Abstract
Background
Brain imaging studies have revealed abnormal function in the prefrontal cortex (PFC) of alcoholics that may contribute to the impulsive behavior and lack of control over drinking that characterizes this disorder. Understanding how ethanol affects the physiology of PFC neurons may help explain this loss of control and lead to better treatments for alcohol addiction. In a previous study from this laboratory, we showed that ethanol inhibits complex patterns of persistent activity (known as “up-states”) in medial PFC (mPFC) neurons in a reversible and concentration-dependent manner.
Methods
In the current study, whole-cell patch clamp recordings were used to directly examine the effects of ethanol on the glutamatergic and GABAergic components that underlie persistent activity.
Results
In deep-layer mPFC pyramidal neurons, ethanol reversibly attenuated electrically evoked N-methyl-d-aspartate-type glutamate receptor (NMDAR)-mediated EPSCs. Significant inhibition was observed at concentrations as low as 22 mM, equivalent to a blood ethanol concentration (0.1%) typically associated with legal limits for intoxication. In contrast to NMDA responses, neither evoked nor spontaneous EPSCs mediated by α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-type glutamate receptor were affected by ethanol at concentrations as high as 88 mM, a concentration that can be fatal to non-tolerant individuals. At similar concentrations, ethanol also had little effect on spontaneous or evoked IPSCs mediated by a-type γ-amino-butyric acid receptor. Finally, mPFC neurons showed little evidence of GABAR-mediated tonic current and this was unaffected by ethanol.
Conclusions
Together, these results suggest that NMDAR-mediated processes in the mPFC may be particularly susceptible to disruption following the acute ingestion of ethanol.
Keywords: Alcohol, Addiction, GABA, AMPA, Electrophysiology
Despite years of research and education about the adverse effects of excessive drinking, alcohol abuse and alcoholism remain leading causes of premature death and morbidity in the U.S. and other countries. Acutely, ethanol slows reaction time, leading to reduced motor control, and impairs perception and judgment regarding the outcome of potentially risky behaviors. Deficits in cognitive processing and higher order functions may persist in individuals that are alcohol-dependent or those who have undergone multiple cycles of dependence and withdrawal. These changes have been documented by brain imaging and behavioral studies and suggest that areas of the prefrontal cortex (PFC) in particular may be especially susceptible to alcohol-induced damage (for review, see Moselhy et al., 2001). Reduced prefrontal function following long-term alcohol use may contribute to the loss of control over drinking that is a hallmark of alcoholism (Goldman-Rakic, 1999; Kalivas et al., 2005).
While imaging and behavioral studies have identified the PFC as an ethanol-sensitive brain region, relatively little is known regarding the mechanisms by which these changes take place. Among the various ethanol-sensitive cellular processes that have been investigated to date, the large family of ligand-gated ion channels appears to be a particularly relevant target for many of the acute actions of ethanol. Several reports have demonstrated that ion flux through both native and recombinant channels gated by various neurotransmitters is altered by concentrations of ethanol associated with behavioral signs of intoxication. Most of these studies have focused on brain regions associated with reward or learning and memory pathways and have identified alcohol sensitive conductances in regions such as the nucleus accumbens (Nie et al., 1994), hippocampus (Lovinger et al., 1990), and amygdala (Roberto et al., 2004). The PFC, which exerts modulatory control over many of these regions, has received relatively little attention as an important site of action for alcohol.
In a recent study from this laboratory, we reported that ethanol inhibited persistent network activity in the PFC both in vivo and in a novel slice culture system (Tu et al., 2007). Persistent activity is characterized by spontaneous membrane depolarizations, termed “up-states”, that are driven by synaptic activity. These “up-states” involve both glutamatergic and GABAergic ion channels that have previously been shown to be sensitive to behaviorally relevant concentrations of ethanol (Seamans et al., 2003). Ethanol was shown to reduce both the amplitude and duration of up-states in PFC neurons, suggesting that conductances that underlie the onset and duration of up-states are sensitive to ethanol (Tu et al., 2007).
In the present study, we examined the effects of ethanol on pharmacologically isolated glutamatergic and GABAergic responses in acutely dissected slices of rat PFC. The results of these studies reveal that N-methyl d-aspartate-type glutamate receptor (NMDAR)-mediated events appear especially sensitive to ethanol while those generated by α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid-type glutamate receptor (AMPARs) or a-type γ-aminobutyric acid receptor (GABAARs) are relatively resistant. These findings provide additional evidence to support the hypothesis that the PFC is an important target for the actions of ethanol.
