Abstract
Background
Adipose tissue dysfunction is an important feature of obesity characterized by enlarged adipocytes and marked changes in secretion of cytokines. These changes result in insulin resistance, chronic vascular inflammation, oxidative stress, and activation of the renin–angiotensin system (RAS), eventually leading to type 2 diabetes, obesity-related hypertension, and cardiovascular disease (CVD). Several trials have shown that bariatric surgery significantly reduces these comorbidities. However, there is a gap in knowledge regarding the mechanisms whereby bariatric surgery reduces the burden of CVD in obese individuals.
Method
Mesenchymal stem cells (MSCs) were isolated from adipose tissue collected from three groups: (1) nonobese control subjects, (2) obese subjects undergoing gastric bypass surgery (GBS), and (3) subjects 1 year or more after GBS. In the study, MSCs were induced to adipogenic differentiation, and RAS-related gene expressions were determined by quantitative polymerase chain reaction. The effect of angiotensin II (Ang II) on adipogenic differentiation of MSCs also was investigated.
Results
Angiotensinogen mRNA levels in MSCs and differentiated adipocytes were significantly higher in the obese group than in the nonobese control subjects. Renin mRNA levels were significantly higher in the obese group MSCs than in the nonobese and post-GBS groups. Angiotensin–converting enzyme mRNA levels were significantly lower in the MSCs derived from the post-GBS group than in the obese and nonobese control subjects. Serum Ang II levels were significantly lower in the post-GBS group (52.1 ± 4.2 pg/ml) than in the nonobese (85.4 ± 12.4 pg/ml) and obese (84.7 ± 10.0 pg/ml) groups. Ang II treatment inhibited adipogenesis of MSCs in a dose-dependent manner. The inhibitory effect of Ang II was mainly abolished by PD123319, a receptor 2 blocker.
Conclusions
The adipogenesis of MSCs is inhibited by Ang II treatment. Obese individuals are characterized by an upregulation of the RAS-related gene expressions in adipose tissue. This upregulation resolves in post-GBS subjects.
Keywords: Adipogenesis, Bariatric surgery, Cardiovascular disease, Gastric bypass, Mesenchymal stem cells, Renin-angiotensin system
Obesity is intimately linked to the occurrence of type 2 diabetes mellitus and cardiovascular diseases [1]. Excess fat stored in adipose tissue results in adipose tissue dysfunction [2]. Hypertrophy of adipocytes and marked changes in secretion of inflammatory cytokines are important features of adipocyte dysfunction. The resulting chronic inflammatory status is the pathogenic mechanism by which obesity causes endothelial dysfunction, insulin resistance, oxidative stress, and activation of the reninangiotensin system (RAS), eventually leading to type 2 diabetes mellitus, obesity-related hypertension, and cardiovascular disease (CVD) [3].
The formation of RAS components in human adipose tissue has been well described [4]. Angiotensin II (Ang II), the main effector of RAS components, is produced by the rate-limiting enzyme renin and angiotensin-converting enzyme (ACE) on the substrate angiotensinogen (AGT) [5]. The primary functions of RAS components produced in adipose tissue are to serve as a source of these components for circulating Ang II and to exert an autocrine/paracrine effect on adipose tissue [6]. Locally, Ang II not only represses differentiation of precursor cells in adipose tissue but also impairs fatty acid storage, resulting in the formation of poorly differentiated adipocytes. In mature adipocytes, Ang II inhibits lipolysis [7], causing cell hypertrophy.
All these effects contribute to the pathogenesis of insulin resistance, oxidative stress, and endothelial dysfunction. In addition, Ang II is a positive regulator of plasminogen activator inhibitor type 1 (PAI-1) expression in human adipocytes [8]. The elevated circulating levels of PAI-1 are a biochemical hallmark of obesity and likely contribute to the increased risk of atherothrombotic events in obese patients [9].
