is only over the last few years that the Sig-1R has been found to inhibit Na+ channels both directly and indirectly, an effect that has been observed in different type of cells and preparations. One of the first studies was performed on prefrontal cortex slice preparation [23] and showed that the Sig-1R activation by the neurosteroid dehydroepiandrosterone sulphate (DHEAS) inhibits persistent Na+ current—an effect that seems to occur via the activation of Gi protein and protein kinase C (PKC). However, over the last two years, several studies have revealed that the Sig-1R can also modulate Na+ current through direct interaction [24–26] (Table 1). Although unequivocal evidence is still needed, Sig-1R activation in mouse cardiac myocytes and fibroblast-like cell lines [eg. COS-7 and human embryonic kidney cells (HEK 293)] by SKF-10047, (+)-pentazocine [25] or N,N-dimethyltryptamine (DMT) [24] (a newly discovered Sig-1R endogenous ligand), inhibited Nav1.5-mediated currents without alterations in channel kinetics or shifts in voltage dependence [25]. This effect was attenuated in cells from Sig-1R knock-out mice [25]. Further analysis showed that Sig-1Rs inhibited Nav1.5-mediated currents without requiring ATP or GTP [25], which suggests that its inhibitory action is not dependent on G-proteins and protein kinases, and is likely through direct association between Sig-1Rs and Nav1.5 channels. Further supporting DMT as an endogenous ligand that can efficiently and quickly activate Sig-1Rs, a recent study performed in mouse motoneurons (MN) revealed subsurface cisternae colocalization of the Sig-1R and indole-N-methyl transferase (INMT), an enzyme that synthesizes DMT [27]. So far, using cultured intracardiac ganglion neurons, only one study has revealed a similar effect of Sig-1Rs activation on Na+ currents in the nervous system [26] (Figure 1b). The inhibitory effect of the Sig-R agonists 1,3-di(2-tolyl)guanidine (DTG) and (+)–pentazocine on Na+ currents —an effect blocked by the specific Sig-1R antagonist BD 1063—resulted in delayed AP latency, decreased firing rate, and a shift in steady-state inactivation of Na+ channels to more negative potentials [26]. Thus, not only can Sig-1R activation decrease intrinsic excitability by decreasing Na+ current, but also by decreasing Na+ channel availability when needed. Taken together - and in contrast to bidirectional action of Sig-1R on Ca2+ currents - the effect of Sig-1R on Na+ currents to date has been shown to be inhibitory (Figure 2a).
Voltage-gated K+ channels
Sig-1R inhibition of various K+ channels in both non-neuronal and neuronal cells can involve direct or indirect interaction with channels (for reviews see Refs [3, 9]). Similar to the effect of Sig-1R on Na+ channels [26], Sig-1R activation shifts the steady-state inactivation curve toward more negative potentials, which could decrease K+ channels availability upon excitation and delay neuronal repolarization. To exacerbate this effect, the Sig-1R can also delay K+ channels recovery from inactivation. But in contrast to its effect on Na+ channels, Sig-1R activation changes the channel kinetics by accelerating current decay, which in total would lead to increased cell excitability.
To date, a few studies have suggested a possible physical interaction between the Sig-1R and K+ channels ([28–30], reviewed in Refs [3]) (Table 1), yet the evidence is indirect. However, unequivocal evidence for a direct physical interaction between Sig-1Rs and K+ channels is accumulating [31–33] (Figure 1a). A seminal study [31], combining electrophysiological recordings and co-immunoprecipitation data from posterior pituitary and Xenopus oocytes, showed that the Sig-1R inhibits K+ currents formed by Kv1.4 through a direct protein-protein interaction. Interestingly, depending on the absence or presence of ligand, the Sig-1R differentially inhibited the Kv1.4-mediated current, which suggests that the Sig-1R may act as a ligand-regulated auxiliary K+ channel subunit. Since Kv1.4 is an important contributor to transient A-type K+ currents (IA), regulation of Kv1.4 currents may have profound effects on dendritic excitability and therefore on intrinsic and synaptic plasticity [34]. Two recent studies have provided additional evidence for K+ currents regulation involving a direct interaction mechanism [32, 33]. The first showed that in HEK cells, the Sig-1R co-immunoprecipitates with human ether-à-gogo K+ channel (hERG) [32] (Figure 1c), a voltage-dependent K+ channel that regulates cardiac repolarization [35, 36]. Interestingly and in contrast to previous studies, when expressed in Xenopus oocytes, the interaction between Sig-1Rs and hERG channels resulted in increased hERG-mediated K+ currents—a mechanism that seemed to occur through a regulation of channels subunits maturation and stability [32]. The second study, reminiscent of previous findings [31], also showed a ligand-independent modulation of K+ channels function [33] (Figure 1c). Specifically, co-expression in Xenopus oocytes of Sig-1Rs and Kv1.3 channels, a slowly-inactivating outward voltage-gated K+ channel that is predominantly expressed in T lymphocytes [37] and in cerebellum [38], resulted in Kv1.3-mediated currents inhibition by accelerating channel inactivation [33].