MATERIALS AND METHODS
Preparation of Brain Slices
All procedures were carried out according to MUSC Institutional Animal Care and Use Committee protocols. Male (22- to 25-day-old) Sprague–Dawley rats were deeply anesthetized with isoflurane and decapitated. The brains were quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) sucrose (200), KCl (1.9), Na2HPO4 (1.2), MgCl2 (6), CaCl2 (0.5), ascorbate (0.4), glucose (10), and NaHCO3 (25). Coronal slices (350 μm) containing PFC were prepared using a Leica VT1000S vibratome. Slices were placed in holding chambers containing warmed (30 to 32°C) ACSF containing (in mM) NaCl (125), KCl (2.5), Na2HPO4 (1.25), MgCl2 (1.3), CaCl2 (2.0), ascorbate (0.4), glucose (10), and NaHCO3 (25). Following 30 to 60 minutes of incubation, slices were maintained at room temperature for a minimum of 30 minutes until use.
Electrophysiology
Whole-cell recordings were carried out using an Axon MultiClamp 700B amplifier (Molecular Devices, Union City, CA). Pyramidal neurons from deep layers of the prelimbic cortex were identified using an Olympus BX51WI microscope equipped with infrared Dodt gradient contrast imaging (Luigs and Neumann, Ratingen, Germany). Experiments were conducted at 25 to 28°C and temperature was controlled by in-line and bath heaters (Warner Instruments, Hamden, CT). Neurons were voltage-clamped (–60 to –70 mV) using borosilicate glass recording microelectrodes (3 to 5 MΩ). For NMDAR- and AMPAR-mediated currents, the pipette filling solution contained (in mM) K-gluconate (130), KCl (10), HEPES (10), MgCl2 (2), EGTA (1), Na2ATP (2), and NaGTP (0.3). For GABAR-mediated currents, K-Gluconate and KCl were replaced with CsCl (140 mM). QX-314 (1 mM) was added to the internal solution when recording GABAAR-mediated currents to block GABABR-mediated transmission in the recorded neuron (Nathan et al., 1990). Series resistance (Rs) was ~10 to 30 MΩ and was monitored throughout the experiment by applying a small hyperpolarizing voltage pulse. Experiments were discontinued if the Rs changed by more than 25% over the course of the recording.
NMDAR-mediated currents were recorded in a modified ACSF (0.1 mM MgCl2, 3.2 mM CaCl2) supplemented with picrotoxin (100 μM) and NBQX (10 μM) to block GABAA and AMPA receptors, respectively. To record AMPAR-mediated currents, (R,S)-amino-5-phosphonovaleric acid (DL-APV) (100 μM) and picrotoxin (100 μM) were added to normal ACSF while GABAR-mediated currents were measured in ACSF containing DL-APV (100 μM) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) (10 μM). In some cases, (2S)-3-[[(1S)-1-(3,4-dichloro-phenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid (CGP-55845) (1 μM) was added to the recording solution to inhibit all GABABR-mediated transmission. Miniature and spontaneous GABAAR- and AMPAR-mediated events were recorded in normal ACSF containing DL-APV (100 μM) and either NBQX (10 μM) or picrotoxin (100 μM), respectively. GABAR-mediated tonic current (in the presence of APV and NBQX) was assessed by monitoring the change in the average holding current following application of bicuculline or ethanol.
Three types of stimulating electrodes were used to evoke currents: concentric wire electrodes, formvar-coated nichrome wire electrodes, and theta glass electrodes. Theta glass electrodes (1 mm diameter glass pulled to a tip with a ~1 to 5 μM diameter) were placed adjacent to the recorded cell and used exclusively when evoking AMPAR-mediated EPSCs. This was done because they produced a consistent monosynaptic response without the large uncontrolled currents often observed when using wire electrodes placed either in the deep or superficial layers of the prelimbic cortex. In all cases, stimulus pulses were delivered at a rate of 0.05 Hz, ranged from 10 to 1000 μA in intensity, and elicited a reliable, submaximal response from the recorded neuron.
Statistical Analysis
Data were acquired using an ITC-18 digital interface controlled by AxographX software (Axograph Scientific, New South Wales, Australia) running on a G4 Macintosh computer. Current amplitudes and areas were measured relative to the pre-stimulus baseline value and are expressed as means ± SEM. For miniature and spontaneous events, recordings were filtered at 4 kHz and acquired at 10 kHz. Individual events were detected and analyzed using a sliding template algorithm in AxographX software (Axograph Scientific, Sydney, New South Wales, Australia). Individual events (200 to 500) were averaged and used to generate measurements of amplitude, frequency, rise time and decay time.
Statistical analysis of data was carried out using Prism (GraphPad Software, San Diego, CA) and SAS software (Cary, NC). For analysis of evoked synaptic currents, log-transformed data were analyzed as a mixed model (SAS PROC MIXED) with an autoregressive covariance matrix and individual time-points nested within each time interval (i.e. “baseline”, “in ethanol” and “washout”). This analysis is similar to ANOVA, but has less stringent assumptions and is insensitive to non-informative missing data, making it ideal for analyzing experiments that were discontinued prematurely due to a disruption in the cell patch or an unacceptable increase in series resistance (Little and Rubin, 2002).