The endocrine function of RAS in human adipose tissue still is not fully understood, but several human studies have linked RAS dysfunction with obesity-related hypertension and insulin resistance [10]. A significant positive correlation between body mass index (BMI) and blood pressure levels with plasma AGT concentration has been reported [11]. In the treatment of hypertension, ACE-inhibitors are one of the first-line medications used, and blockade of the RAS is used to reduce the incidence of new-onset type 2 diabetes mellitus among high-risk patients [12].
An earlier study showed that significant weight loss decreased the level of circulating AGT, renin and ACE activity, and AGT expression in adipose tissue [13]. Several trials have demonstrated that bariatric surgery significantly resolves hypertension, resulting in an overall reduction of the risk for CVD [14, 15]. However, there is a gap in knowledge about the molecular mechanisms whereby bariatric surgery reduces the burden of CVD for obese individuals, and to date, there are no data about the effect of bariatric surgery on RAS expression in adipose tissue.
Therefore, the current study aimed to investigate the effect of surgically induced weight loss on the expression of RAS-related genes in adipose-derived MSCs and differentiated adipocytes and the effect of Ang II on the adipose adipogenic differentiation of MSCs.
Materials and methods
Subjects
The study enrolled 45 subjects, who were assigned to three different groups: (1) nonobese control subjects (BMI ≥ 30), (2) obese subjects (BMI C 35), and (3) post–Roux-en-Y gastric bypass surgery (GBS) subjects with more than 1 year of postoperative follow-up evaluation. In the study, BMI was calculated as weight in kilograms divided by the square of height in meters. Plasma samples were stored at −80°C until their analysis. The concentration of Ang II was determined by Ang II Human ELISA kit (Abcam, Cambridge, MA, USA) according the manufacturer’s instructions. The insulin level was determined by radioimmunoassay, and the glucose concentration was determined by Clinical Chemistry Glucose/HK (Roche Diagnostic, Indianapolis, IN, USA). The Homeostasis Model Assessment (HOMA) index was determined as previously described [16]. The study was approved by the Institutional Review Board at Duke University, and informed written consent was obtained from all subjects before their participation.
Isolation of MSCs
For this study, MSCs were isolated from abdominal subcutaneous adipose tissue biopsies collected in a subset of the entire cohort according to the following group distribution: group 1 (nonobese control subjects: BMI, 28.2 ± 1.1 kg/m2; n = 11), group 2 (obese subjects undergoing GBS: BMI, 50.6 ± 9.0 kg/m2; n = 5), group 3 (post-GBS subjects with an average follow-up period of 19 months after the procedure: BMI, 30.0 ± 0 kg/m2; n = 3).
Briefly, subcutaneous adipose tissue biopsies were washed in Hank’s balanced salt solution (HBSS) three times, and the tissue was digested for 1 h at 37 °C in HBSS with collagenase. Adipocytes were separated from stromal vascular cells after centrifugation at 4009g for 10 min and removed by aspiration. Erythrocytes were removed by resuspension of stromal vascular cell pellets in lysis buffer (2.06 mg/ml Tris base, pH 7.2, 7.49 mg/ml NH4Cl) for 10 min.
After centrifugation, the pellets were resuspended in HBSS containing 2 % fetal bovine serum (FBS) and filtered through a 70- and 40-μm sieves. The cells were incubated with biotin-conjugated mouse antihuman CD31, CD45, and CD11b monoclonal antibodies (Invitrogen, Carlsbad, CA, USA). The positive cells were removed by paramagnetic beads (MACS; Miltenyi Biotech, Cambridge, MA). CD31, The CD45 and CD11b negative stem cells were collected and expanded in growth medium [Dulbecco modified Eagle medium (DMEM)-F12 supplemented with 10 % FBS and antibiotics].
