Abstract
Introduction
To estimate the prevalence of parasitic infection and nutritional status, and to evaluate the extent to which the two are associated among schoolchildren in rural Ethiopia.
Methods
This is a cross sectional study of 664 students aged from 6 to 19 years old from Angolela, Ethiopia. Socio-demographic information was collected using a structured questionnaire. Anthropometric measurements were taken at the time of interview. Examinations of fecal samples for helminthic and protozoan parasitic infections were performed. Logistic regression procedures were employed to evaluate the association between stunting, underweightedness, and wasting with parasitic infections.
Results
One-third of the participants were found to have a protozoan infection, while 7.1% were found to have a helminthic infection. Approximately 11% of the students were stunted, 19.6% were wasted, and 20.8% were underweight. Severely underweight boys were 3.88-times more likely than boys of adequate weight (OR=3.88, 95%CI: 1.12–13.52) to be diagnosed with protozoan infections. Among girls, those who were severely stunted were approximately 12 times (OR=11.84, 95%CI: 1.72–81.62) as likely to be infected with a helminthic parasite, than those who were not. Overall, there was a deficit in normal growth patterns as indicated by lower than average anthropometric measures.
Discussion and conclusion
There is a high prevalence of intestinal parasitic infections. Stunting, wasting, and underweightedness were also prevalent, and showed patterns of associations with intestinal parasitic infections. Efforts should be made to strengthen and expand school and community-based programs that promote inexpensive, though effective, practices to prevent the spread of parasitic diseases. Initiatives aimed at improving the nutritional status of school children are also needed.
Keywords: intestinal parasitic infection, helminth infection, schoolchildren, nutrition, anthropometric measures
INTRODUCTION
Gastrointestinal parasitic infections are amongst the most common infections worldwide. These cases are attributed to three common intestinal parasites: Ascaris lumbricoides, hookworm, and Trichuris trichiura. The global prevalence of parasitic diseases is estimated to be 478 million children for A. lumbricoides; 280 million for hookworms and 347 million children for T. trichiura [1–3]. These infections are most prevalent in populations with low household income, poor handling of personal and environmental sanitation, overcrowding, and limited access to clean water [4]. Reports have shown that access to basic sanitation facilities in Ethiopia, especially in rural areas, is quite poor [5]. Only 11% of the total population has access to sustainable sanitation, and less than 42% have access to a source of clean water [6]. These factors, as well as poor knowledge, attitudes, and practices in personal hygiene, have added to this growing public health concern.
The best global indicator of a child's well-being is growth. Irregular growth patterns in children are known to be indicative of underlying risk factors, including low household income and recourses, inadequate food consumption, increased burden of diseases, particularly communicable diseases, inadequate sanitation, and poor hygienic conditions [7].
In Ethiopia, intestinal parasitic infections are of serious public health concern [4]. According to a report by the Ministry of Health, helminthiasis is the third leading cause of outpatient visits in health institutions in 2005–2006 [8]. Recently, it was discovered that the prevalence of intestinal parasitic infections in Jimma, Ethiopia [9] was as high as 86.2%; whereas in Tigray, the overall prevalence was 48.1% [10]. The range, though wide, still illustrates a high prevalence of these infections in Ethiopia.
A study conducted by the Central Statistical Agency (CSA) of Ethiopia in 2005, found that underweight, wasting, and stunting in children aged under-five years in Ethiopia was 36%, 10%, and 51%, respectively [11]. Family/household income appeared to be significantly associated with the nutritional status of young children. Other factors have also been found to be related to undernutrition, included lack of sanitation infrastructure, and limited knowledge about proper hygiene practices [12].
Since intestinal parasitic infections are associated with poor socioeconomic class and unsanitary conditions, people living in such settings in rural Ethiopia are at substantially increased risk for developing intestinal parasitic infections and suffering its diverse sequlae. Previous studies conducted in Ethiopia focused on the prevalence of parasitic infections themselves, [4,12–14], but not the association with undernutrition. Therefore, we sought to assess the prevalence of parasitic infection and nutritional status, and to evaluate the extent to which the two are associated among schoolchildren in a rural village in Ethiopia.