In summary, the Sig-1R bidirectionally modulates K+ currents, an effect that can occur through direct protein-protein interaction and resulting in either the modulation of K+ channels function [31, 33] or the regulation of subunits trafficking [32] (Figure 2a). These findings raise the possibility that the Sig-1R acts as an auxiliary subunit for voltage-gated K+ channels.
Ligand-gated channels: glutamate and GABA ionotropic receptors
The capability of Sig-Rs ligands to modulate excitatory transmission in the brain is now well established. Although specific antagonists were not yet available at that time, by combining Sig-Rs agonists and antagonists with electrophysiological recordings, the first studies show that the Sig-1R has the potential to modulate NMDA receptor (NMDAR) transmission bidirectionally. This phenomenon has been shown in both the CNS and PNS, including the CA3 field of rat dorsal hippocampus [39–41], cultured neuronal cells from fetal rat telencephalon [42], pyramidal cell of medial prefrontal cortex [43], and spinal cord [44, 45]. However, modulation of AMPA receptor (AMPAR) transmission by the Sig-1R seems very modest [43]. More specifically, Sig-1R agonists (e.g. (+)-pentazocine, DTG and SR 31742A) induce a biphasic modulatory action on NMDAR transmission wherein a low dose is excitatory and a high dose inhibitory, resulting in bidirectional modulation on evoked excitatory transmission [43].
The effect of sigma receptors activation on AMPAR-mediated currents has also been studied and has generated mixed results. In contrast to the lack of effect of the Sig-1R activation on AMPAR current amplitude [43], a study performed on cultured pyramidal neurons from the CA1 field of hippocampus showed that PREGS increased the frequency of AMPAR-mediated miniature EPSCs—an effect mimicked by Sig-1R agonists ((+)-pentazocine and DHEAS) and blocked by Sig-1R antagonists (haloperidol and BD 1063). However, the effect on AMPAR appears to be relatively modest when compared to NMDARs [43]. Thus, we speculate that changes in excitatory transmission due to the Sig-1R likely originated primarily from its modulation of NMDAR-mediated currents. While the cellular basis of this has been studied, the mechanistic picture is far from clear. To date, some data suggest that it might be either through a NMDAR phosphorylation mechanism [44, 45] and/or indirectly through blockade of the small conductance Ca2+-activated K+ (SK) channel [46]. Indeed, (+)-pentazocine and apamin, a specific Sig-1R agonist and SK channel blocker respectively, similarly blocked the SK channel, which in turn potentiated NMDAR currents and LTP [46]. Furthermore, the Sig-1R can also regulate AMPAR and NMDAR mRNA and protein expression levels [47]. For example, repeated exposure to E-5842, a putative atypical antipsychotic and preferential Sig-1R ligand, differentially regulated levels of AMPAR and NMDAR subunits (ie. GluA2 and GluN2A) in a regionally-specific manner [47].
Little is known about the relationship between the Sig-1R and GABAAR-mediated transmission, but the few studies published on this topic have all shown a decrease in GABAAR-mediated currents via a Sig-1R-dependent presynaptic mechanism [48, 49].
In the sections above, we have provided a brief overview on the many effects of the Sig-1R on several aspects of neuronal transmission including generation and conduction of APs, release of neurotransmitters from presynaptic terminals, and postsynaptic receptors function. The challenge is to understand how neuronal activity will be affected by a combination of various effects of sigma receptors on such a diverse variety of targets. This complexity has been the main issue limiting our understanding on how the Sig-1R function affects CNS activity. However, in the next section, we would like to propose a few possibilities that may explain this variety of outcomes.
How does Sig-1R activity affect overall neuronal excitability?
