Abstract
Objective
To assess whether combination treatment with lithium and divalproex is more effective than lithium monotherapy in prolonging the time to mood episode recurrence in patients with rapid-cycling bipolar disorder (RCBD) and comorbid substance abuse and/or dependence.
Method
A 6-month, double-blind, parallel group comparison was carried out in recently manic/hypomanic/mixed patients who had demonstrated a persistent bimodal response to combined treatment with lithium and divalproex. Subjects were randomly assigned to remain on combination treatment or to discontinue divalproex and remain on lithium monotherapy.
Results
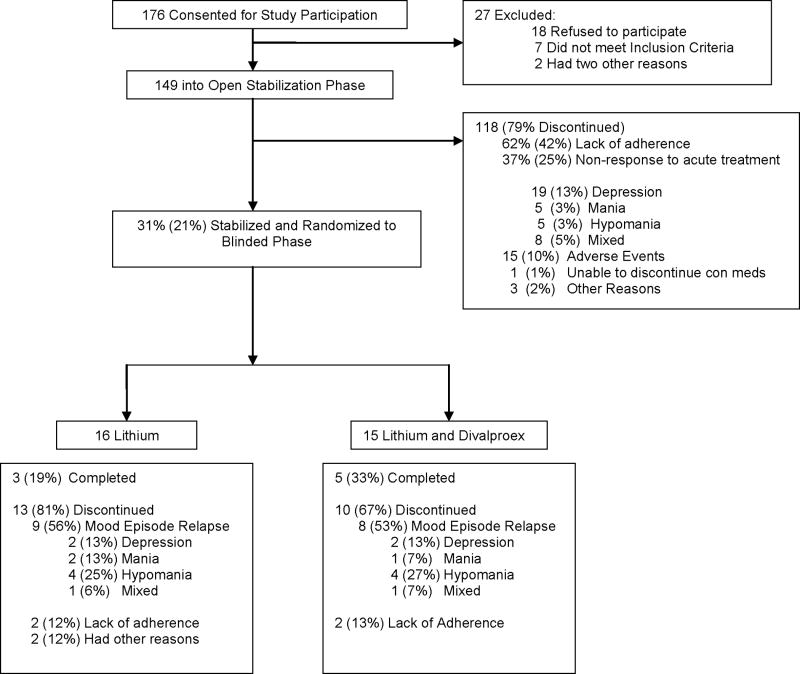
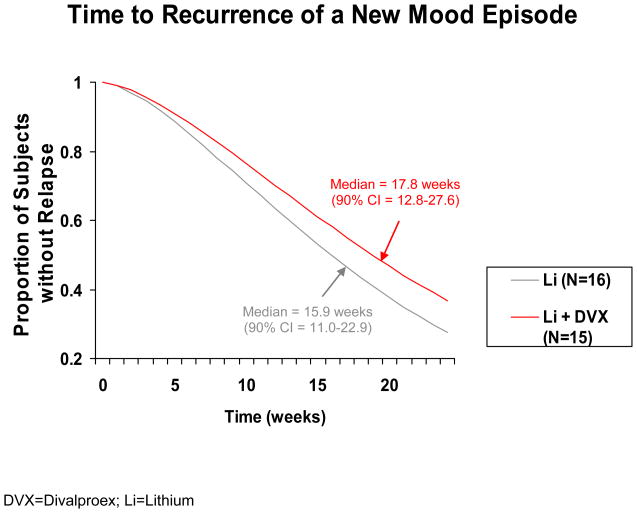
Of 149 patients enrolled into the open-label acute stabilization phase, 79% discontinued prematurely (poor adherence: 42%; nonresponse: 25%; intolerable side effects: 10%). Of 31 patients (21%) randomly assigned to double-blind maintenance treatment, 55% relapsed (24% into depression and 76% into a manic/hypomanic/mixed episode), 26% completed the study, and 19% were poorly adherent or exited prematurely. The median time to recurrence of a new mood episode was 15.9 weeks for patients receiving lithium monotherapy and 17.8 weeks for patients receiving the combination of lithium and divalproex (p=NS). The rate of relapse into a mood episode for those receiving lithium monotherapy or the combination of lithium and divalproex was 56% and 53%, respectively. The rate of depressive relapse in both arms was 13%, while the rate of relapse into a manic, hypomanic, or mixed episode was 44% for lithium monotherapy and 40% for the combination of lithium and divalproex.
Conclusion
A small subgroup of patients in this study stabilized after six months of treatment with lithium plus divalproex. Of those who did, the addition of divalproex to lithium conferred no additional prophylactic benefit over lithium alone. Although depression is regarded as the hallmark of RCBD in general, these data suggest that recurrent episodes of mania tend to be more common in presentations accompanied by comorbid substance use.