RESULTS
Ethanol-Sensitivity of NMDAR-Mediated Currents in the PFC
NMDAR-mediated EPSCs were elicited in deep-layer pyramidal neurons in acute slices isolated from rat PFC. To facilitate detection of NMDAR-mediated responses, slices were bathed in ACSF containing 0.1 mM magnesium supplemented with 100 μM picrotoxin and 10 μM NBQX to block GABAARs and AMPARs, respectively. Neurons were voltage-clamped at –70 mV and NMDA EPSCs were evoked (eEPSCs) by local stimulation at a frequency of 0.05 Hz (Fig. 1A). In some experiments, to confirm that this response was NMDAR-mediated, DL-APV (100 μM) was applied to eliminate the remaining current (data not shown). Following 5 minutes of stable baseline activity, the ACSF was switched to one containing various concentrations of ethanol (11 to 66 mM) for 10 minutes followed by a washout period.
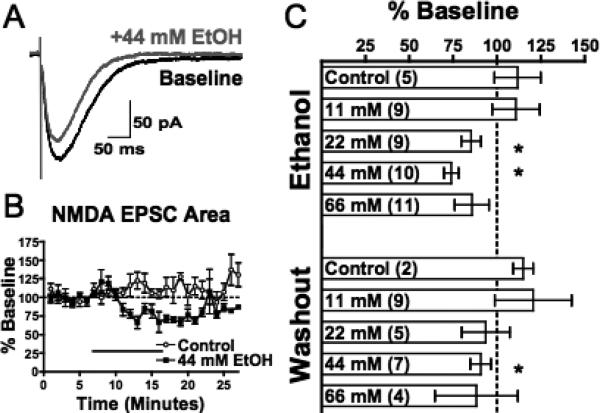
Fig. 1.
Acute ethanol attenuates evoked NMDAR-mediated EPSCs (eEPSCs) in mPFC pyramidal neurons. (A) Representative averaged traces of NMDA eEPSCs before (black) and during (gray) application of acute ethanol (44 mM). (B) Normalized timecourse of the NMDA eEPSC area in response to sham solution exchange (open circles) or 44 mM ethanol application (black squares). Black bar indicates period of solution exchange. (C) Normalized response of NMDA eEPSC area during ethanol exposure (top) and following 10 to 15 minutes of ethanol washout (bottom). Values are expressed as averages ± SEM. *p < 0.001 compared with baseline values.
As shown in Fig. 1B and C, deep-layer PFC neurons displayed stable NMDA eEPSCs over the recording period. Perfusion of slices with 11 mM ethanol (approximately 0.05% blood alcohol concentration) had no significant effect on NMDA responses (Fig. 1C). However, in the presence of higher concentrations of ethanol (22 and 44 mM), the area of NMDA eEPSCs was significantly reduced (22 mM: 85.5 ± 5.7%, p < 0.0001; Fig. 1C; 44 mM: 74.1 ± 4.2%, p < 0.0001; Fig. 1B, C). At 66 mM ethanol, the eEPSC area was similarly reduced, but due to increased variability, this attenuation was not statistically significant.
NMDA eEPSCs returned towards pre-ethanol baseline levels during washout from ethanol. Following 15 to 20 minutes of washout from 22 mM ethanol, responses were no longer significantly different from control responses. During this same period of washout in slices exposed to 44 mM ethanol, NMDA eEPSCs had recovered to approximately 91% of control responses, but were still significantly attenuated. Periods of washout greater than 20 minutes may be needed to fully recover at higher doses; however, this is complicated by the inherent difficulty in maintaining stable whole-cell responses for extended periods of time.