A substantial amount of information on the effects of Sig-1R activation has been reported. However, little is known about how these effects will modulate intrinsic and synaptic excitability, and thus how they will affect overall neuronal excitability. Because of the various effects of the Sig-1R on individual channels, this task might be difficult to resolve. For example, inhibition of Na+ currents by the Sig-1R should decrease AP firing, whereas inhibition of K+ currents should, in contrast, increase AP firing. To add a supplementary level of complexity, depending on the action site, the Sig-1R can facilitate or inhibit voltage-gated Ca2+ channels. Thus, how would a combination of such opposite effects on voltage-gated ion channels affect intrinsic excitability? It is difficult to infer the changes in firing capacity from changes in individual (or even several) currents [50], and thus, a direct measurement of firing properties is necessary to determine Sig-1R-induced changes in intrinsic excitability.To date, the few studies that measured the effect of the Sig-1R activation on basal firing have provided mixed results, showing both excitation and inhibition [23, 51–53].
Why does Sig-1R activation lead to various consequences on neuronal excitability? Although difficult to assess, experimental conditions are likely to affect functional outcomes of Sig-1Rs activation. For example, in vitro and in vivo preparations exhibit different levels of neuronal network integrity, which is absent in cultured neurons, semi-preserved in brain slices, and intact in in vivo studies. More importantly, one might consider intra-, membrane and extra-cellular environments as biological factors that may dictate how a neuron will respond to the Sig-1R stimulation. Specifically:
The Sig-1R is differentially expressed throughout the brain [54–57]. We speculate that a neuronal type that highly expresses the Sig-1R will respond differently to Sig-1R activation when compared to a neuron that exhibits a low level of expression. Along the same line, a dose-curve analysis revealed that modulation of NMDAR currents by some sigma receptor ligands follows a bell-shaped curve [43, 58]. Does this indicate that the levels of activation and/or expression of Sig-1Rs influence target functions? Or perhaps, it reflects the existence of several subtypes of Sig-Rs and/or simply off-targets effects of ligands when used at high doses.
The availability of the targets. Subtypes of Na+, Ca2+, and K+ channels are heterogeneously and not proportionately distributed throughout the nervous system. Combined with variability in their subcellular distribution [59, 60], these factors are likely to influence the resulting effect of the Sig-1R activation on neuronal excitability.
In the same line as the previous point, the intracellular milieu and molecular substrates available at the membrane may also play important roles. Indeed, it appears that protein kinases [ e.g. extracellular signal-regulated kinase (ERK)] and Sig-1Rs can mutually regulate one another [61, 62]. Protein kinases are strong modulators of VGICs [63]. Therefore, variations in the levels and types of protein kinases, an effect that is directly related to levels of neuronal activity, may lead to differential effects of Sig-1R activation on neuronal excitability. This also likely provides a powerful way for Sig-1R to fine tune neuronal excitability. This is a critical point because one of the characteristics that define neuronal types is the level of tonic activity, which suggests that modulatory effects mediated through the Sig-1R are likely to be influenced by neuronal and/or neural system basal activity as well.
Finally, a factor that is often underestimated is age. Whether Sig-1Rs levels vary with age have provided mixed results [64, 65]; however, the levels of neuroactive steroids - important endogenous ligands for the Sig-1R - decrease with age [10]. As mentioned earlier, the action of neurosteroids on Sig-1Rs can affect both VGICs and LGICs, which indicate that endogenous age-related variations in neurosteroids levels could also contribute to physiological regulation of neuronal activity. This raises concerns when investigating the effects of Sig-1Rs stimulation on neuronal excitability using preparations from animal of different ages. In other words, exogenous experimental conditions may interact with the endogenous level of neuroactive steroids and lead to different functional outcomes. For example, administration of the same concentration of neuroactive steroids to preparations from animals of different ages may lead to different results when neuronal excitability is investigated, a situation that may also in case of in vivo administration lead to different behavioral effects.
Taken together, the net effect of Sig-1Rs activation on neuronal activity may not only depend on Sig-1Rs levels of expression but also on several other factors, including availability, anatomical and subcellular localization of voltage-gated ion channels, and the intracellular milieu, a factor that is influenced by the level of basal neuronal activity and age.