Keywords: Bipolar disorder, Rapid cycling, Dual-diagnosis, Substance use disorder, Maintenance trial, Placebo-controlled trial, Lithium, Divalproex, Combination pharmacotherapy
INTRODUCTION
Rapid cycling is a variant of bipolar disorder characterized by four or more mood episodes during a 12-month period. A rapid cycling course affects approximately 20% of patients with bipolar disorder1,2 and occurs more commonly among females and the bipolar II subtype.1 Rapid-cycling presentations are frequently accompanied by other Axis I comorbidities, including alcohol and drug use disorders.3,4 In fact, more than 40% of RCBD patients enrolled into the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) met criteria for comorbid substance abuse.2
The added morbidity associated with substance use disorders (SUDs) poses important public health implications. Substance abuse is widely known to negatively affect treatment outcomes in bipolar disorder, contributing to greater treatment non-adherence5, increased hospitalizations,6 lower remission rates,7 more lifetime mood episodes,8 and decreased quality of life.9
Although recognized as a cornerstone in the maintenance treatment of bipolar disorder, lithium is ineffective for up to 40% of patients.10 In addition, both rapid cycling11 and co-occurring substance abuse12 have been associated with lithium nonresponse. Open-label data suggest patients with RCBD may respond better to divalproex than to lithium,11,13 and divalproex has shown efficacy in the acute treatment of bipolar mood episodes complicated by substance abuse.14,15 One small (N=12) unblinded study found the combination of lithium and divalproex superior to lithium monotherapy during maintenance treatment of non-rapid cycling bipolar disorder.16
The present study was conceptualized while undertaking the first double-blind maintenance trial in RCBD to evaluate outcomes with lithium and divalproex.17 During the conduct of that trial, it became apparent that a critical mass of patients was being excluded from participation due to active substance use. This practice is common and serves to reduce potential sources of variance, yet substantially decreases clinical trial generalizability. The present study was intended to improve generalizability by focusing on a cohort with active SUDs. To our knowledge, it is one of only 3 placebo-controlled intervention trials to specifically evaluate mood outcomes in subjects with bipolar disorder and co-occurring SUDs;18–20 it is the first entirely composed of patients with rapid cycling.
We hypothesized that lithium in combination with divalproex would be superior to lithium monotherapy in preventing maintenance phase relapse. The trial was designed to address the following questions:
Does the combination of lithium and divalproex compared with lithium monotherapy prolong the time to mood episode recurrence among patients with RCBD and co-occurring SUDs?
Do differences exist in the frequency and polarity of mood relapse among patients with RCBD and co-occurring SUDs?
How do stabilization and relapse rates during treatment with lithium and divalproex affect the design and feasibility of conducting future, large-scale maintenance trials in RCBD and co-occurring SUDs?
PATIENTS AND METHODS
The study was conducted at the outpatient Mood Disorders Program of Case Western Reserve University/University Hospitals Case Medical Center between October 1997 and October 2006. The University Hospitals Case Medical Center Institutional Review Board approved all study procedures. Written informed consent was obtained from each subject before any study-related procedures were performed. Patients could discontinue or be discontinued from any phase of the study for poor tolerance of study medications, lack of medication efficacy, investigator or patient unwillingness to continue the study for any reason, or nonadherence with study procedures. Participation could last up to 12.5 months, including a 2-week screening period, a 6-month open-label acute stabilization phase, and a 6-month double-blind, parallel-group maintenance phase.
Study Subjects
Patients eligible for participation were men and women, between 16 and 65 years of age, who met DSM-IV criteria for the following: 1) bipolar I or II disorder; 2) alcohol, cannabis, or cocaine abuse within the last 3 months or dependence within the last 6 months; 3) rapid cycling during the 12 months preceding study entry (confirmed by retrospective mood charting)21; and 4) a history of at least one manic, hypomanic, or mixed episode within 3 months of study entry. Patients were required to be in good physical health according to medical history, physical examination, and laboratory analyses conducted at the screening visit. Patients were excluded from participation if they had previous intolerance to lithium or divalproex, were completely non-responsive to past lithium treatment, had alcohol-related liver disease as reflected by diffuse elevations in liver function tests exceeding the upper limits of the normal range by 50%, were pregnant or planning to become pregnant, were taking exogenous steroids, required anticoagulant drug therapy, or were actively suicidal as evinced by a score ≥3 on item 3 of the 17-item Hamilton Depression Rating Scale (HAM-D).22
Subjects met DSM-IV criteria for rapid-cycling bipolar disorder type I or II as ascertained by Extensive Clinical Interview (ECI) and the Mini-International Neuropsychiatric Interview (MINI) performed by a research psychiatrist and research assistant.23 For the diagnosis of SUDs, the Structured Clinical Interview for the DSM-IV, Patient Edition24 was used instead of the MINI. The ECI consists of questions and criteria for the diagnosis of DSM-IV Axis I disorders, which is similar to the SCID-P, but also contains items to assess mental status, severity of suicidality, demographics, and other variables of interest. To substantiate the clinical history, all subjects were required to bring a significant other to the initial diagnostic assessment.
Pretreatment psychiatric assessments included the following measures: HAM-D, Young Mania Rating Scale (YMRS),25 Global Assessment Scale (GAS),26 and the Addiction Severity Index.27 Eligible patients were then enrolled in the open-label acute stabilization phase.
Open-Label Acute Stabilization Phase
During this phase, patients were seen by a research psychiatrist every 2 weeks and treated with the combination of lithium carbonate and divalproex sodium. Lithium monotherapy was initiated at 300 mg twice daily and titrated over 3–6 weeks to minimum blood levels of 0.8 meq/L. Divalproex was then initiated at 250 mg twice daily and increased over 3–6 weeks to minimum blood levels of 50 ug/ml. If patients were already taking lithium, but not divalproex, divalproex was initiated as described. If patients were already taking divalproex, but not lithium, lithium was initiated and titrated as described. Any other psychotropic medications that patients were taking at study entry were gradually discontinued a minimum of 4 weeks before random assignment to double-blind treatment.