Ethanol-Sensitivity of AMPAR-Mediated Currents in the PFC
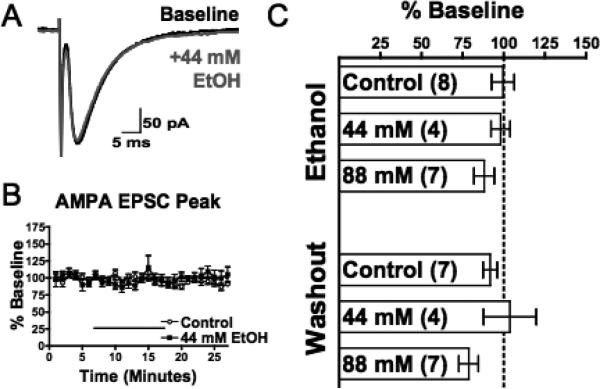
The inhibition of NMDAR-mediated eEPSCs is consistent with a direct effect on the post-synaptic receptor. However, it is also possible that ethanol may attenuate the release of glutamate through a presynaptic action. We reasoned that if this were the case, we would also detect changes in isolated AMPAR-mediated transmission in the PFC in response to acute ethanol application. Deep-layer pyramidal neurons were voltage clamped at –70 mV and electrically stimulated with local stimulation at a rate of 0.05 Hz. AMPA eEPSCs were isolated by perfusing slices with ACSF containing 100 μM picrotoxin and 100 μM DL-APV to inhibit GABAAR- and NMDAR-mediated transmission, respectively. In some experiments, to confirm that this response was AMPAR-mediated, NBQX (30 μM) was applied to eliminate the remaining current (data not shown). At a concentration of ethanol that reliably and significantly inhibited NMDA eEPSCs (44 mM), AMPAR-mediated eEPSCs were unaffected (Fig. 2B and C). Increasing the concentration to 88 mM produced a slight trend towards inhibition, but this effect was not statistically significant (Fig. 2C). Although lethal levels of alcohol cannot be determined in humans, studies suggest that blood alcohol concentrations in this range (approximately 0.4%) are often associated with coma and respiratory failure in nontolerant individuals (Poikolainen, 1984).
Fig. 2.
Acute ethanol has no effect on evoked AMPAR-mediated EPSCs (eEPSCs) in mPFC pyramidal neurons. (A) Representative averaged traces of AMPA eEPSCs before (black) and during (gray) application of acute ethanol (44 mM). (B) Normalized timecourse of AMP eEPSC peak in response to sham solution exchange (open circles) or 44 mM ethanol application (black squares). Black bar indicates period of solution exchange. (C) Normalized response of AMPA eEPSC peak during ethanol exposure (top) and following 10 to 15 minutes of ethanol washout (bottom). Values are expressed as averages ± SEM.
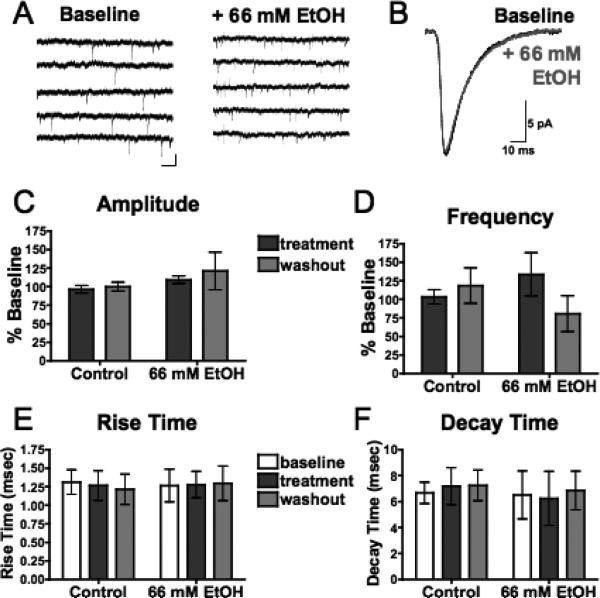
As an additional test of a presynaptic site of action, the effect of ethanol on spontaneous AMPAR-mediated events was determined. Deep-layer pyramidal neurons, voltage clamped at –70 mV in the presence of 100 μM picrotoxin and 100 μM DL-AP5, displayed spontaneous EPSCs (sEPSCs) that were approximately 20 to 30 pA in amplitude (Fig. 3A and B). These events were completely blocked by the AMPAR antagonist NBQX (data not shown). Application of 66 mM ethanol had no significant effect on the amplitude (Fig. 3C), rise time (Fig. 3E), or decay time (Fig. 3F) of these sEPSCs. A slight increase in frequency of these events was observed with 66 mM ethanol, but this effect was not statistically significant (Fig. 3D). Together, these data suggest that ethanol primarily affects the postsynaptic NMDAR component of glutamatergic responses in deep-layer pyramidal neurons.
Fig. 3.
Acute ethanol has no effect on spontaneous AMPAR-mediated EPSCs (sEPSCs) in mPFC pyramidal neurons. (A) Representative traces containing sEPSCs from PFC pyramidal neurons in the presence of picrotoxin (100 μM) and DL-APV (100 μM) before (left) and after (right) acute exposure to ethanol (66 mM). Scale bar: 25 pA, 5 ms. (B) Averaged sEPSCs before (black) and after (gray) acute exposure to ethanol (66 mM). (C,D) Average sEPSC amplitude (C) and frequency (D) normalized to baseline during (dark gray) and 15 to 20 minutes following (light gray) sham solution exchange (n=7) or acute exposure to 66 mM ethanol (n=5). (E,F) sEPSC rise time (E) and decay time (F) before (white), during (dark gray), and 15 to 20 minutes following (light gray) sham solution exchange or acute exposure to 66 mM ethanol. Values are expressed as averages ± SEM.