Cellular neurobiology of Sig-1Rs
At the MAM, Sig-1Rs reside in the ceramide-enriched microdomains where Sig-1Rs apparently bind ceramide [56]. Sig-1Rs at the MAM have also been shown to bind to BiP (Binding immunoglobulin Protein), another ER chaperone protein that normally prevents the Sig-1R from translocation [8, 20]. The exact relation among the binding of the Sig-1R, BiP, and ceramide is unknown at present. As mentioned earlier, Sig-1Rs can regulate the dendrite arborization and dendritic spine formation in hippocampal neurons [7]. Whether Sig-1Rs have other functions at the MAM remains to be clarified. Accumulating evidence indicates however that, upon stimulation by the Sig-1R agonist like cocaine, Sig-1Rs can dissociate from BiP and translocate to the nucleus, the plasma membrane, and even the extracellular space [8, 9] (Figure 2).
Sig-1Rs in CNS diseases
Sig-1Rs have been implicated in a multitude of CNS disorders, including amnesia, depression, stroke, Alzheimer’s disease, age-related cognitive impairments, neuropathic pain, and cocaine and alcohol addiction (Table 2). We will review here only neurological studies that clearly implicate the translocation of Sig-1Rs in those disorders. For example, a recent study has indicated that the anti-ischemic action of Sig-1Rs in stroke involves the increased translocation of Sig-1Rs to the lipid raft of neurons [66]. As lipid rafts are cholesterol-enriched specialized regions of the plasma membrane that serve as platforms for functional proteins [67], this anti-ischemic finding suggests that Sig-1Rs recruit synaptic proteins required for brain repair to the lipid rafts to stimulate brain plasticity (Figure 2b). As another example, the translocation of Sig-1Rs from the ER into the nucleus has been found to correlate with the development of frontotemporal lobar degeneration-motor neuron disease [2].
Table 2.
Neurological and psychiatric disorders associated with changes in the expression and/or function of Sig-1Rs: clinical and preclinical findings1
| Sig-1R expression | |||
|---|---|---|---|
| Disorder | Human studies | Rodent studies | Ligand with therapeutic potential |
| Drug addiction | |||
| Alcohol | ↓ TT-241-240/Pro2 polymorphism [72] | ↓ (NAc) [73] | Antagonist [73,74] |
| ↓ ratio of A-485 allele or TT-241-240/Pro2 polymorphism [72] | |||
| Cocaine | ↑ (whole brain, striatum, cortex) [74, 75] | Antagonist [11, 76] | |
| Methamphetamine | No significant difference in polymorphism (GC-241-240TT, A61C) [77] | ↑ (midbrain) [66] [78–80] | Antagonist [78–80] |
| Psychiatric disorders | |||
| Schizophrenia | binding site (↓ occipital, ↓ frontal, ↓temporal, ↑ cingulate cortices, ↓ cerebellum)b [81, 82] | Positive symptoms: antagonist (panamesine, eliprodil, rimcazole, BMY14802, DuP734)g [83–87] | |
| Negative symptoms/cognitive deficits: agonist (fluvoxamine) [83–87] | |||
| Depression | Agonist [igmesine, opipramol, fluvoxamine, DHEA, OPC-14523, YKP10A, cutamesine (SA4503)]d [3] [83, 88–90] | ||
| Anxiety disorders | Agonist/agonist-like ligands (opipramol [91], afobazole [92]) | ||
| Neurodegenerative disorders | |||
| Stroke (including neurosurgery) | Agonist (cutamesine [66] (SA4503), DMd [93] | ||
| Alzheimer’s disease | ↓ binding sites (frontal, temporal, occipital lobes, cerebellum, thalamus, hippocampus)b [3] [94, 95] | Agonist | |
| ↓ (polymorphism: TT-241-240P2)c [3] [96, 97] | |||
| Juvenile ALS | (mutation: c.304G>C) e [98] | Agonist? | |
| Pseudobulbar affect (e.g. ALS, MS) | Agonist (carbetapentane, DMf) [99] | ||
| Frontotemporal lobar degeneration-motor neuron disease (FTLD-MND) | ↑ (mutation: 3’UTR672*51G>T) [2] | Agonist? [2] | |
| Others | |||
| HIV-associated neurocognitive disorder (HAND) | Antagonist [68, 100] | ||
| Pain | Agonist (DMd) [101] or antagonist [102] | ||
Abbreviations: ALS, amyotrophic lateral sclerosis; DHEA, dehydroepiandrosterone; DM, dextromethorphan; MS, multiple sclerosis; NAc, nucleus accumbens; PRE-084, 2-(4-morpholino) ethyl 1-phenylcyclohexane-1-carboxylate.