POPULATION AND METHODS
Population
The total study population consisted of a sample totaling 664 students, aged 6–19 years, from Angolela Primary School, a government operated, primary school in Angolela Woreda, now called Basona Worena (9° 37' 60 N, 39° 25' 60 E). All students in grades 1–6 and who were in attendance during the study period of October 20th – 25th, 2008 were included, and absentees (n=52) were not. Students were given unique identification numbers for the socio-demographic, stool examinations, and anthropometric measures.
Ethical approval for all study procedures was granted by the Institutional Review Board of Addis Continental Institute of Public Health (Addis Ababa, Ethiopia). The Human Subjects Division of the University of Washington, USA granted approval to use the de-identified and anonymised data set for analysis. Approval from the Woreda Health Office and the Woreda Education Office was also granted prior to the commencement of this study.
Anthropometric measurement
Height was measured, using a tape meter fixed on a wood board (15 cm × 3cm × 175cm) posted vertically on the wall, by appropriately trained research nurses who were unaware of the student's infection status. Each student was instructed to stand barefoot, with head in standard anatomical position, or Frankfurt plane (where the margins of the orbital and the upper margin of each ear canal was most nearly parallel to the ground) [15], and was measured to the closest centimeter (cm). Weight was measured using a calibrated Soehnle model 61319 scale (Soehnle Waagen GmbH & Co., Germany) to the closest kilogram (kg), with the student barefoot and wearing light clothing. These anthropometric measurements were made according to the World Health Organization (WHO) guidelines [16].
Socioeconomic and personal risk factors survey
A structured questionnaire was used to collect data (translated from English and printed in the local Amharic language), consisting of information on socioeconomic, sanitary, environmental, and demographic risk factors. Prior to use, the questionnaire was pre-tested in Dalcha Elementary School in Basona Worena Woreda, Ethiopia to assess the suitability of the questionnaire with regard to duration, language appropriateness, and question comprehensibility. The questionnaire was administered by trained research interviewers who performed in-person interviews of all participating students. The questionnaire included questions concerning mother and father literacy, students' gender, age, and grade in school.
Parasitological examination
A total of 664 fresh stool samples were collected in plastic cups and were labeled with the students' unique study identification number. Labeled cups and directions were given to the students by trained laboratory technicians. Following stool collection, the samples were preserved in a tube containing 10% formalin in 0.85% saline. The samples were then taken to Debre Birhan Hospital, 10 km from the study site, for processing, using a formal ether sedimentation technique for fecal examination to diagnose infections with intestinal worms and protozoa, following WHO standard operating procedures for the parasitological examination of feces [17].
To evaluate accuracy, 5% of the fecal samples were randomly re-processed by a separate lab technician from Debre Birhan Hospital and the results were compared with the results made by the original lab technician.
Data Entry
Data were entered in the program EPI-INFO (Version 3.3.2), a public access software made available from the US Centers for Disease Control and Prevention (CDC Atlanta, GA, USA), the analysis was completed using SPSS (version 17.0, SPSS, Inc., Chicago, USA), and growth chart comparisons were made using WHO AnthroPlus software using the 2007 reference data (version 1.02, World Health Organization, Geneva, Switzerland).
Statistical analysis
We first explored frequency distributions of socio-demographical and behavioral characteristics of subjects. Continuous variables were expressed as mean ± standard error of mean. Categorical variables were expressed as number (percentage, %). Chi-Square tests were used to evaluate the differences in the distribution of categorical variables for study groups.
Anthropometric measures expressed using the “height-for-age, weight-for-age, BMI-for-age” z-scores. Students who were overnourished, with a z-score value greater than or equal to +2 standard deviations (s.d.) above the expected mean, were categorized as eutrophic, above-average, and overweight, respectively; whereas students who were undernourished, with a z-score value less than or equal to −2 s.d. were characterized as stunted(low height-for-age), wasted(low weight-for-age), and underweight(low BMI-for-age), respectively. In the weight-for-age statistical analysis, students who were ≥ 10 years (n=326) of age were excluded. The weight-for-age measure is regarded as an inappropriate indicator of nutritional status for older children experiencing the pubertal growth spurt [18].
Logistic regression was employed to evaluate the association between nutritional status and intestinal parasitic infections. Confidence intervals (CI) of 95% were reported for each odds ratio (OR). All reported p-values were two-tailed and statistical significance was set at P = 0.05.