Ethanol-Sensitivity of GABAAR-Mediated Currents in the PFC
In addition to glutamatergic transmission, ethanol has also been shown to alter GABAR-mediated responses. These effects are believed to contribute to some of the behavioral effects that result from acute and chronic exposure to ethanol. Many reports have demonstrated enhancement of GABAAR-mediated responses in the presence of behaviorally relevant concentrations of ethanol, although this effect is variable and is influenced by factors such as the brain region investigated, the concentration of ethanol, and the mode of recording (for review, see Weiner and Valenzuela, 2006). As the effects of acute ethanol on GABAergic responses in deep-layer PFC neurons have not been previously reported, we conducted several studies to establish the ethanol sensitivity of these receptors.
Deep-layer pyramidal neurons were voltage clamped at –70 mV and GABAR-mediated IPSCs were isolated by perfusing slices with standard ACSF containing 10 μM NBQX and 100 μM DL-APV to block glutamatergic responses. Pairs of GABAR evoked inhibitory postsynaptic currents (eIPSCs; inter-stimulus interval of 50 ms) were elicited by local electrical stimulation at a rate of 0.05 Hz (Fig. 4A). In some experiments, to confirm that this response was GABAAR-mediated, picrotoxin (100 μM) was applied to eliminate the remaining current (data not shown). Following 5 minutes of stable baseline activity, various concentrations of ethanol (22 to 88 mM) were bath-applied for 10 minutes followed by a period of washout.
Fig. 4.
Acute ethanol has no effect on evoked GABAR-mediated IPSCs (eIPSCs) in mPFC pyramidal neurons. (A) Representative averaged traces of GABA eIPSCs before (black) and during (gray) application of acute ethanol (44 mM). (B) Normalized timecourse of the first GABA eIPSC peak in response to sham solution exchange (open circles) or 44 mM ethanol application (black squares). Black bar indicates period of solution exchange. (C) Normalized response of GABA eIPSC peak during ethanol exposure (top) and 10 to 15 minutes of ethanol washout (bottom). (D) Average paired-pulse ratio before (white bars), during (dark gray bars) and 10 to 15 minutes following (light gray bars) exposure to various doses of ethanol. Values are expressed as averages ± SEM.
Ethanol, at concentrations from 22 to 88 mM had no significant effect on GABAR-mediated eIPSCs (Fig. 4B and C), although there was slight, but non-significant decrease in peak amplitude at 88 mM. Several studies have reported that the ethanol-sensitivity of GABAAR-mediated IPSCS is enhanced when GABABR-mediated receptors are blocked (Ariwodola and Weiner, 2004; Wan et al., 1996). To test this possibility in deep-layer PFC neurons, we recorded eIPSCs in some experiments in the presence of the GABABR-specific antagonist CGP-55845 (1 μM). No significant effects of ethanol on GABAAR eIPSCs were observed under these conditions (data not shown).
Recent evidence suggests that, in some brain regions, ethanol may enhance GABAR-mediated transmission by increasing presynaptic GABA release (Ariwodola and Weiner, 2004; Siggins et al., 2005). To examine whether ethanol affects GABA release, we first calculated the effects of acute ethanol exposure on the paired-pulse ratio (PPR) of GABA eIPSCs. PPR is commonly used to assess a presynaptic locus of action and changes in PPR are inversely related to effects on release probability. We calculated the PPR by dividing the average amplitude of the first IPSC peak over a period of 5 minutes by the average amplitude of the second IPSC peak over the same 5-minute period. As reported by Kim and Alger (2001), this technique reduces the chances of detecting changes in PPR due to random fluctuations in GABA-mediated responses. As shown in Fig. 4D, there was no significant effect of any concentration of ethanol tested (22 to 88 mM) on the PPR of GABA IPSCs.
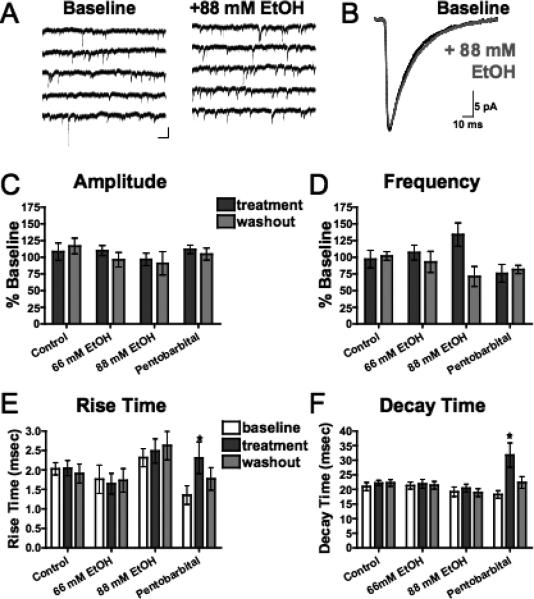
To more carefully examine whether ethanol has effects on GABA-mediated transmission, we monitored spontaneous GABA IPSCs in ACSF containing 100 μM DL-APV and 10 μM NBQX. In deep-layer pyramidal neurons voltage clamped at –70 mV, inward currents with amplitudes between 20 and 30 pA were detected (Fig. 5A and B). These events were completely blocked by bicuculline (data not shown), indicating that they were mediated by GABAARs. Neither 66 mM nor 88 mM ethanol had any significant effect on the amplitude (Fig. 5C), rise time (Fig. 5E), or decay time (Fig. 5F) of these sIPSCs. The frequency of GABA sIPSCs was also unaffected by ethanol, although at 88 mM, a slight, but non-significant enhancement was observed (Fig. 5D).