Post-mortem study.
Conflicting results.
Fluvoxamine’s efficacy against psychotic depression needs further verification.
This mutation did not affect the level of Sig-1Rs but relocated Sig-1Rs to detergent-resistant membranes of lower density. Inasmuch as Sig-1R agonists are well known to be neuroprotective, it is speculated that the agonist may serve as a therapeutic agent.
Whether this clinical effect of DM involves Sig-1Rs is not determined.
Some studies suggested that Sig-1R antagonists may not possess antipsychotic action.
It is known that cocaine causes the translocation of Sig-1Rs from the ER to the other parts of the cell [8]. In primary human brain microvascular endothelial cells, it was shown that cocaine “hijacks” Sig-1Rs from the ER to the plasma membrane lipid rafts and causes the transmigration of microglia across the blood brain barrier (BBB). This results in the enhancement of neuroinflammation associated with the HIV infection and the exacerbation of HIV-associated neurological dementia [68, 69].
Cocaine binding to Sig-1Rs may have a bearing on the development of cocaine addiction. For example, cocaine causes a dissociation of the Sig-1R from its binding partner at the ER, BiP, and thus allows Sig-1Rs to translocate and interact with other functional proteins that may play a role in cocaine addiction [9, 20]. For example, in rat brains and in cell lines, cocaine was reported to trigger translocation of Sig-1Rs from the ER to the plasma membrane to interact with (D1Rs), which are well known to be important for cocaine addiction [70]. Thus, inasmuch as Sig-1Rs are intracellular ER proteins, cocaine may exert its effects both intracellularly and extracellularly (Figure 2). The extracellular action of cocaine, of course, leads to the increase of DA at the synapse by the blockade of dopamine transporters.
Conclusion
In summary, the Sig-1R, through various means and diverse targets, is capable of affecting each stage of neuronal transmission. This may explain why the Sig-1R is associated with many brain functions and neurological disorders. A clear, region-specific understanding of how Sig-1Rs can regulate neuronal activity through the modulation of VGICs and glutamate/GABA transmission will not only provide information on how Sig-1Rs participate in shaping neuronal activity but also how its disruption can lead to symptoms observed in brain disorders. In this regard, the clarification of many outstanding questions on the fundamental property of Sig-1Rs will certainly help in advancing our understanding of how Sig-1R dysfunction plays a role in these disorders (Box 2).
Box 2. Outstanding questions.
How can a two-transmembrane protein like Sig-1Rs move quickly upon stimulation by agonists? For example, Sig-1Rs are seen in the extracellular space and the nucleus just 10–15 minutes after cocaine stimulation [8]. It takes a tremendous amount of energy to move proteins along the biological membrane, let alone moving a two-transmembrane protein. How could the energy barrier be so easily overcome?
Chaperone proteins are known to be able to accommodate many client proteins and thus have a great degree of tolerance in binding clients of different structures [108]. What is the motif or domain of the Sig-1R that allows it to play such a role?
Almost a mystery is why the Sig-1R has many interacting proteins, in that Sig-1Rs are known to bind many different classes of compounds including neurosteroids [20], antidepressants [83], benzomorphans [3], and cocaine [20]. Why can the binding pocket of Sig-1Rs accept so many different classes of ligands? What is the biological implication of this?
One peculiar property of Sig-1Rs is that they can translocate or efflux into the outer space of cells when, for example, stimulated by cocaine [8]. So far, this has only been reported in cell culture systems, and is still unclear whether this phenomenon is relevant in vivo. Although some other chaperone proteins exist outside of the cell as well [108], most of them do not have transmembrane regions. Thus, how do Sig-1Rs cross the plasma membrane? Is it through exocytosis? Furthermore, how could a two-transmembrane protein like Sig-1Rs exist outside of the cell? Is it in the form of an exosome? What is the Sig-1R doing in the extracellular space? Is it working as a chaperone protecting the conformation of client proteins' motifs in the extracellular space? Could Sig-1R interact not just with the intracellular portion of its client proteins but also with the extracellular domain of the same protein? Could Sig-1Rs serve as retrograde chaperones, i.e., they are released from postsynaptic site to chaperone the presynaptic proteins across the synaptic cleft?