Adipogenic differentiation of MSCs and treatment
The MSCs were cultured to confluence before differentiation in a 24-well plate. For adipogenesis, the cells cultured in 1 ml of DMEM/F-12 with 10 % FBS were stimulated with a differentiation cocktail, which included 0.5 mmol/l 1-methyl-3 isobutylxanthine (IBMX), 1 lmol/l dexamethasone, 10 lg/ml human insulin, 5 lmol/l troglitazone, 33 lmol/l biotin, and 170 mmol/l pantothenate (all reagents from Sigma, St. Louis, MO, USA) for 1 week [17]. Then 0.8 ml of a maintaining cocktail that included 1 lmol/l dexamethasone and 10 lg/ml insulin was replaced every 3 days. During differentiation, 1 lmol/l Ang II, Ang II in combination with Ang II receptor type 1 (AT1) blocker, 10 lmol/l losartan or 10 lmol/l telmisartan and/or Ang II receptor type 2 (AT2) blocker, and 10 lmol/l PD123319 were added. For PAI-1 expression in adipocytes, differentiated cells were starved for 24 h and exposed to 0, 0.5, and 1 lmol/l of Ang II for 16 h. The cells were washed with cold PBS, then harvested into TRIzol solution (Qiagen, CA).
Oil Red-O staining
Differentiated cells were washed with PBS three times and fixed by 4 % formaldehyde for 45 min using 0.3 % Oil Red O stock (in isopropyl alcohol) diluted with water (3:2) to prepare an Oil Red O working solution. The cells were incubated with the filtered fresh solution for 5 min at room temperature and visualized under light microscope (Leica, Wetzlar, Germany).
Immunoblot analysis
Whole-cell lysates were prepared using RIPA lysis buffer supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN, USA). The lysates were resolved on 4–12 % Bis-Tris–HCl-buffered polyacrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes. The membranes were blocked for 1 h with 5 % nonfat milk in Tris-Tween Buffered Saline (TTBS) buffer at room temperature. Anti-fatty acid-binding protein (1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was blotted with membranes overnight at 4 °C. Horseradish peroxidase-linked secondary antibodies were detected and visualized by ECL Advance (GE Healthcare BioSciences, Piscataway, NJ, USA) on Versadoc 5000 (Bio-Rad, Hercules, CA, USA). The membrane then was stripped (ReBlot, Billerica, MA, USA) and re-probed with anti-glyceraldehyde phosphate dehydrogenase (GAPDH) antibody (1:10,000; Abcam).
RNA extraction and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Using the Rneasy Lipid Tissue Kit (Qiagen, Valencia, CA), RNA was extracted from both the MSCs and the differentiated adipocytes. The Rnase-Free Dnase Set (Qiagen, Valencia, CA) was used to remove the genomic DNA, and cDNA synthesis was carried out by SuperScript III Reverse Transcriptase (Invitrogen) and analyzed by quantitative realtime PCR (MyIQ Single Color System, Bio-Rad, Hercules, CA). The expression level was normalized to GAPDH, and the data were processed by Gene Expression Analysis Software (Bio-Rad Life Science, Hercules, CA, USA).
Human RAS-related gene quantitative PCR primers were adopted from Janke et al. [18] for AGT, renin, and ACE. Primers for GAPDH (accession no. NM_002046) were designed by Beacon Designer (Premier Biosoft International, Palo Alto, CA) for use as a control condition, and the sequences were forward 5′CCCATGTTCGTCAT GGGTGT3′ and reverse 5′TGGTCATGAGTCCTTCCAC GATA3′. The specificity of the amplification product was confirmed by a melting curve profile and agarose gel electrophoresis.
Statistical analysis
Values are presented as the mean ± standard deviation unless otherwise indicated. Data were analyzed by GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Student’s t test and one-way analysis of variance (ANOVA) were used for group comparisons as appropriate. Statistical significance was assumed for p values lower than 0.05.