Fig. 5.
Acute ethanol has no effect on spontaneous GABAR-mediated IPSCs (sIPSCs) in mPFC pyramidal neurons. (A) Representative traces containing sIPSCs from PFC pyramidal neurons in the presence of NBQX (10 μM) and DL-APV (100 μM) before (left) and after (right) acute exposure to ethanol (88 mM). Scale bar: 25 pA, 5 ms. (B) Averaged sEPSCs before (black) and after (gray) acute exposure to ethanol (88 mM). (C,D) Average sIPSC amplitude (C) and frequency (D) normalized to baseline during (dark gray) and 15 to 20 minutes following (light gray) sham solution exchange (n=8) or exposure to 66 mM ethanol (n = 7), 88 mM ethanol (n = 8), or 50 μM pentobarbital (n=7). (E,F) sIPSC rise time (E) and decay time (F) before (white), during (dark gray), and 15 to 20 minutes following (light gray) sham solution exchange or exposure to 66 mM ethanol, 88 mM ethanol, or 50 μM pentobarbital. Values are expressed as averages ± SEM. *p < 0.001 compared with baseline values.
As a positive control for detecting changes in sIPSC kinetics, we recorded sIPSCS during application of the positive GABAAR partial agonist pentobarbital. Consistent with previous findings (Quilichini et al., 2006; Rovira and Ben-Ari, 1999), pentobarbital (30 μM) produced a significant and reversible increase in the decay time of sIPSCs (p < 0.005, Fig. 5F) but had no significant effect on amplitude (Fig. 5C) or frequency (Fig. 5D). This application of pentobarbital also produced a significant increase in rise time (p < 0.001, Fig. 5E).
Ethanol Effects on Tonic GABA Current
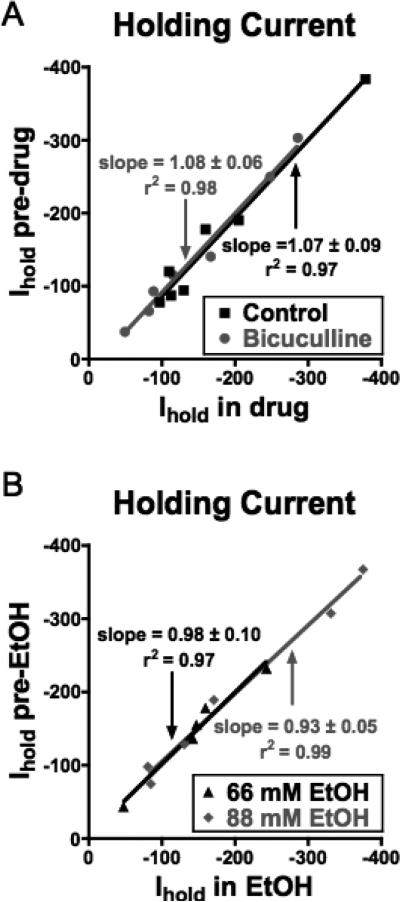
In addition to the reported effects of ethanol on synaptically evoked “phasic” GABA responses, recent studies have demonstrated that extrasynaptic GABAARs that mediate a “tonic” current may be especially sensitive to ethanol (for review, see Mody et al., 2007). Tonic current may arise from spillover of synaptically released GABA and, if present, is detected as a bicuculline-sensitive reduction in the holding current of voltage-clamped neurons. Bath application of bicuculline (20 μM) to voltage-clamped PFC neurons eliminated sIPSCs but had no significant effect on the holding current (Fig. 6A). This lack of effect is consistent with a previous report showing negligible GABA-mediated tonic currents in deep-layer pyramidal neurons within the neocortex (Drasbek and Jensen, 2006). To examine whether a tonic GABA current may be unmasked by ethanol, we monitored the holding current before and during application of various concentrations of ethanol. As shown in Fig. 6B, no significant change in holding current was observed at ethanol concentrations of 66 or 88 mM.
Fig. 6.