Patients meeting stabilization criteria for a minimum of 4 consecutive weeks were eligible for random assignment to double-blind maintenance treatment. Entry criteria included a 17-item HAM-D score ≤ 20, YMRS score ≤ 12.5, GAS score ≥ 51, lithium levels ≥ 0.8 meq/L, and valproate levels ≥ 50 μg/ml. Patients not meeting these criteria by 24 weeks were discontinued from the study.
Double-Blind Maintenance Phase
Patients were assigned in a 1:1 ratio to treatment with lithium monotherapy or the combination of lithium and divalproex after stratification for illness type (bipolar I versus bipolar II ). At randomization, patients were continued on the same lithium dose as during the acute stabilization phase and on equal capsules of double-blind divalproex or matching placebo. Patients assigned to lithium monotherapy underwent divalproex-placebo substitution at a rate of 250 mg decrements every week until discontinued. Patients assigned to the combination group were continued on lithium and blinded divalproex. The maintenance phase and survival analysis began at the point of randomization, coinciding with the beginning of the medication taper.
After the taper was completed, the number of capsules of lithium and blinded divalproex or placebo remained unchanged for the remainder of the maintenance phase, except for adjustments made by the unblinded medical monitor when blood levels decreased to less than 0.8 meq/L for lithium and 50 μg/ml for valproate. Trough divalproex and lithium levels were performed twice monthly during the first 3 months of the maintenance phase and monthly thereafter. Dose adjustments were made according to blood levels. To maintain the blind and the exact number of capsules being administered during the maintenance phase, each change in the dose of active divalproex was accompanied by a matching change in the placebo dose. The number of placebo capsules was decreased commensurately if the number of capsules of the active compound was increased, and vice versa for decreases. Dosing of lithium or divalproex could be decreased if patients were believed to be experiencing dose-related side effects (such as tremors) as long as minimum blood levels were maintained. If this was not possible, patients reached study endpoint due to intolerable side effects. Patients were seen by the research psychiatrist every 2 weeks during the first 3 months of the maintenance phase and monthly thereafter for up to 6 months.
Concomitant Medications
Patients could receive lorazepam in doses up to 2 mg/day for anxiety, agitation, and insomnia. For severe insomnia, zolpidem up to 10 mg/day could be prescribed. Initiation of psychotherapy was not permitted during the study.
Safety Monitoring
For each study phase, the safety population comprised all patients who received at least one dose of study drug. Safety was assessed by summarizing treatment-emergent adverse events and evaluating clinical laboratory test results, including white blood cell count, platelet count, free thyroxine index, thyroid stimulating hormone, and liver function tests (ALT and AST).
Data Analysis
Time to treatment for a mood episode, (i.e., time to treatment for emerging symptoms of a mood relapse) was the primary outcome measure. This was evaluated by statistical methods designed for the time-to-event data. Weibull distribution curves were generated to plot the survival function, and differences between treatment groups were compared using log rank tests at an alpha = 0.05 level of significance. Patients who did not relapse, including those who discontinued early for other reasons, were censored on the date of their last efficacy evaluation or the last dose of study medication. Secondary efficacy outcome measures were evaluated using log rank tests and included time to study discontinuation for any reason, time to depressive relapse, and time to manic/hypomanic/mixed relapse. A Cox proportional hazards model was used to evaluate differences for the following predictors of outcome: treatment arm assignment, bipolar subtype, index episode at study entry, and substance use disorder diagnosis. A repeated measures mixed-effects model was used to analyze mean changes in symptom severity scores.
Baseline clinical characteristics were compared using a Fisher’s exact test or chi-square for nominal or ordinal data, and Student’s t test or Wilcoxon’s rank sum test for continuous data.
RESULTS
Demographics and Baseline Clinical Characteristics
Patients enrolled into this study were more likely to be male and have a diagnosis of bipolar I disorder (Table 1). Study patients exhibited severe illness as reflected by the number of mood episodes in the last 12 months, lifetime history of physical and/or sexual abuse, and number of comorbid Axis I disorders (Table 1). Fifty-eight percent (N=86) of the sample had a prior psychiatric hospitalization, 46% had attempted suicide (N=69), and 53% (N=79) had experienced past psychotic episodes.
TABLE 1.