Inasmuch as cocaine can also cause the translocation of Sig-1Rs from the ER into the nucleus [8], it is tempting to speculate that Sig-1Rs may mediate certain aspects of cocaine-induced gene regulation. Of course, experiments are needed in the future to confirm this speculation.
Because the Sig-1R is a chaperone protein, an important question that needs to be addressed is: What is/are the atomic structural changes that occur in the receptor when selective agonist or antagonists bind to the Sig-1R, and what are the conformers that allow interaction(s) with various protein partners?
Figure I.
A schematic diagram illustrating the topology of the Sig-1R and associated ligand binding sites. The orange dots are amino acids reported to be critical for the binding of Sig-1R ligands [103]. The dashed blue regions are important for cholesterol binding [107].
Acknowledgements
This work is supported by the Intramural Research Program of NIDA, NIH/DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al Faleh HF, et al. In-hospital adverse clinical outcomes of ST elevation myocardial infarction patients with renal dysfunction. Insights from the Saudi Project for Assessment of Coronary Events. Saudi Med J. 2011;32:806–812. [PubMed] [Google Scholar]
- 2.Luty AA, et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68:639–649. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- 3.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shioda N, et al. Expression of a Truncated Form of the Endoplasmic Reticulum Chaperone Protein, sigma1 Receptor, Promotes Mitochondrial Energy Depletion and Apoptosis. J Biol Chem. 2012;287:23318–23331. doi: 10.1074/jbc.M112.349142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto M, Hayashi T. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. Int Rev Cell Mol Biol. 2011;292:73–117. doi: 10.1016/B978-0-12-386033-0.00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, et al. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai SY, et al. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc Natl Acad Sci U S A. 2009;106:22468–22473. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 9.Su TP, et al. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurice T, et al. The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 11.Fishback JA, et al. Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol Ther. 2010;127:271–282. doi: 10.1016/j.pharmthera.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 14.Hille B. Ionic Channels of Excitable Membranes. 3rd ed. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 15.Jegla TJ, et al. Evolution of the human ion channel set. Comb Chem High Throughput Screen. 2009;12:2–23. doi: 10.2174/138620709787047957. [DOI] [PubMed] [Google Scholar]
- 16.Catterall WA. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci Lett. 2010;486:107–116. doi: 10.1016/j.neulet.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Tchedre KT, et al. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- 19.Sabeti J, et al. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. doi: 10.1002/hipo.20273. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Carnally SM, et al. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys J. 2010;98:1182–1191. doi: 10.1016/j.bpj.2009.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera Y, et al. sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- 23.Cheng ZX, et al. Neurosteroid dehydroepiandrosterone sulphate inhibits persistent sodium currents in rat medial prefrontal cortex via activation of sigma-1 receptors. Exp Neurol. 2008;210:128–136. doi: 10.1016/j.expneurol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Fontanilla D, et al. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannessen M, et al. Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol. 2009;296:C1049–C1057. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, et al. Sigma receptor activation inhibits voltage-gated sodium channels in rat intracardiac ganglion neurons. Int J Physiol Pathophysiol Pharmacol. 2010;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Mavlyutov TA, et al. Development of the Sigma-1 Receptor in C-Terminals of Motoneurons and Colocalization with the N,N'-Dimethyltryptamine Forming Enzyme, Indole-N-Methyl Transferase. Neuroscience. 2012;206:60–68. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupardus PJ, et al. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526(Pt 3):527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavlyutov TA, Ruoho AE. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J Mol Signal. 2007;2:8. doi: 10.1186/1750-2187-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Cuevas J. sigma Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1387–1396. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]
- 31.Aydar E, et al. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 32.Crottes D, et al. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J Biol Chem. 2011;286:27947–27958. doi: 10.1074/jbc.M111.226738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita M, et al. Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res. 2012;1452:1–9. doi: 10.1016/j.brainres.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah MM, et al. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanguinetti MC, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 36.Trudeau MC, et al. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 37.Panyi G, et al. Ion channels and lymphocyte activation. Immunol Lett. 2004;92:55–66. doi: 10.1016/j.imlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Vacher H, et al. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergeron R, et al. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- 40.Bermack JE, Debonnel G. Distinct modulatory roles of sigma receptor subtypes on glutamatergic responses in the dorsal hippocampus. Synapse. 2005;55:37–44. doi: 10.1002/syn.20085. [DOI] [PubMed] [Google Scholar]