RESULTS
The study population was comprised of 341 (51.4%) boys and 323 (48.6%) girls. Approximately, 61.3% of students were enrolled in grades 1–3, and 39.7% were enrolled in grades 4–6 (Table 1). Infection with any protozoan (Giardia lamblia and Entamoeba histolytica) was identified in 30.2% (14.7% boys and 15.7% girls) of the study population. Infection with any helminth (A. lumbricoides, hookworm, Hymenolopis nana, T. trichiura, and Enterobius vermicularis, and Hymenolepis diminuta) was also identified in 7.0% (4.3% boys and 2.7% girls) of the study population.
Table 1.
Sociodemographic Characteristics and Prevalence of Parasitic Infections of Study Participants, Primary School, Angolela, Ethiopia, October 2008
| Characteristics | n (Total=664) | % |
|---|---|---|
| Grade | ||
| 1 | 105 | 15.8 |
| 2 | 188 | 28.3 |
| 3 | 114 | 17.2 |
| 4 | 70 | 10.5 |
| 5 | 109 | 16.4 |
| 6 | 78 | 11.7 |
| Gender | ||
| Female | 323 | 48.6 |
| Male | 341 | 51.4 |
| Mother literate | ||
| No | 210 | 31.6 |
| Yes | 432 | 65.1 |
| Father literate | ||
| No | 396 | 59.6 |
| Yes | 261 | 39.3 |
| Intestinal parasitic infection | Number infected | % |
| A. lumbricoides | 23 | 3.5 |
| Hookworm | 3 | .5 |
| H. nana | 10 | 1.5 |
| T. trichiura | 0 | 0 |
| H. dimunita | 28 | 4.2 |
| E. vermicularis | 11 | 1.7 |
| Any helminithic infection | 47 | 7.0 |
| G. lamblia | 48 | 7.2 |
| E. histolytica | 177 | 26.7 |
| Any protozoa infection | 202 | 30.4 |
Total numbers may not add to reported population size
Mean height, weight, and BMI for the boys of the study population were, 139.96 cm ± 0.80, 31.13 kg ± 0.54, and 15.43 ± 0.12, respectively; and for girls, 140.66 cm ± 0.81, 31.72 kg ± 0.58, and 15.58 ± 0.13, respectively (Table 2). Stunting was found among 10.2% of the boys and 12.1% of the girls, while wasting was found in 19.5% of boys and 19.7% of girls. Finally, underweight was found in 20.5% of the boys and 21.1% of the girls, as shown in Table 3.
Table 2.
Mean and Standard Error of Mean of Height, Weight and Body Mass Index, Primary School, Angolela, Ethiopia, October 2008
| Among Boys | Among Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Age groups | n | Height (cm) | Weight (kg) | BMI (kg/m2) | n | Height (cm) | Weight (kg) | BMI (kg/m2) |
| 6 | 2 | 134.00 ± 2.00 | 26.50 ± 1.50 | 14.74 ± 0.40 | 4 | 135.00 ± 6.36 | 28.00 ± 2.61 | 15.40 ± 1.22 |
| 7 | 24 | 131.08 ± 2.99 | 26.04 ± 1.80 | 14.73 ± 0.35 | 24 | 138.33 ± 2.52 | 29.58 ± 1.50 | 15.26 ± 0.41 |
| 8 | 47 | 136.49 ± 2.03 | 27.83 ± 1.24 | 14.57 ± 0.24 | 36 | 134.61 ± 2.09 | 27.28 ± 1.14 | 14.80 ± 0.26 |
| 9 | 30 | 136.07 ± 2.78 | 27.77 ± 1.68 | 14.59 ± 0.39 | 26 | 136.15 ± 2.75 | 29.12 ± 2.00 | 15.23 ± 0.49 |
| 10 | 68 | 140.06 ± 1.70 | 30.88 ± 1.21 | 15.23 ± 0.27 | 66 | 139.71 ± 1.82 | 30.80 ± 1.26 | 15.33 ± 0.28 |
| 11 | 35 | 139.46 ± 2.12 | 30.54 ± 1.35 | 15.39 ± 0.29 | 39 | 140.82 ± 2.31 | 32.38 ± 1.81 | 15.77 ± 0.39 |