Results
Subjects’ characteristics and plasma Ang II concentration
As shown in Table 1, the subjects who completed GBS and the nonobese control subjects had similar levels of insulin sensitivity expressed as HOMA index. As expected, obese individuals undergoing GBS had significant insulin resistance, as demonstrated by their higher HOMA indexes (p < 0.05 vs nonobese and post-GB groups). It is striking that plasma Ang II levels were significantly lower (p < 0.05) in the post-GBS group (52.1 ± 4.2 pg/ml) than in the nonobese (85.4 ± 12.4 pg/ml) and obese (84.7 ± 10 pg/ml) groups.
Table 1.
Subjects’ characteristics and plasma angiotensin (Ang II) concentration analysis
| Variable | Nonobese | Obese | Post-GBS |
|---|---|---|---|
| n | 12 | 24 | 9 |
| Age (years) | 46.1 ± 14.2 | 44.8 ± 10.8 | 39.0 ± 10.5 |
| BMI (kg/m2) | 26.9 ± 2.6 | 48.9 ± 10.4a | 29.1 ± 7.8 |
| HOMA-IR | 3.7 ± 1.5 | 6.9 ± 5.2a | 3.6 ± 2.9 |
| Ang II (pg/ml) | 85.4 ± 24.7 | 84.7 ± 28.3 | 52.1 ± 8.3b |
BMI body mass index, HOMA-IR Homeostasis Model Assessment-Insulin Resistance
p<0.05, obese versus nonobese control subjects and post-gastric bypass surgery (GBS) patients
p<0.05, post-gastric bypass surgery (GBS) patients vs obese and nonobese control subject
RAS-related gene expression during adipogenic differentiation of MSCs
Expressions of RAS-related gene were determined in both MSCs and differentiated mature adipocytes. As shown in Fig. 1a, AGT mRNA levels in MSCs and adipocytes were significantly higher in the obese group than in the nonobese control subjects (p < 0.05). Renin mRNA levels were significantly higher in obese group MSCs than in the nonobese and post-GBS groups (p < 0.05) (Fig. 1b). However, MSCs obtained from post-GBS subjects still had a higher level of AGT and renin expression than those obtained from nonobese control subjects (p < 0.05). Interestingly, ACE mRNA levels were significantly lower in the MSCs derived from the post-GBS group than in those obtained from the obese and nonobese control subjects (p < 0.05). The ACE mRNA levels were significantly downregulated in differentiated adipocytes of the post-GBS subjects compared with those of the nonobese control subjects (p < 0.05) (Fig. 1c). These results show that GBS significantly decreased ATG, renin, and ACE expressions in MSCs and ACE expression in differentiated adipocytes.
Fig. 1.
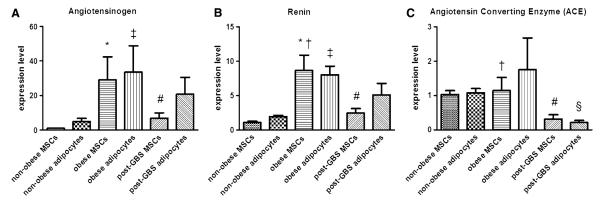
Renin–angiotensin system (RAS)-related gene expression during adipogenesis of mesenchymal stem cells (MSCs). Adipogenic differentiation was performed for MSCs isolated from adipose tissue collected from nonobese, obese, and post-gastric bypass surgery (GBS) groups. The total RNA was extracted from the MSCs and the differentiated adipocytes. Measurement of RAS-related gene expression was performed by quantitative polymerase chain reaction (PCR). a Angiotensinogen (AGT). b Renin. c Angiotensin-converting enzyme (ACE). Data are expressed as mean ± standard error of the mean (SEM). *p < 0.05, nonobese versus obese in MSCs. #p < 0.05, nonobese versus post-GBS in MSCs. †p < 0.05, obese versus post-GBS in MSCs. ‡p < 0.05, nonobese versus obese in adipocytes. §p < 0.05, nonobese versus post-GBS in adipocytes
Adipogenic differentiation of MSCs inhibited by Ang II
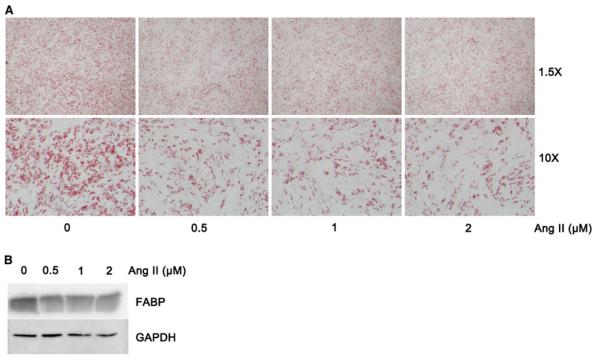
Adipose-derived MSCs from nonobese control subcutaneous adipose tissue were subjected to adipogenesis and exposed to different concentrations of Ang II for 13 days. The adipogenic differentiation of MSCs was significantly inhibited by Ang II in a dose-dependent manner. As shown in Fig. 2a and b, the lipid drop formation exhibited by Oil Red O staining and the adipose differentiation specific marker (FABP) detected by Western blot were significantly decreased in Ang II-treated cells compared with the vehicle control. Ang II inhibited MSCs adipogenic differentiation predominantly through the angiotensin (AT2) receptor.