Demographic and Clinical Characteristics of Rapid-Cycling Bipolar Disorder Patients Treated with a Lithium/Divalproex Combination Regimen Followed by Double-Blind Maintenance with Lithium Monotherapy or Lithium/Divalproex Combination Upon Stabilization
| Characteristic | Open-Label Acute Stabilization (N=149) | Double-Blind Maintenance Therapy (N=31) | ||||
|---|---|---|---|---|---|---|
| Lithium (N=16) | Lithium/Divalproex (N=15) | |||||
| N | % | N | % | N | % | |
| Female | 54 | 36 | 4 | 25 | 6 | 40 |
| Male | 95 | 64 | 12 | 75 | 9 | 60 |
| Illness Type | ||||||
| Bipolar I disorder | 112 | 75 | 13 | 81 | 13 | 87 |
| Bipolar II disorder | 37 | 25 | 3 | 19 | 2 | 13 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 36.2 | 10.1 | 40 | 10.6 | 37.1 | 10.9 |
| Age at first diagnosis (years) | 33.4 | 10.3 | 34.8 | 10.6 | 36.5 | 11.2 |
| Age at first manic/hypomanic/mixed episode (years) | 15.5 | 7.2 | 15.7 | 9.2 | 14.4 | 6.5 |
| Age at first depression (years) | 13.6 | 6.9 | 11.5 | 7.7 | 12.1 | 5.7 |
| Number of mood episodes past year | 12.2 | 7.7 | 11.5 | 7.4 | 7.9 | 3.1 |
| Depression | 6 | 3.9 | 5.8 | 4 | 4 | 1.6 |
| Hypomania/mania/mixed | 6.1 | 3.9 | 5.8 | 3.7 | 4 | 1.6 |
| Number of psychiatric admissions (lifetime) | 2.1 | 3.7 | 2.1 | 3.1 | 1.2 | 1.4 |
| Number of alcohol or drug rehabilitation admissions (lifetime) | 1.4 | 2.2 | 1.0 | 2.2 | 1.7 | 2.6 |
| Mood state at screening | N | % | N | % | N | % |
| Depressed | 70 | 47 | 5 | 31 | 6 | 40 |
| Hypomanic | 28 | 19 | 3 | 19 | 3 | 20 |
| Manic | 25 | 17 | 5 | 31 | 2 | 13 |
| Mixed | 21 | 14 | 2 | 13 | 4 | 27 |
| Euthymic | 5 | 3 | 1 | 6 | 0 | 0 |
| Clinical History (lifetime) | ||||||
| Anxiety disorder comorbidity | 67 | 45 | 5 | 31 | 10 | 67 |
| Psychotic episode | 79 | 53 | 10 | 63 | 10 | 67 |
| Suicide attempt | 69 | 46 | 8 | 50 | 8 | 53 |
| Sexual abuse | 33 | 22 | 5 | 31 | 3 | 23 |
| Physical abuse | 50 | 34 | 5 | 31 | 4 | 20 |
| Previous treatment with bipolar medications | 99 | 66 | 10 | 63 | 8 | 53 |
| Medications received (lifetime) | ||||||
| Lithium | 72 | 48 | 6 | 38 | 6 | 40 |
| Divalproex | 78 | 52 | 7 | 44 | 8 | 53 |
| Antidepressants | 104 | 70 | 12 | 75 | 8 | 53 |
| Antipsychotics | 37 | 25 | 7 | 44 | 1 | 7 |
Disposition
Of 149 patients enrolled into the open-label acute stabilization phase, 42% (N=62) exited because of poor adherence, 25% (N=37) exhibited nonresponse to the combination of lithium plus divalproex and exited because of the need for additional treatment, and 10% (N=15) exited because of adverse events. Only 21% (N=31) completed the stabilization phase and were randomly assigned to double-blind maintenance treatment for up to 6 months (lithium: N=16, lithium plus divalproex: N=15). Of the 37 subjects not responding to the combination of lithium plus divalproex, 51% (N=19) exhibited refractory depression, 14% refractory hypomania (N=5), 14% (N=5) refractory mania, and 21% (N=8) a refractory mixed state (Figure 1). Of 31 patients entering the 6-month double-blind maintenance phase, 26% (N=8) completed the phase, 55% (N=17) required treatment for a mood episode, and 19% (N=6) discontinued prematurely for reasons other than a mood episode (Figure 1). Among the patients requiring treatment for a mood episode (N=17), relapse into a manic/hypomanic/mixed state (N=13) was more common than relapse into depression (N=4; p = 0.029).
Figure 1. Enrollment and Outcome.
Disposition of patients with Rapid-Cycling Bipolar Disorders and Co-Occuring Substance Use Disorders Treated with a Lithium/Divalproex Combination Regimen Followed by Double-Blind Maintenance with Lithium Monotherapy or Lithium/Divalproex Combination Upon Stabilization.
Intervention
The mean dose of lithium during the double-blind maintenance phase was 1440 mg/day (range=900mg–2400mg) for the lithium monotherapy group, and the mean lithium level was 0.88 meq/L. For the combination group, the mean dose of lithium was 1400 mg/day (range=600mg–2100mg) and the mean dose of divalproex was 1583 mg/day (range=1000mg–3250mg). The mean lithium level was 0.89 meq/L and the mean valproate level was 67 ug/ml.
During the double-blind maintenance phase, lorazepam use occurred in 2 of 16 patients assigned to lithium monotherapy and 1 of 15 patients assigned to the combination of lithium and divalproex. Zolpidem use occurred in 1 subject in each of the treatment arms.
Efficacy Data
There were no significant differences in time to treatment for a mood episode (Figure 2), time to premature discontinuation for any reason, time to treatment for depression, and time to treatment for a manic/hypomanic/mixed episode. The median time to a mood episode recurrence was 15.9 weeks for lithium and 17.8 weeks for the combination of lithium and divalproex (hazard ratio=0.72 [p = 0.44; 95% CI = 0.32–1.65]). The median time to discontinuation for any reason was 12.3 weeks for lithium and 16.1 weeks for the combination of lithium and divalproex. The Cox regression predictors of outcome analysis yielded no effect for treatment arm assignment, bipolar subtype, substance use disorder diagnosis, or index episode.