| 12 | 39 | 139.77 ± 2.33 | 31.92 ± 1.61 | 15.85 ± 0.36 | 43 | 141.58 ± 2.21 | 32.16 ± 1.63 | 15.59 ± 0.34 |
| 13 | 29 | 147.52 ± 2.47 | 35.79 ± 1.79 | 16.11 ± 0.35 | 26 | 147.54 ± 2.32 | 37.54 ± 2.12 | 16.85 ± 0.53 |
| 14 | 25 | 144.60 ± 2.91 | 36.28 ± 2.01 | 17.09 ± 0.65 | 21 | 140.90 ± 3.45 | 31.24 ± 2.36 | 15.24 ± 0.49 |
| 15 | 18 | 150.28 ± 3.42 | 38.22 ± 2.31 | 16.54 ± 0.43 | 21 | 151.19 ± 2.84 | 38.57 ± 2.15 | 16.60 ± 0.46 |
| ≥16 | 15 | 140.13 ± 3.29 | 31.33 ± 2.64 | 15.53 ± 0.60 | 9 | 143.44 ± 4.45 | 34.33 ± 3.15 | 16.36 ± 0.73 |
| Total | 332 | †139.96 ± 0.80 | †31.13 ± 0.54 | †15.43 ± 0.12 | 315 | †140.66 ± 0.81 | †31.72 ± 0.58 | †15.58 ± 0.13 |
| Unreported | 9 | — | — | — | 8 | — | — | — |
Values are mean ± standard error of mean.
Total means by gender
Table 3.
Distribution of Children According to Z-score, Primary School, Angolela, Ethiopia, October 2008
| Among Boys | Among Girls | |||||
|---|---|---|---|---|---|---|
| Z-score | *Weight-for-Age n (%) | Height-for-Age n (%) | BMI-for-Age n (%) | *Weight-for-Age n (%) | Height-for-Age n (%) | BMI-for-Age n (%) |
| >0 | 28 (16.6) | 133 (39.0) | 36 (10.6) | 35 (22.3) | 117 (36.2) | 50 (15.5) |
| 0 to −1 | 55 (32.5) | 84 (24.6) | 120 (35.2) | 49 (31.2) | 99 (30.7) | 99 (30.7) |
| −1 to −2 | 53 (31.4) | 80 (23.5) | 106 (31.1) | 42 (26.8) | 61 (18.9) | 99 (30.7) |
| −2 to −3 | 22 (13.0) | 24 (7.0) | 53 (15.5) | 22 (14.0) | 33 (10.2) | 50 (15.5) |
| < −3 | 11 (6.5) | 11 (3.2) | 17 (5.0) | 9 (5.7) | 6 (1.9) | 18 (5.6) |
| Total Valid | 169 (100) | 332 (97.4) | 332 (97.4) | 157 (100) | 316 (97.8) | 316 (97.8) |
| Unreported | 0 (0) | 9 (2.6) | 9 (2.6) | 0 (0) | 7 (2.2) | 7 (2.2) |
Weight-for-age reference data are not available beyond age 10 because this indicator does not distinguish between height and body mass in an age period where many children are experiencing the pubertal growth spurt and may appear as having excess weight (by weight-for-age) when in fact they are just tall.
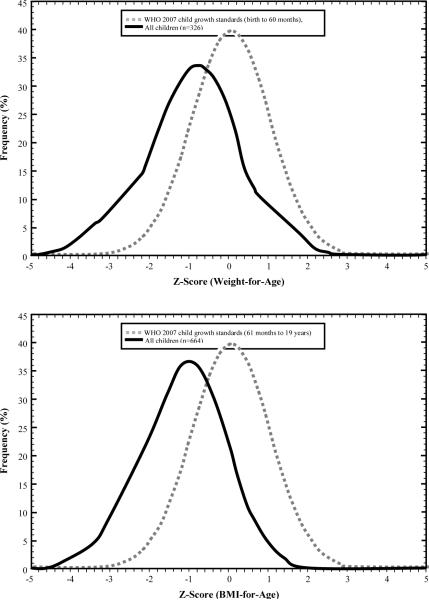
The growth patterns of the total study population, as well as the WHO 2007 reference data for weight-for-age and BMI-for-age are shown in Figure 1. The mean weight, height, and BMI of the total study population were found to be −0.91, −0.38, and −1.16 standard deviations below the WHO 2007 reference median for each respective anthropometric measure. The mean weight, height, and BMI for boys were −0.95, −0.41, and −1.28 standard deviations below the median WHO 2007 reference data, respectively. For girls, the mean weight, height, and BMI were −0.86, −0.35, and −1.02 standard deviations below the median WHO 2007 reference data, respectively. Overall (figures not presented), there appeared to be no significant gender difference in the distribution of the three anthropometric growth measures.