- 41.Monnet FP, et al. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto H, et al. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J Neurosci. 1995;15:731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang X, Wang RY. Biphasic modulatory action of the selective sigma receptor ligand SR 31742A on N-methyl-D-aspartate-induced neuronal responses in the frontal cortex. Brain Res. 1998;807:208–213. doi: 10.1016/s0006-8993(98)00797-5. [DOI] [PubMed] [Google Scholar]
- 44.Kim HW, et al. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon SY, et al. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology. 2010;59:460–467. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Martina M, et al. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guitart X, et al. Regulation of ionotropic glutamate receptor subunits in different rat brain areas by a preferential sigma(1) receptor ligand and potential atypical antipsychotic. Neuropsychopharmacology. 2000;23:539–546. doi: 10.1016/S0893-133X(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 48.Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- 49.Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 51.Lucas G, et al. Further evidence for an antidepressant potential of the selective sigma1 agonist SA 4503: electrophysiological, morphological and behavioural studies. Int J Neuropsychopharmacol. 2008;11:485–495. doi: 10.1017/S1461145708008547. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Cuevas J. sigma Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J Pharmacol Exp Ther. 2005;313:1387–1396. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, et al. Sigma receptor activation inhibits voltage-gated sodium channels in rat intracardiac ganglion neurons. Int J Physiol Pathophysiol Pharmacol. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 54.Alonso G, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 55.Gundlach AL, et al. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi T, Su TP. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell Biochem. 2010;51:381–398. doi: 10.1007/978-90-481-8622-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitaichi K, et al. Expression of the purported sigma(1) (sigma(1)) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat. 2000;20:375–387. doi: 10.1016/s0891-0618(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 58.Bergeron R, et al. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:252–260. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- 59.Lujan R. Organisation of potassium channels on the neuronal surface. J Chem Neuroanat. 2010;40:1–20. doi: 10.1016/j.jchemneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Cormaci G, et al. Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: Implication for neuroplasticity. J Pharmacol Exp Ther. 2007;320:202–210. doi: 10.1124/jpet.106.108415. [DOI] [PubMed] [Google Scholar]
- 62.Moriguchi S, et al. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J Neurochem. 2011;117:879–891. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- 63.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett. 2010;486:60–67. doi: 10.1016/j.neulet.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishiwata K, et al. Age-related changes of the binding of [3h]SA4503 to sigma1 receptors in the rat brain. Ann Nucl Med. 2003;17:73–77. doi: 10.1007/BF02988264. [DOI] [PubMed] [Google Scholar]
- 65.Phan VL, et al. Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol Aging. 2003;24:865–881. doi: 10.1016/s0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- 66.Ruscher K, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–746. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- 67.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 68.Yao H, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31:5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao H, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro G, et al. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yagasaki Y, et al. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- 72.Miyatake R, et al. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol Psychiatry. 2004;55:85–90. doi: 10.1016/j.biopsych.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Sabino V, et al. The sigma-Receptor Antagonist BD-1063 Decreases Ethanol Intake and Reinforcement in Animal Models of Excessive Drinking. Neuropsychopharmacology. 2009;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, et al. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. Journal of Pharmacology and Experimental Therapeutics. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- 76.Romieu P, et al. Sigma 1 receptor-related neuroactive steroids modulate cocaine-induced reward. J Neurosci. 2003;23:3572–3576. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inada T, et al. No association found between the type 1 sigma receptor gene polymorphisms and methamphetamine abuse in the Japanese population: a collaborative study by the Japanese Genetics Initiative for Drug Abuse. Ann N Y Acad Sci. 2004;1025:27–33. doi: 10.1196/annals.1316.003. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi S, et al. MS-377, a novel selective sigma(1) receptor ligand, reverses phencyclidine-induced release of dopamine and serotonin in rat brain. Eur J Pharmacol. 2001;427:211–219. doi: 10.1016/s0014-2999(01)01254-7. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen EC, et al. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 80.Kitanaka J, et al. Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology (Berl) 2009;203:781–792. doi: 10.1007/s00213-008-1425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weissman AD, et al. Selective loss of cerebral cortical sigma, but not PCP binding sites in schizophrenia. Biol Psychiatry. 1991;29:41–54. doi: 10.1016/0006-3223(91)90209-5. [DOI] [PubMed] [Google Scholar]