Lack of tonic GABAergic current in mPFC neurons or modulation by ethanol. Graphs show holding currents (Ihold) of voltage-clamped PFC neurons before (y-axis) and during (x-axis) application of either bicuculline (30 μM) (A) or ethanol (B). Lines show best fit through the plotted points under each condition. All lines had slope values that were not significantly different from one indicating no effect on holding currents.
DISCUSSION
Ethanol-Sensitivity of Glutamatergic EPSCs
The major finding of the present study was that ethanol significantly inhibited NMDAR-mediated EPSCs in deep-layer pyramidal neurons from the mPFC while having little effect on currents carried by AMPARs or GABAARs.
The inhibition of NMDA eEPSCs by ethanol is consistent with findings from previous studies showing that both recombinant and native NMDARs are inhibited by ethanol. For example, ethanol directly inhibits recombinant NMDARs expressed in oocytes (Mirshahi and Woodward, 1995) and in HEK 293 cells (Jin and Woodward, 2006) as well as in primary cultures of neurons (Lovinger et al., 1989; Marszalec et al., 1998). Similar findings have also been reported for NMDA responses measured in brain slices from a variety of regions, including hippocampus (Lovinger et al., 1990), nucleus accumbens (Nie et al., 1994), posterior cingulate cortex (Li et al., 2002), amygdala (Roberto et al., 2004), bed nucleus of the stria terminalis (Kash et al., 2007; Weitlauf et al., 2004) and superficial (2/3) layers of the mPFC (Yaka et al., 2003). The results of the present study add deep-layer pyramidal neurons of the mPFC to the list of ethanol-sensitive NMDARs in the brain. Because these neurons are the major output neurons of the mPFC (Sesack et al., 1989), disruption of NMDAR-dependent transmission in this layer would also predicted to impact both the cortical and sub-cortical processes regulated by the mPFC.
Despite the widely accepted view that NMDARs are an important and sensitive target for the actions of ethanol, the magnitude of ethanol inhibition of NMDA currents is relatively modest. In both recombinant expression systems and in native neurons, ethanol inhibition of NMDA responses is generally less than 50% at concentrations achieved during moderate to heavy drinking in humans (20 to 100 mM). In the present study, the maximal degree of inhibition of NMDA eEPSCs was approximately 25%, a value similar to that observed in other brain areas (see above references). As discussed below, despite these modest effects, studies with the competitive NMDAR antagonist APV show that even small decreases in NMDA ion channel activity may have dramatic effects on brain function.
In contrast to its effect on NMDAR eEPSCs, ethanol had no effect on events mediated by AMPARs even at concentrations (88 mM) associated with severe intoxication or death in non-tolerant individuals. This lack of effect was observed for AMPA responses evoked by direct electrical stimulation (Fig. 2) as well as those generated by the spontaneous release of glutamate (Fig. 3). Despite the lack of appreciable ethanol inhibition of AMPARs observed for mPFC neurons in the present study, ethanol-sensitive AMPA responses have been reported in other brain regions such as the nucleus accumbens (Nie et al., 1994), central amygdala (Roberto et al., 2004), somatosensory cortex (Lu and Yeh, 1999) and the CA3 of the hippocampus (Mameli et al., 2005). In CA3 neurons, this effect was only found in very young (post-natal day 4) rats while AMPA EPSCs were insensitive to ethanol in older (post-natal day 23) rats (Mameli et al., 2005). In the somato-sensory cortex, ethanol's effects on AMPA-mediated events were variable ranging from significant enhancement to attenuation. As in CA3 neurons, these actions were also developmentally regulated (Lu and Yeh, 1999). Whether AMPAR-mediated transmission in PFC neurons from very young animals is ethanol-sensitive is not currently known.
Ethanol-Sensitivity of GABAARs in the PFC
Ethanol had little effect on GABAAR-mediated IPSCs in mPFC pyramidal neurons. These findings are consistent with a report by Criswell and Breese (2005) that measured the effect of ethanol on miniature IPSCs (mIPSCs) in isolated nerve bouton preparations from neurons of the PFC. A similar lack of effect was reported by Mihic et al. (1992) who examined the effect of acute ethanol exposure on GABA-evoked ion flux in cortical microsacs. However, not all studies have found that GABAAR-mediated events in cortical neurons are ethanol insensitive. Several studies from the Dunwiddie laboratory reported that ethanol enhanced GABAAR-mediated transmission in cortical neurons (Proctor et al., 1992; Soldo et al., 1998), while Signore and Yeh (2000) reported a neuron-specific response to 25 mM ethanol in the cortex that ranged from significant enhancement to significant attenuation. These conflicting reports are not unique to cortical neurons. Indeed, numerous studies have reported a wide range of responses regarding ethanol modulation of GABAR-mediated transmission (reviewed by Weiner and Valenzuela, 2006; Lovinger and Homanics, 2007).