Fig. 2.
Angiotensin II (Ang II)-inhibited adipogenic differentiation of adipose mesenchymal stem cells (MSCs). For 13 days, MSCs isolated from nonobese control subcutaneous adipose tissue were subject to adipogenic differentiation and exposed to 0, 0.5, 1, 2 lmol/l of Ang II. a Cellular lipid drop was stained by Oil Red O. b Differentiated MSCs were harvested, and western blot was used to detect fatty acidbinding protein (FABP), the adipose differentiation-specific marker
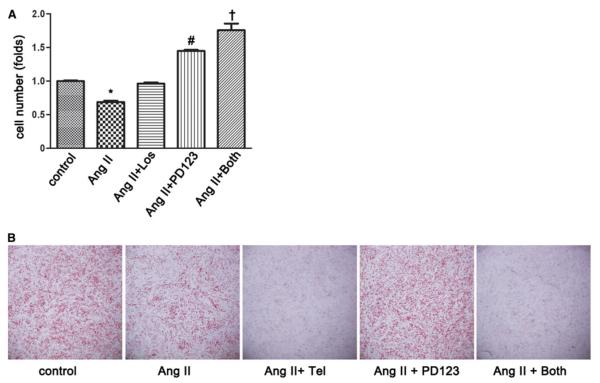
Next, we determined which Ang II receptors are responsible for the anti-adipogenenic effects of Ang II. For this purpose, MSCs were induced to adipogenic differentiation and treated with Ang II in the presence or absence of the AT1 receptor blockers losartan and telmisartan and the AT2 receptor-blocker PD123319. Cellular lipids content was stained by Oil Red O, and the differentiated adipocytes were counted. As shown in Fig. 3a, the inhibitory effect of Ang II on the adipogenesis of MSCs was abolished by the AT1 receptor-blocker losartan. However, the adipogenic differentiation of MSCs was significantly increased when cells were treated with Ang II and AT2 receptor-blocker (PD123319) compared with the vehicle control (p < 0.05). Moreover, blocking of both Ang II receptors further enhanced differentiation of MSCs (p < 0.05), suggesting a significant role for endogenously produced Ang II as a vigorous restrainer of MSCs’ differentiation. Unexpectedly, telmisartan dominantly enhanced the inhibitory effect of Ang II on the adipogenic differentiation of MSCs, showing an inhibitory effect independent of the AT1 receptor blockade (Fig. 3b) and likely secondary to its known Peroxisome proliferator-activated receptor (PPAR) γ-modulating activity [19].
Fig. 3.