- 82.Shibuya H, et al. Sigma receptors in schizophrenic cerebral cortices. Neurochem Res. 1992;17:983–990. doi: 10.1007/BF00966825. [DOI] [PubMed] [Google Scholar]
- 83.Hayashi T, et al. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2011;15:557–577. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iyo M, et al. Fluvoxamine as a sigma-1 receptor agonist improved cognitive impairments in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1072–1073. doi: 10.1016/j.pnpbp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Niitsu T, et al. Fluvoxamine improved cognitive impairments in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1345–1346. doi: 10.1016/j.pnpbp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Silver H. Selective serotonin re-uptake inhibitor augmentation in the treatment of negative symptoms of schizophrenia. Expert Opin Pharmacother. 2004;5:2053–2058. doi: 10.1517/14656566.5.10.2053. [DOI] [PubMed] [Google Scholar]
- 87.Silver H, et al. Fluvoxamine augmentation of antipsychotics improves negative symptoms in psychotic chronic schizophrenic patients: a placebo-controlled study. Int Clin Psychopharmacol. 2000;15:257–261. doi: 10.1097/00004850-200015050-00002. [DOI] [PubMed] [Google Scholar]
- 88.Gatti F, et al. Fluvoxamine alone in the treatment of delusional depression. Am J Psychiatry. 1996;153:414–416. doi: 10.1176/ajp.153.3.414. [DOI] [PubMed] [Google Scholar]
- 89.Serretti A, et al. Patterns of symptom improvement during antidepressant treatment of delusional depression. Psychiatry Res. 2000;94:185–190. doi: 10.1016/s0165-1781(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 90.Stahl SM. Antidepressant treatment of psychotic major depression: potential role of the sigma receptor. CNS Spectr. 2005;10:319–323. doi: 10.1017/s1092852900022641. [DOI] [PubMed] [Google Scholar]
- 91.Moller HJ, et al. Opipramol for the treatment of generalized anxiety disorder: a placebo-controlled trial including an alprazolam-treated group. J Clin Psychopharmacol. 2001;21:59–65. doi: 10.1097/00004714-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 92.Neznamov GG, et al. Clinical study of the selective anxiolytic agent afobazol. Eksp Klin Farmakol. 2001;64:15–19. [PubMed] [Google Scholar]
- 93.Werling LL, et al. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist. 2007;13:272–293. doi: 10.1097/NRL.0b013e3180f60bd8. [DOI] [PubMed] [Google Scholar]
- 94.Jansen KL, et al. Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer's disease correlates with CA1 pyramidal cell loss. Brain Res. 1993;623:299–302. doi: 10.1016/0006-8993(93)91441-t. [DOI] [PubMed] [Google Scholar]
- 95.Mishina M, et al. Low density of sigma1 receptors in early Alzheimer's disease. Ann Nucl Med. 2008;22:151–156. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- 96.Uchida N, et al. A variant of the sigma receptor type-1 gene is a protective factor for Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:1062–1066. doi: 10.1176/appi.ajgp.13.12.1062. [DOI] [PubMed] [Google Scholar]
- 97.Maruszak A, et al. Sigma receptor type 1 gene variation in a group of Polish patients with Alzheimer's disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:432–438. doi: 10.1159/000101990. [DOI] [PubMed] [Google Scholar]
- 98.Al-Saif A, et al. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70:913–919. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- 99.Rosen H. Dextromethorphan/quinidine sulfate for pseudobulbar affect. Drugs Today (Barc) 2008;44:661–668. doi: 10.1358/dot.2008.44.9.1258664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roth MD, et al. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78:1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 101.Siu A, Drachtman R. Dextromethorphan: a review of N-methyl-d-aspartate receptor antagonist in the management of pain. CNS Drug Rev. 2007;13:96–106. doi: 10.1111/j.1527-3458.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mei J, Pasternak GW. Sigma1 receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- 103.Pal A, et al. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J Biol Chem. 2008;283:19646–19656. doi: 10.1074/jbc.M802192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhuiyan MS, Fukunaga K. Targeting sigma-1 receptor signaling by endogenous ligands for cardioprotection. Expert Opin Ther Targets. 2011;15:145–155. doi: 10.1517/14728222.2011.546350. [DOI] [PubMed] [Google Scholar]
- 105.Xu J, et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun. 2011;2:380. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCann DJ, et al. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- 107.Palmer CP, et al. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- 108.Rothbard JB, et al. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708–9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]