FIGURE 2.
Time to Treatment Intervention for Any Mood Episode Among Stabilized Rapid-Cycling Bipolar Disorder Patients Randomly Assigned to Double-Blind Lithium Monotherapy or the Combination of Lithium and Divalproex.
Using a repeated measures mixed-effects model, the mean (±SE) change in HAM-D total score from baseline to endpoint of the double-blind phase did not differ between lithium monotherapy and the combination of lithium and divalproex (0.4 ± 1.1 vs. −0.2 ± 1.3; p=0.3). Likewise, the mean change from baseline to endpoint in the YMRS total score did not differ between lithium monotherapy and the combination of lithium and divalproex (1.4 ± 1.1 vs. 1.5 ± 1.3; p=0.7).
Effects on Alcohol and Substance Use
A completer analysis of subjects assigned to a double-blind treatment arm found the rate of active substance use disorders to diminish from study entry to completion of the acute stabilization phase. Of 19 subjects abusing or dependent on alcohol, 58% (N=11) no longer met criteria for active abuse or had entered into early full remission after receiving up to 6 months of open-label treatment with lithium and divalproex. Similarly, among 15 subjects with cannabis use disorders, 53% (N=8) no longer met criteria for active cannabis abuse or had entered into early full remission. Among 9 subjects with cocaine use disorders, 78% (N=7) no longer met criteria for active cocaine abuse or had entered into early full remission.
Results from the Addiction Severity Index confirmed that bimodal responders to the combination of lithium and divalproex were less likely to be troubled by alcohol problems (0.3 vs. 1.2; p=0.005) and to experience fewer days of alcohol use in the month prior to randomization (0.9 vs. 5.8; p=0.001) as compared with treatment non-responders. However, given the limited sample size, no statistical difference was found when comparing bimodal responders and non-responders in the number of days of cannabis (3.2 vs. 4.3; p=0.6) or cocaine (0 vs. 1; p=0.06) use in the month prior to randomization, nor were treatment responders less likely to report being troubled by drug problems (0.3 vs. 0.5; p=0.5).
Changes in Symptom Severity and Overall Function
For those subjects entering the study in a depressive episode and eventually assigned to a double-blind maintenance group, HAM-D-based symptom severity at baseline (mean ± SD) diminished substantially by the time of random assignment, from 19.6 (± 6.0) to 8.2 (± 4.5) (p=0.001). For those patients entering the study in a manic/hypomanic/mixed state, YMRS-based symptom severity at baseline diminished by the time of random assignment from 16.6 (± 5.44) to 4.53 (± 2.48) (p=0.001). Among all subjects randomized to the double-blind maintenance phase, GAS-based functional impairment improved from 52.4 (± 5.13) to 74.9 (± 10.78) (p<0.001).
Adverse Events
Of 149 subjects enrolled, 15 (10%) discontinued during the open-label phase because of adverse events. Weight gain (33%), gastrointestinal discomfort (27%), tremors (20%), dizziness (7%), cognitive difficulties (7%), and polyuria/polydipsia (7%) were the most common adverse events leading to premature discontinuation. Table 2 summarizes the adverse events observed in at least 10% of patients during the double-blind maintenance phase; none of the subjects discontinued because of adverse events. Tremors and polyuria/polydipsia were the most common adverse events in both treatment groups. A significant increase in alanine transaminase levels occurred in the lithium and divalproex combination group (+19.60 U/L) compared with the lithium monotherapy group (−30.83 U/L; p=0.029). There were no differences in white blood cell count, platelet count, free thyroxine index, thyroid stimulating hormone, or aspartate transaminase levels between treatment groups during the randomized phase.
TABLE 2.
Common Adverse Events Experienced by > 10% of Rapid-Cycling Bipolar Disorder Patients During the Double-Blind Maintenance Phase
| Double-Blind Maintenance Phase | ||||
|---|---|---|---|---|
| Lithium (N = 16) | Lithium and Divalproex (N = 15) | |||
| Adverse Event | N | % | N | % |
| Tremors | 10 | 63 | 10 | 67 |
| Polyuria/Polydipsia | 5 | 31 | 6 | 40 |
| Diarrhea | 6 | 38 | 4 | 27 |
| Weight Gain | 5 | 31 | 2 | 13 |
| Fatigue | 1 | 6 | 5 | 33 |
| Nausea | 3 | 19 | 2 | 13 |
| Alopecia | 1 | 6 | 3 | 20 |
| Dry Mouth | 0 | 0 | 3 | 20 |
| Sexual Dysfunction | 2 | 13 | 2 | 13 |
| Cognitive Dysfunction | 2 | 13 | 2 | 13 |
| Blurred Vision | 1 | 6 | 2 | 13 |
| Increased Appetite | 2 | 13 | 0 | 0 |
| Acne | 2 | 13 | 0 | 0 |
DISCUSSION
This 6-month, randomized, double-blind, placebo-controlled trial is the first maintenance study in RCBD to compare combination mood stabilizer treatment (lithium and divalproex) with lithium monotherapy. To our knowledge, it is also the first double-blind maintenance trial of RCBD to be conducted in subjects with a co-occurring alcohol and/or drug use disorder. This study complements a 20-month maintenance trial previously conducted by our group comparing double-blind treatment with lithium or divalproex monotherapy in RCBD uncomplicated by SUDs.17
After random assignment, no significant difference was observed on the primary outcome measure of time to relapse into a new mood episode or on the secondary outcome measure of time to discontinuation for any reason. Although a numeric benefit was observed in the estimated hazard ratio (0.72), this value was non-significant and suggests the difference in prophylactic efficacy between lithium monotherapy and the combination of lithium and divalproex is small when used in the maintenance treatment of RCBD comorbid with SUDs.