Figure 1.
Mean weight (kg), and BMI (kg/m2) of total Angolela Primary School with WHO (2007) Reference Data, Angolela, Ethiopia, October 2008
Undernutrition was observed to be prevalent among both boys and girls, as shown in Figure 2. Compared with the WHO 2007 growth reference, the mean weight-for-age, mean height-for-age, and mean BMI –for-age of Angolela school children were found to be in the lowest percentiles.
Figure 2.
WHO child growth standards of Weight-for-Age, Height-for-Age and BMI-for-age among Primary School students, Angolela, Ethiopia, October, 2008
Severely underweight boys (greater than −3 s.d. from the median of the WHO 2007 growth reference data) were 3.88-times more likely than boys of normal weight (OR=3.88, 95%CI: 1.12–13.52) to be diagnosed with protozoan infections (Table 4). However, this association did not reach statistical significance among girls. Severely stunted girls (greater than −3 s.d. from the median of the WHO 2007 growth reference) were approximately 12 times (OR=11.84, 95%CI: 1.72–81.62) more likely to have helminthic infections compared with those with normal height (Table 4). This association was not statistically significant among boys. Overall, those with poor nutritional status were more likely to have any intestinal parasitic infection.
Table 4.
Association between Nutritional Status and Intestinal Parasitic Infection, Primary School, Angolela, Ethiopia, October 2008
| Among Boys | Among Girls | |||
|---|---|---|---|---|
| Z-score | Any Protozoa Infection OR (95% CI) | Any Heliminitic Infection OR (95% CI) | Any Protozoa Infection OR (95% CI) | Any Heliminitic Infection OR (95% CI) |
| WAZ | ||||
| >0 | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) |
| 0 to −1 | 0.48(0.13 – 1.83) | 0.64 (0.12 – 3.31) | 0.56 (0.18 – 1.77) | 0.7(0.09 – 5.24) |
| −1 to −2 | 0.57(0.16 – 2.03) | 0.39 (0.07 – 2.24) | 0.97 (0.28 – 3.30) | 0.77(0.1–5.74) |
| −2 to −3 | 0.56(0.12 – 2.60) | 0.64 (0.09 – 4.56) | 0.51 (0.12 – 2.16) | †-- |
| < −3 | 1.77(0.31 – 10.03) | 0.63 (0.05 – 7.32) | 2.83 (0.42 – 19.00) | 2.06(0.17–25.68) |
| HAZ | ||||
| >0 | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) |
| 0 to −1 | 0.79(0.415 – 1.526) | 0.66 (0.241 – 1.79) | 0.89 (0.50 – 1.59) | 1.55 (0.47 – 5.15) |
| −1 to −2 | 1.72(0.944 – 3.127) | 0.66 (0.242 – 1.80) | 1.01 (0.53 – 1.95) | 1.13 (0.26 – 4.91) |
| −2 to −3 | 2.00(0.812 – 4.956) | 0.71 (0.149 – 3.35) | 0.85 (0.37 – 1.95) | 0.66 (0.075 – 5.91) |
| < −3 | 0.29(0.035 – 2.328) | 0.81 (0.09 – 6.88) | †-- | 11.83 (1.72 – 81.62) |
| BAZ | ||||
| >0 | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) | 1.00(Reference) |
| 0 to −1 | 1.04(0.44 – 2.47) | 1.98 (0.18 – 21.29) | 1.16 (0.57 – 2.37) | 0.52 (0.08 – 3.42) |
| −1 to −2 | 1.02(0.42 – 2.46) | 2.29 (0.28 – 19.07) | 0.77 (0.370 – 1.59) | 0.62 (0.12 – 3.25) |
| −2 to −3 | 0.99(0.37 – 2.68) | 1.69 (0.19 – 14.45) | 0.91 (0.39 – 2.09) | 0.17 (0.02 – 1.27) |
| < −3 | 3.88(1.11 – 13.52) | 1.04 (0.10 – 10.75) | 0.56 (0.16 – 1.95) | 0.74 (0.12 – 4.52) |
Adjusted for age (continuous)
OR could not be calculated because of small sample size
DISCUSSION
Our study found that the overall prevalence of any parasitic infection (protozoan or helminthic) was 37.2% and mean weight-for-age, height-for-age, and BMI-for-age of the study population were lower in this study population than WHO 2007 reference values. We also noted that severe underweightedness was associated with a statistically significant increased risk of protozoan parasitic infection among boys (OR=3.88, 95%CI: 1.12–13.52). Girls who were severely stunted had a 12-fold increased risk (OR=11.84, 95%CI: 1.72–81.62) of helminthic parasitic infections.