Exogenous angiotensin II (Ang II) receptor blockers on adipogenesis of mesenchymal stem cells (MSCs). The MSCs were isolated from adipose tissue collected from nonobese individuals and subjected to adipogenic differentiation exposure to Ang II combined with the indicated angiotensin receptor blockers losartan and PD123319 (panel A) and telmisartan and PD123319 (panel B). Cellular lipids content was stained by Oil Red O, and the differentiated adipocytes were counted. *p < 0.05, Ang II versus all others. #p < 0.05, Ang II? PD123 versus control, Ang II, and Ang II? PD123. †p < 0.05, Ang II? both versus all others. Tel temisartan, los losartan, PD123 PD123319
Ang II effects on PAI-1 expression in mature adipocytes
We further investigated the effect of Ang II on PAI-1 gene expression. Adipose-derived MSCs from nonobese control subcutaneous adipose tissue were subjected to adipogenic differentiation for 13 days. The differentiated cells were starved and exposed to Ang II for 16 h. The quantitative PCR results showed that untreated adipocytes have significantly lower PAI-1 expression levels than cells treated with Ang II (p < 0.05) (Fig. 4).
Fig. 4.
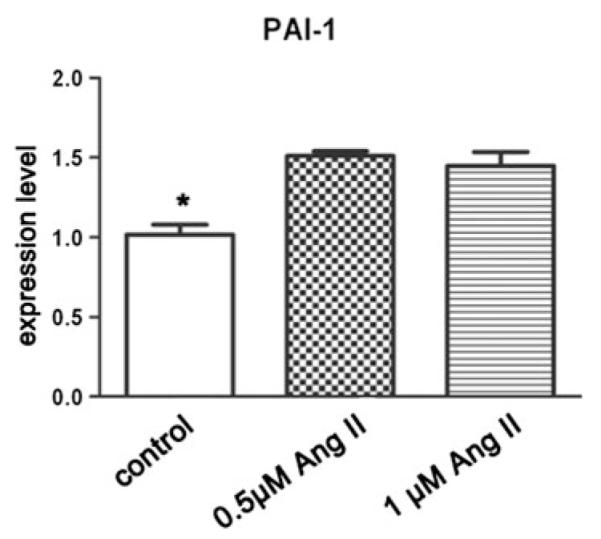
Angiotensin II (Ang II) effect on plasminogen activator inhibitor type 1 (PAI-1) expression in mature adipocytes. Adipose-derived mesenchymal stem cells (MSCs) from nonobese subcutaneous adipose tissue were subjected to adipogenic differentiation for 13 days. Differentiated cells were starved for 24 h and exposed to Ang II for 16 h. Cells washed with cold phosphate-buffered saline (PBS) then were harvested into TRIzol solution. Quantitative polymerase chain reaction (PCR) was used to measure the PAI-1 mRNA level. *p < 0.05, control subjects versus all others Table 1 Subjects’ characteristics and plasma angiotensin (Ang II) concentration analysis
Discussion
Our study demonstrated that AGT mRNA levels in MSCs and differentiated adipocytes were significantly higher in the obese group than in the nonobese control subjects. Renin mRNA levels also were significantly higher in MSCs derived from the obese group than in the nonobese and post-GBS groups. The ACE mRNA levels were significantly lower in the MSCs derived from the post-GBS group than in the obese and nonobese control subjects. Accordingly, circulating levels of Ang II were significantly decreased in the post-GBS group. Furthermore, Ang II dose-dependently inhibited adipogenesis of MSCs mainly through the AT2 receptor and upregulated PAI-1 expression in differentiated adipocytes.
The increase in adipose mass observed in obesity includes a process of hyperplasia that requires a population of pluripotent stem cells to be recruited from the vascular stroma of adipose tissue [20]. Therefore, using this niche of stem cells to support more cell differentiation is essential when there is a caloric surplus need for urgent storage of lipids.