Similar to other controlled maintenance studies of subjects with bipolar disorder, this trial employed an enriched design requiring subjects to respond to lithium and divalproex prior to entering the double-blind, placebo-controlled phase.17,28–29 This served to enrich the randomized population with compliant subjects tolerant of the medications under investigation, effectively reducing bias due to differences in tolerability. Consistent with this expectation, no dropouts due to adverse events were observed during the double-blind maintenance phase.
In maintenance studies conducted over 6–18 months, rates of discontinuation due to lack of efficacy are higher among trials that employ more rigorous stabilization criteria.28–29 In the present trial, 25% of subjects discontinued due to lack of efficacy, reflective of the requirement for stabilization to be sustained over 4 consecutive weeks (HAM-D ≤ 20, YMRS ≤ 12.5, and GAS ≥51), as well as the difficulty encountered when treating subjects with co-occurring SUDs. Of 149 patients who received open lithium and divalproex, 118 (79%) exited the study prior to randomization to the blinded maintenance phase. The attrition rate in previously conducted maintenance trials of non-substance abusing bipolar patients has ranged from 50–72%.28–30
During open stabilization, 37 patients demonstrated mood symptoms non-responsive to combination treatment with lithium and divalproex. Approximately equal numbers experienced refractory depression or refractory manic/hypomanic/mixed states. This finding is novel and stands in contrast to RCBD populations without co-occurring SUDs, where the predominant mood presentation is depression.17 Furthermore, the majority of subjects relapsed into manic/hypomanic/mixed states as opposed to depression during maintenance phase treatment. This also distinguishes RCBD populations with co-occurring SUDs, as relapse into depression is more prevalent in populations without substance use comorbidity.17
Although depression is regarded as the hallmark of RCBD, manic, hypomanic, or mixed states appear to be the major mood morbidity in the setting of alcohol and/or other SUDs. This observation is supported by other authors who identified a higher rate of mixed states and dysphoric mania among bipolar patients who abuse substances.7,31 Mania was identified as the most common index episode in a post hoc analysis of patients with bipolar disorder and comorbid cannabis abuse,32 and manic or mixed states were the most common presentations among patients with comorbid alcoholism.20
The prevalence of rapid cycling is believed to be highest among females1,33 and those with bipolar II disorder.1 The positive association between females and rapid-cycling may partly be attributed to the greater depressive morbidity experienced by women over their lifetime.34 Contrarily, in this trial the patient composition was weighted toward males and subjects with bipolar I disorder, consistent with previous research identifying these variables to be associated with higher rates of alcohol and drug abuse.2 This suggests the tendency for relapse into manic/hypomanic/mixed states may be more heavily influenced by comorbid substance use than rapid-cycling status per se.
The present study has several strengths, perhaps foremost its attempt to assess mood outcomes in a population with bipolar disorder and co-occurring SUDs. This area has recently been articulated as a priority for clinical investigation by a “call to action” report, given the scarcity of clinical trials addressing substance use comorbidity.35 In fact, bipolar patients with SUDs have been identified as a population exhibiting the greatest level of unmet need, followed closely by those with rapid-cycling.36 The present trial addresses both understudied areas and is highly generalizable to clinical practices, providing the first estimate of the difference in median survival for two commonly used drug regimens.
There is evidence that clinicians under-treat bipolar disorder when a co-morbid SUD is present, as STEP-BD data show nearly half of patients do not receive adequate mood stabilizer treatment. 37 Our findings suggest that combination mood stabilizer therapy can be efficacious and safe when prescribed to substance-abusing individuals with bipolar disorder. Relatedly, Salloum and colleagues20 found the addition of valproate to lithium among individuals with alcohol dependence reduced the number of heavy drinking days and prolonged the time to relapse to sustained heavy drinking. However, divalproex did not reduce depressive or manic symptoms more effectively than lithium alone.20 Collectively, these results emphasize that clinicians should not abandon or delay mood stabilizer treatment as a result of substance use.