The prevalence of intestinal parasitic infections in our study was inconsistent with other published findings. In southern Ethiopia, it was found that the prevalence of infection for A. lumbricoides and T. trichiura among schoolchildren were 83.4% and 86.4%, respectively [19]. In Asendabo, also in southern Ethiopia, the prevalence of intestinal parasitic infection was found to be 86.2% [9]. Separately, in a national survey of amoebiasis, the overall prevalence of E. histolytica infections in schoolchildren was 15.0% [20], which was lower than our findings. In Gorgora, Ethiopia, the prevalence of S. mansoni infection was found to be in 50.8% of the schoolchildren population [21]. These findings show that there appears to be a varied, yet high prevalence of intestinal parasites in Ethiopia. Collectively, these findings were found to be higher than estimates reported from a rural population of Thai schoolchildren, where the prevalence for intestinal parasites was 22.7% [22]. The prevalence of parasitic infection in Ethiopia however, closely matches prevalence estimates from Northeastern Brazil, where 55.1% of schoolchildren were found to have S. mansoni infections [7]. Differences in reported prevalence of intestinal parasitic infections may be indicative of differences in geographical distributions of parasites and other risk factors associated with poor hygiene and other environmental factors [7,22–24].
The nutritional status of students in the present study showed that undernutrition was prevalent, compared to the 2007 WHO international reference standards [18]. Nearly eleven percent, 20.8%, and 19.6% of students were found to be stunted, underweight and wasted, respectively. Our findings are lower than what is reported by others. In Tigray, northern Ethiopia, the prevalence of stunting and wasting were 26.5% and 58.3%, respectively [24]. Also, in a region-specific study conducted by Lemma & Mariam in Agaro, Ethiopia, 18.6% of schoolchildren were found to be either wasted or stunted [23]. A national survey conducted by Hall, et al. reported stunting and wasting among schoolchildren to be 22.3% and 23.1%, respectively [14]. In rural Nepal, more than half (53.3%) of the children were found to be wasted and more than one third (36.6%) were stunted [25]. The variation in the reported prevalence of undernutrition across studies may be explained by the difference in study setting, socio-demographic and cultural differences.
Possible reasons for the observed positive association of poor nutritional status with intestinal parasitic infection include morbidity-associated weight loss, reduced appetite, deliberate dietary restrictions and altered absorption of micro and macronutrients [26].
In this study, poor nutritional status was associated with intestinal parasitic infections. The consequences of this may result in undesirable costs on an individual's mortality, morbidity, growth, education, and cognitive function, as suggested by Jardim-Botelho, et al. [27]. These factors, if not dealt with at a young age, may eventually contribute to reduced school attendance and higher susceptibility to disease, thereby compromising physical capacity and work opportunities in adulthood. Intestinal parasitic infections, coupled with other risk factors, may inevitably depress an individual's overall well-being, and that of his/her community's as well [28].
Treatments against parasitic infections have shown to be associated with significant improvements on a child's overall well-being. Latham, et al., observed that Kenyan school children, who were infected with intestinal parasites and then treated with albendazole or pyrantel pamoate, all showed significant improvement in appetite and physical performance just 3 weeks to 4 months after treatment. As a result, these children significantly improved their weight (1.0 kg greater than the placebo group, P < 0.0002), height (0.6 cm more, P < 0.003), arm circumference (0.3 cm more, P < 0.0002), and tricep and subscapular skinfolds (1.0 mm more, P <0.0002) compared to the control group [29]. In Brazil, it was found that helminthic infection during the first 2 years of life is associated with impaired growth, and that a single treatment of albendazole may benefit the child nutritionally in the long run [26].