One of the mechanisms responsible for insulin resistance derives from the storage of impaired fatty acids in adipose tissue, which facilitates overflow of lipids to ectopic storage such as liver and muscle [21-23]. The RAS components produced in the adipose tissue exhibit an autocrine/paracrine effect that modulates the differentiation of stem cells and the lypolysis of mature adipocytes, ultimately affecting fatty acids storage. We demonstrated that the expression levels of renin and AGT were significantly higher in MSCs and adipocytes of obese subjects than in those of nonobese control subjects. These findings are consistent with previously reported data suggesting that RAS is upregulated and that high levels of Ang II are produced in obesity [13].
Although MSCs are a major source of adipocyte generation, very few studies have determined the role of RAS in differentiation of MSCs from adipose tissue [24, 25]. Moreover, data are controversial, especially regarding the effect of Ang II on adipogenesis.
In this study, we used adipose-derived MSCs and confirmed that exogenous Ang II inhibits the differentiation of adipose stem cells, as previously shown by Matsushita et al. [24]. Instead, Saint-Marc et al. [25] proposed that Ang II enhances the formation of GPDH-expressing cells from preadipocytes in response to prostacyclin released from adipocytes. These differences are likely due to the use of a different precursor cell line and differentiation medium.
Clinical and experimental evidence suggests that the upregulation of RAS plays a significant role in metabolic syndrome. According to our published data and those of others, the upregulation of adipose RAS in the obese can inhibit the adipogenic differentiation of the adiposederived MSCs, resulting in the formation of poorly differentiated adipocytes with impaired fatty acid storage. Simultaneously the autocrine/paracrine action of Ang II inhibiting the lipolysis of the adipocytes allows the formation of enlarged mature adipocytes [7]. In fact, the copresence of poorly differentiated adipocytes and hypertrophic mature adipocytes is the pathologic hallmark of adipose tissue dysfunction in metabolic syndrome.
Our results clearly indicate that after GBS, the niche for the adipogenesis of MSCs is characterized by a diminished production of endogenous Ang II. This significant change allows correction of the adipose tissue dysfunction, resulting in amelioration/resolution of insulin resistance.
The pathogenesis of obesity-related hypertension is complicated, and the mechanisms may involve insulin resistance, sodium retention, increased sympathetic nervous system activity, activation of renin-angiotensin-aldosterone, and altered vascular function [26]. Patients undergoing bariatric surgery experience remarkable improvements in systemic chronic inflammation, insulin sensitivity, lipids profile, and glycemic control. Longitudinal data show that GBS significantly lowers the rate of high blood pressure [27]. The circulating RAS is the major determinant of blood pressure regulation.
In our study, circulating Ang II data were consistent with a previous report that levels of plasma Ang II did not differ between lean and obese subjects [10]. However, in the post-GBS individuals, circulating Ang II levels were lower than in the obese and nonobese control subjects. This novel finding is striking, especially when coupled with the significant changes in the RAS-related gene expression observed in the adipose tissue of the post-GBS subjects. These data are supported by a previous report showing that significant weight loss reduced the level of circulating AGT, renin and ACE activity, and AGT expression in adipose tissue [13]. We further confirmed that Ang II increased PAI-1 expression in differentiated mature adipocytes. Elevated plasma levels of PAI-1 are a biochemical hallmark of obesity and likely contribute to the increased risk of atherothrombotic events experienced by obese patients [11].
Overall, our data suggest that bariatric surgery induces significant changes in the adipose tissue RAS-related gene profile that may result in a noteworthy reduction of risk for CVD events. However, our study had some limitations including the small population of MSCs isolated from post-GBS patients and a study design not aimed at correlating changes in the adipose RAS components with levels of blood pressure. These limitations clearly warrant further study.
In summary, obesity is characterized by an upregulation of RAS-related gene expressions in adipose tissue. This upregulation resolves after GBS, suggesting that this change is one of the mechanisms whereby bariatric surgery reduces the risk of CVD for obese individuals.
Acknowledgments
The study was supported by a SAGES Research Grant Award and an NIH K23 DK07597 award to A.T. The authors thank all the participants in this study.
Footnotes
Disclosures
Jie-Gen Chen, Anna Spagnoli, and Alfonso Torquati have no conflicts of interest or financial ties to disclose.