The intent of this single-site pilot trial was to generate an estimate of the difference in overall survival between treatment arms for designing a future, large scale maintenance study. Consequently, the lack of power to detect a small difference in overall survival between treatments is a valid but expected methodological limitation. The estimated hazard ratio of 0.72 indicates that patients randomly assigned to combination treatment had a tendency toward lower risk of discontinuation for any reason. Thus, a future study would need to enroll 193–225 subjects per arm in order to achieve statistical power of 0.80 with an alpha set at 0.05, two-tailed. Given the large sample size sample requirement and relatively small effect size demonstrated by this pilot study, it is unlikely that a future, large-scale trial involving these two first-line mood stabilizers will ever be conducted. Furthermore, the high drop-out rate during the open stabilization phase highlights the complexity of treating patients with comorbid RCBD. A further limitation concerns the nominal data on substance use outcomes. We were not able to quantify the change in frequency of alcohol or drug use. In addition, toxicology screens were not collected to verify the presence of a SUD or relapse into substance use. Future maintenance trials of RCBD with co-occurring SUDs should specifically evaluate the quantity and frequency of substance use as a primary outcome measure, in addition to measuring relapse rates and time to relapse.
There is a need for future maintenance studies that combine conventional mood stabilizers with agents able to reduce depression symptoms and also protect against mood elevation. Members of the atypical antipsychotic drug class offer promise of satisfying this unmet need and may also reduce drug and alcohol consumption.38–40
In conclusion, this is the first controlled investigation to compare lithium monotherapy with combination mood stabilizer therapy (lithium plus divalproex) in rapid-cycling presentations of bipolar I or II disorder and co-occurring substance use disorders. The similar time to relapse between treatment arms suggests a small effect size when combining divalproex with lithium. The high rate of dropout observed during the open-label stabilization phase suggests combination therapy with lithium and divalproex will be inadequate for the majority of patients with RCBD and comorbid substance abuse or dependence. Greater efforts are needed to identify adjunctive pharmacotherapy or psychosocial interventions that are effective in such highly comorbid populations.
Acknowledgments
Funding: Supported by NIH grants R01 MH-50165 to Dr. Calabrese, P20 MH-66054 to Drs. Calabrese and Findling, and in part by 1KL2RR024990 to Dr. Kemp.
None.
Footnotes
Previous Presentations:
Presented in part at the 45th annual meeting of the American College of Neuropsychopharmacology, Hollywood, Florida, December 3–7, 2006.
Disclosures:
Dr. Kemp has acted as a consultant to Abbott, Bristol-Myers Squibb, and Wyeth; and has received honoraria from Servier.
Dr. Gao has received grant support and/or honoraria from Abbott, AstraZeneca, and GlaxoSmithKline.
Dr. Rapport is a consultant to and/or on the advisory boards of Bristol-Myers Squibb, Forest, and Novartis; and is on the speaker’s bureaus of and receives honoraria from GlaxoSmithKline and Pfizer.
Dr. Elhaj has received honoraria from Abbott, AstraZeneca, and Pfizer
Dr. Findling has received research support, acted as a consultant, and/or served on a speaker’s bureau for Abbott, AstraZeneca, Bristol-Myers Squibb, Celltech-Medeva, Cypress Biosciences, Forest, GlaxoSmithKline, Johnson & Johnson, Eli Lilly, Neuropharm, New River, Novartis, Organon, Otsuka, Pfizer, Sanofi-Aventis, Serpacore, Shire, Solvay, Supernus Pharmaceuticals, Wyeth, and Validus.
Dr. Calabrese has received research support, acted as a consultant, and/or served on an advisory board for Abbott, AstraZeneca, Bristol-Myers Squibb, France Foundation, GlaxoSmithKline, Janssen, Johnson & Johnson, Lilly, Pfizer, Servier, and Solvay/Wyeth.
Dr. Ganocy, Ms. Bilali and Ms. Conroy have no relevant disclosures to report.
References
- 1.Kupka RW, Luckenbaugh DA, Post RM, Leverich GS, Nolen WA. Rapid and non-rapid cycling bipolar disorder: a meta-analysis of clinical studies. J Clin Psychiatry. 2003;64:1483–94. doi: 10.4088/jcp.v64n1213. [DOI] [PubMed] [Google Scholar]
- 2.Schneck CD, Miklowitz DJ, Calabrese JR, et al. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161:1902–8. doi: 10.1176/ajp.161.10.1902. [DOI] [PubMed] [Google Scholar]
- 3.Kupka RW, Luckenbaugh DA, Post RM, et al. Comparison of rapid-cycling and non-rapid-cycling bipolar disorder based on prospective mood ratings in 539 outpatients. Am J Psychiatry. 2005;162:1273–80. doi: 10.1176/appi.ajp.162.7.1273. [DOI] [PubMed] [Google Scholar]
- 4.Gao K, Bilali S, Conroy C, Ganocy S, Elhaj O, Calabrese JR. Clinical impacts of comorbid anxiety disorder and substance use disorder on patients with rapid cycling bipolar disorder [poster]. Presented at the 159th American Psychiatric Association Annual Meeting; May 20–25, 2006; Toronto, Canada. [Google Scholar]
- 5.Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–41. doi: 10.1111/j.1399-5618.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy F, Ahearn EP, Carroll BJ. Substance abuse in bipolar disorder. Bipolar Disord. 2001;3:181–8. [PubMed] [Google Scholar]