Some limitations must be considered when interpreting the results of our study. First, the cross-sectional design of the study precludes causal inference. Second, our study was limited to students in grades 1–6 and those who were present in school. Students absent due to illness or other circumstances were not included, thus results may not be generalized to all schoolchildren. Lastly, inferences from this analysis are limited by our relatively small sample size as reflected by the wide 95% CI.
In Ethiopia, despite governmental efforts to address infection and malnutrition, our study documented a high prevalence of intestinal parasitic infections, as well as evidence of undernutrition, as shown by our anthropometric indicators. With chronic food insecurity being a major issue in Ethiopia due to structural cause, poverty, and aggravated by recurring droughts [30], children are often the first to be denied adequate nutrition [31]. This is illustrated by the high prevalence of undernutrition, as indicated by reports from the EDHS national survey, which found stunting and wasting in children under-five years to be 46.5% and 38.4%, respectively [11]. Thus, initiatives aimed at improving the nutritional status of school children, particularly younger children, are pivotal. The results of our study also underscore the importance of incorporating measures to ensure that existing nutrition program ensure that school-aged children receive diets with adequate amounts of total calories and protein in order to improve nutritional status. Furthermore, efforts should be made to strengthen and expand school and community-based programs that promote inexpensive, yet effective, practices aimed at preventing the spread of parasitic diseases by promoting the use and distribution of prophylaxis and other de-worming medications while making substantial improvements in school and community-based sanitation facilities. Comprehensive school and community-based early hygiene education, water treatments and proper waste disposal programs, as well as strategies from UNICEF's water, sanitation, and hygiene (WASH) Program [32], should be fully implemented in rural communities with child health and nutrition profiles similar to those observed in our present study. Coordination of these efforts are likely to yield appreciable and sustainable gains in improving the health and welfare of Ethiopia's children, and securing a prosperous future for its people.
Acknowledgments
This research was completed while Mr. Nam Nguyen was a research training fellow with the Multidisciplinary International Research Training (MIRT) Program of the University and Washington, School of Public Health. The MIRT Program is supported by an award from the National Institutes of Health, National Center on Minority Health and Health Disparities (T37-MD001449). The authors wish to thank Feed the Children Ethiopia, Angolela Primary School, and Addis Continental Institute of Public Health for providing facilities and logistics support throughout the research process. The authors would also like to thank Debre Birhan Hospital, the Woreda Health Office, and the Woreda Education Office for providing testing facilities and granting access to conduct the study.
Funding: This study was supported by an award from National Institutes of Health (T37-MD001449).
Footnotes
Conflicts of interest
None declared
References
- 1.Musgrove P. Investing in health: the 1993 World Development Report of the World Bank. Bull Pan Am Health Organ. 1993;27:284–6. [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 4.Mengistu A, Gebre-Selassie S, Kassa T. Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiopian Journal of Health Development. 2007;21:12–7. [Google Scholar]
- 5.Admassu M, Wubshet M, Tilaye T. Sanitary Survey in Gondar Town. Ethiopian Journal of Health Development. 2004;18:39–42. [Google Scholar]
- 6.World Health Organization . Country Health System Fact Sheet: Ethiopia. Geneva: 2006. [Google Scholar]
- 7.Assis AM, Prado MS, Barreto ML, et al. Childhood stunting in Northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr. 2004;58:1022–9. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health . Health and health related indicators: Ministry of Health. 2006. [Google Scholar]