This paper was presented at the SAGES 2012 Annual Meeting, March 7–10, 2012, San Diego, CA
Contributor Information
Jie-Gen Chen, Department of Surgery, Duke University Medical Center, 407 Crutchfield St, Durham, NC 27704, USA.
Anna Spagnoli, Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Alfonso Torquati, Department of Surgery, Duke University Medical Center, 407 Crutchfield St, Durham, NC 27704, USA.
References
- 1.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 2.Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol. 2010;323:20–34. doi: 10.1016/j.mce.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 5.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens GH, Blaak EE, Arner P, Saris WH, van Baak MA. Angiotensin II: a hormone that affects lipid metabolism in adipose tissue. Int J Obes Lond. 2007;31:382–384. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- 8.Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28:1357–1364. doi: 10.1038/sj.ijo.0802778. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan DE. PAI-1 antagonists: the promise and the peril. Trans Am Clin Climatol Assoc. 2011;122:312–325. [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens GH, Jocken JW, Blaak EE, Schiffers PM, Saris WH, van Baak MA. Endocrine role of the renin-angiotensin system in human adipose tissue and muscle: effect of beta-adrenergic stimulation. Hypertension. 2007;49:542–547. doi: 10.1161/01.HYP.0000256091.55393.92. [DOI] [PubMed] [Google Scholar]
- 11.Schorr U, Blaschke K, Turan S, Distler A, Sharma AM. Relationship between angiotensinogen, leptin, and blood pressure levels in young normotensive men. J Hypertens. 1998;16:1475–1480. doi: 10.1097/00004872-199816100-00011. [DOI] [PubMed] [Google Scholar]
- 12.Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- 13.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angio-tensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 14.Torquati A, Wright K, Melvin W, Richards W. Effect of gastric bypass operation on Framingham and actual risk of cardiovascular events in class II to III obesity. J Am Coll Surg. 2007;204:776–782. doi: 10.1016/j.jamcollsurg.2006.12.038. discussion 782-773. [DOI] [PubMed] [Google Scholar]
- 15.Heneghan HM, Meron-Eldar S, Brethauer SA, Schauer PR, Young JB. Effect of bariatric surgery on cardiovascular risk profile. Am J Cardiol. 2011;108:1499–1507. doi: 10.1016/j.amjcard.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.DeLany JP, Floyd ZE, Zvonic S, Smith A, Gravois A, Reiners E, Wu X, Kilroy G, Lefevre M, Gimble JM. Proteomic analysis of primary cultures of human adipose-derived stem cells: modulation by adipogenesis. Mol Cell Proteomics. 2005;4:731–740. doi: 10.1074/mcp.M400198-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human pre-adipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 19.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 20.Lane MD, Tang QQ. From multipotent stem cell to adi-pocyte. Birth Defects Res A Clin Mol Teratol. 2005;73:476–477. doi: 10.1002/bdra.20150. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 22.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 23.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita K, Wu Y, Okamoto Y, Pratt RE, Dzau VJ. Local renin-angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension. 2006;48:1095–1102. doi: 10.1161/01.HYP.0000248211.82232.a7. [DOI] [PubMed] [Google Scholar]
- 25.Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Angiotensin II as a trophic factor of white adipose tissue: stimulation of adipose cell formation. Endocrinology. 2001;142:487–492. doi: 10.1210/endo.142.1.7883. [DOI] [PubMed] [Google Scholar]
- 26.Rocchini AP. Obesity hypertension. Am J Hypertens. 2002;15:50S–52S. doi: 10.1016/s0895-7061(01)02299-3. [DOI] [PubMed] [Google Scholar]
- 27.Dallal RM, Hatalski A, Trang A, Chernoff A. Longitudinal analysis of cardiovascular parameters after gastric bypass surgery. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.09.020. doi:10.1016/j.soard.2011.09.020. [DOI] [PubMed] [Google Scholar]