- 7.Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L. A history of substance abuse complicates remission from acute mania in bipolar disorder. J Clin Psychiatry. 1999;60:733–40. doi: 10.4088/jcp.v60n1103. [DOI] [PubMed] [Google Scholar]
- 8.Brady KT, Lydiard RB. Bipolar affective disorder and substance abuse. J Clin Psychopharmacol. 1992;12 (Suppl 1):17S–22S. doi: 10.1097/00004714-199202001-00004. [DOI] [PubMed] [Google Scholar]
- 9.Weiss RD, Ostacher MJ, Otto MW, et al. Does recovery from substance use disorder matter in patients with bipolar disorder? J Clin Psychiatry. 2005;66:730–5. doi: 10.4088/jcp.v66n0609. [DOI] [PubMed] [Google Scholar]
- 10.Swann A. Practical management of depressive and manic episodes. In: Garza-Trevino ES, editor. Medical Psychiatry: Theory and Practice. Teaneck, NJ: World Scientific; 1989. [Google Scholar]
- 11.Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;30:229–33. doi: 10.1001/archpsyc.1974.01760080077013. [DOI] [PubMed] [Google Scholar]
- 12.Tohen M, Greenfield SF, Weiss RD, Zarate CA, Jr, Vagge LM. The effect of comorbid substance use disorders on the course of bipolar disorder: a review. Harv Rev Psychiatry. 1998;6:133–41. doi: 10.3109/10673229809000321. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese JR, Woyshville MJ, Kimmel SE, Rapport DJ. Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacol. 1993;13:280–3. [PubMed] [Google Scholar]
- 14.Brady KT, Sonne SC, Anton R, Ballenger JC. Valproate in the treatment of acute bipolar affective episodes complicated by substance abuse: a pilot study. J Clin Psychiatry. 1995;56:118–21. [PubMed] [Google Scholar]
- 15.Tohen M, Waternaux CM, Tsuang MT, Hunt AT. Four-year follow-up of twenty-four first-episode manic patients. J Affect Disord. 1990;19:79–86. doi: 10.1016/0165-0327(90)90012-w. [DOI] [PubMed] [Google Scholar]
- 16.Solomon DA, Ryan CE, Keitner GI, et al. A pilot study of lithium carbonate plus divalproex sodium for the continuation and maintenance treatment of patients with bipolar I disorder. J Clin Psychiatry. 1997;58:95–9. doi: 10.4088/jcp.v58n0301. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152–61. doi: 10.1176/appi.ajp.162.11.2152. [DOI] [PubMed] [Google Scholar]
- 18.Brady KT, Sonne SC, Malcolm RJ, et al. Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol. 2002;10:276–85. doi: 10.1037//1064-1297.10.3.276. [DOI] [PubMed] [Google Scholar]
- 19.Geller B, Cooper TB, Watts HE, Cosby CM, Fox LW. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37:171–8. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2005;62:37–45. doi: 10.1001/archpsyc.62.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry. 1988;145:844–8. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33. [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Patient Version. 4. Washington, DC: American Psychiatric Press; 1997. Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 27.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 29.Keck PE, Jr, Calabrese JR, McQuade RD, et al. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry. 2006;67:626–37. doi: 10.4088/jcp.v67n0414. [DOI] [PubMed] [Google Scholar]
- 30.Tohen M, Calabrese JR, Sachs GS, et al. Randomized, placebo-controlled trial of olanzapine as maintenance therapy in patients with bipolar I disorder responding to acute treatment with olanzapine. Am J Psychiatry. 2006;163:247–256. doi: 10.1176/appi.ajp.163.2.247. [DOI] [PubMed] [Google Scholar]
- 31.Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. J Nerv Ment Dis. 1994;182:349–352. doi: 10.1097/00005053-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Salloum IM, Cornelius JR, Kelly TM, Douaihy A, Kirisci L, Daley DC. Patient characteristics and treatment implications of marijuana abuse among bipolar alcoholics: results from a double blind, placebo-controlled study. Addict Behav. 2005;30:1702–8. doi: 10.1016/j.addbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Coryell W, Solomon W, Turvey C, et al. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60:914–920. doi: 10.1001/archpsyc.60.9.914. [DOI] [PubMed] [Google Scholar]
- 34.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien CP, Charney DS, Lewis L, et al. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: A call to action. Biol Psychiatry. 2005;56:703–13. doi: 10.1016/j.biopsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Chengappa KR, Williams P. Barriers to the effective management of bipolar disorder: a survey of psychiatrists based in the UK and USA. Bipolar Disord. 2005;7 (Suppl 1):38–42. doi: 10.1111/j.1399-5618.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- 37.Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol. 2004;24:512–20. doi: 10.1097/01.jcp.0000138772.40515.70. [DOI] [PubMed] [Google Scholar]
- 38.Beresford TP, Clapp L, Martin B, et al. Aripiprazole in schizophrenia with cocaine dependence: a pilot study. J Clin Psychopharmacol. 2005;25:363–6. doi: 10.1097/01.jcp.0000169419.38899.5b. [DOI] [PubMed] [Google Scholar]
- 39.Smelson DA, Losonczy MF, Davis CW, et al. Risperidone decreases cravings and relapses in individuals with schizophrenia and cocaine dependence. Can J Psychiatry. 2002;47:671–675. doi: 10.1177/070674370204700710. [DOI] [PubMed] [Google Scholar]
- 40.Brown ES, Nejtek VA, Perantie DC, et al. Quetiapine in bipolar disorder and cocaine dependence. Bipolar Disord. 2002;4:406–11. doi: 10.1034/j.1399-5618.2002.02229.x. [DOI] [PubMed] [Google Scholar]