- 9.Ali I, Mekete G, Wodajo N. Intestinal parasitism and related risk factors among students of Asendabo Elementary and Junior Secondary school, South Western Ethiopia. Ethiopian Journal of Health Development. 1999;13:157–61. [Google Scholar]
- 10.Asfaw ST, Giotom L. Malnutrition and enteric parasitoses among under-five children in Aynalem Village, Tigray. Ethiopian Journal of Health Development. 2000;14:67–75. [Google Scholar]
- 11.Central Statistic Agency of Ethiopia . Ethiopia, Demographic and Health Survey. 2005. [Google Scholar]
- 12.Adamu H, Endeshaw T, Teka T, Kifle A, Petros B. The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiopian Journal of Health Development. 2006;20:39–46. [Google Scholar]
- 13.Ayalew D, Boelee E, Endeshaw T, Petros B. Cryptosporidium and Giardia infection and drinking water sources among children in Lege Dini, Ethiopia. Trop Med Int Health. 2008;13:472–5. doi: 10.1111/j.1365-3156.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall A, Kassa T, Demissie T, Degefie T, Lee S. National survey of the health and nutrition of schoolchildren in Ethiopia. Trop Med Int Health. 2008;13:1518–26. doi: 10.1111/j.1365-3156.2008.02168.x. [DOI] [PubMed] [Google Scholar]
- 15.Gershwin ME, Nestel P, Keen CL. Handbook of nutrition and immunity. Humana Press; Totowa, N.J.: 2004. [Google Scholar]
- 16.World Health Organization . Measuring change in nutritional status : guidelines for assessing the nutritional impact of supplementary feeding programmes for vulnerable groups. World Health Organization; Obtainable from WHO Publications Centre USA; Geneva: Albany, N.Y.: 1983. [Google Scholar]
- 17.World Health Organization . Blood safety and clinical technology : 2000–2003 strategy. Dept. of Blood Safety and Clinical Technology, World Health Organization; Geneva: 2001. Dept. of Blood Safety and Clinical Technology. [Google Scholar]
- 18.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erko B, Medhin G. Human helminthiasis in Wondo Genet, southern Ethiopia, with emphasis on geohelminthiasis. Ethiop Med J. 2003;41:333–44. [PubMed] [Google Scholar]
- 20.Erko B, Birrie H, Tedla S. Amoebiasis in Ethiopia. Trop Geogr Med. 1995;47:30–2. [PubMed] [Google Scholar]
- 21.Degu G, Mengistu G, Jones J. Some factors affecting prevalence of and immune responses to Schistosoma mansoni in schoolchildren in Gorgora, northwest Ethiopia. Ethiop Med J. 2002;40:345–52. [PubMed] [Google Scholar]
- 22.Wongstitwilairoong B, Srijan A, Serichantalergs O, et al. Intestinal parasitic infections among pre-school children in Sangkhlaburi, Thailand. Am J Trop Med Hyg. 2007;76:345–50. [PubMed] [Google Scholar]
- 23.Lemma F, Mariam AG. Xerophthalmia and malnutrition among pre-school children in Agaro town south-west Ethiopia. East Afr Med J. 1996;73:179–81. [PubMed] [Google Scholar]
- 24.Mulugeta A, Hagos F, Stoecker B, et al. Nutritional Status of Adolescent Girls from Rural Communities of Tigray, Northern Ethiopia. Ethiopian Journal of Health Development. 2009;23:5–11. [Google Scholar]
- 25.Pramod Singh GC, Nair M, Grubesic R, Connell F. Factors Associated With Underweight and Stunting Among Children in Rural Terai of Eastern Nepal. Asia-Pacific Journal of Public Health. 2009;21:144–52. doi: 10.1177/1010539509332063. [DOI] [PubMed] [Google Scholar]
- 26.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 27.Jardim-Botelho A, Brooker S, Geiger SM, et al. Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop Med Int Health. 2008;13:458–67. doi: 10.1111/j.1365-3156.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisancho AR. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee, WHO Technical Report Series no. 854. The American journal of clinical nutrition. 1996;64:830. [PubMed] [Google Scholar]
- 29.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutr. 1993;123:1036–46. doi: 10.1093/jn/123.6.1036. [DOI] [PubMed] [Google Scholar]
- 30.European Commission on Development Ethiopia's recurring crisis: a problem of food insecurity. Courier-Africa Caribbean Pacific European Union. 2003;197:60–1. [Google Scholar]
- 31.Capote RM, Ramirez DP, Tirado JF. Liver abscess and HIV: report of a case. Rev Cubana Med Trop. 2003;55:47–9. [PubMed] [Google Scholar]
- 32.United Nations Children's Fund . Water, Sanitation and Hygiene. New York: May 22, 2009. 2009. [Google Scholar]