Recently, a number of reports have suggested that receptors containing the ∂ subunit may be extremely sensitive to relatively low concentrations of ethanol (Hanchar et al., 2005, 2006; Sundstrom-Poromaa et al., 2002; Wallner et al., 2003, 2006; but see Borghese et al., 2006). These ∂-containing GABAARs can generate a tonic GABA current and may underlie the effects of ethanol on this current in various brain regions (Brickley et al., 2001; Chandra et al., 2006; Cope et al., 2005; Jia et al., 2005; Stell et al., 2003; but see Carta et al., 2004). In the present study, we found little evidence for a tonic GABAAR-mediated current or its modulation by ethanol under our recording conditions. Drasbek and Jensen (2006) reported a similar lack of tonic GABAAR-mediated current in neurons located in superficial (layer 2/3) or deep (5) layers of the mouse neocortex (the specific cortical subregion was not specified). Interestingly, in that study, current could be revealed if GABA was included in the bath along with a transport inhibitor or if slices were exposed to 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol, a potent agonist at ∂-containing receptors. Consistent with the reported region-dependent pattern of ∂ subunit expression (Pirker et al., 2000), tonic currents were much smaller in neurons from layer 5 compared with those from superficial layers. Together with results of the present study, these findings suggest a limited role for tonic GABAAR-mediated current in the actions of ethanol on deep layer PFC pyramidal neurons.
Implications of Ethanol-Sensitive NMDAR-Mediated Transmission in the PFC
In a previous study from this laboratory (Tu et al., 2007), it was shown that ethanol inhibited persistent activity of deep-layer pyramidal neurons in the PFC. This effect of ethanol was rapid, occurred at ethanol concentrations as low as 17 mM, and was often accompanied by a rebound increase in activity following washout. The generation and maintenance of “up-states” that characterize persistent activity requires a complex interplay between glutamatergic and GABAergic inputs. Data from Seamans et al. (2003) showed that network driven up-states in PFC neurons in slice co-cultures could be blocked by antagonists of either AMPARs or NMDARs or by application of GABAAR agonists such as muscimol. These findings suggest that ethanol-induced disruption of persistent activity may result from its direct effects on glutamatergic and GABAergic transmission. In the Tu et al. (2007) study, the mechanism of action by which ethanol inhibited persistent activity could not be determined since pharmacologically isolating any single glutamate or GABAR-mediated component eliminates persistent activity. However, it was shown that low concentrations of the NMDAR antagonist APV (5 μM) closely mimicked the acute inhibitory effects of 50 mM ethanol on persistent activity in the PFC. Importantly, washout from this antagonist did not induce a rebound increase in activity as seen with ethanol. This, along with the results of the present study suggest that the rebound in persistent activity observed by Tu et al. (2007) following ethanol exposure is not due to enhanced NMDA receptor activity of PFC neurons. NMDAR-mediated extracellular field potentials recorded from superficial layers of the mPFC are also not increased during washout of ethanol, although those recorded from hippocampal slices are (Yaka et al., 2003). As the slice co-cultures used in the Tu et al. (2007) study also contained the hippocampus, these results suggest that the rebound in activity following ethanol washout may be due to increased excitatory input from hippocampal neurons that innervate the mPFC. This hypothesis is currently under study.
As mentioned previously, deep-layer pyramidal neurons from the mPFC make synaptic contact with a variety of sub-cortical structures [including the nucleus accumbens, amygdala and ventral tegmental area (Sesack et al., 1989)] thought to be important in mediating actions of addictive drugs including alcohol (for review, see Gonzales et al., 2004). Disruption of mPFC output by reducing NMDAR function may underlie some of the behavioral effects associated with acute alcohol exposure. These include deficits in decision-making, error detection and judgment, processes all associated with higher cortical cognitive function (for review, see Moselhy et al., 2001). Ethanol-induced inhibition of NMDA receptors in mPFC pyramidal neurons would also be expected to reduce plasticity mechanisms that may underlie the ability of neurons to rapidly adapt to changing environmental conditions. Indeed, NMDAR-dependent forms of long-term potentiation (LTP) have been characterized in slices of PFC (Gemperle et al., 2003; Hirsch and Crepel, 1991; Jay et al., 1996). A disruption of LTP in the PFC, like that of NMDAR-dependent persistent activity, could serve as a molecular mechanism for some of the cognitive deficits associated with both acute and chronic ethanol consumption in human subjects.
ACKNOWLEDGMENT
T32AA007474 (CW), K02AA00238 (JJW), and P50AA 10761 (Charleston NIAAA Alcohol Research Center).
REFERENCES
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Gemperle AY, Enz A, Pozza MF, Luthi A, Olpe HR. Effects of clozapine, haloperidol and iloperidone on neurotransmission and synaptic plasticity in prefrontal cortex and their accumulation in brain tissue: an in vitro study. Neuroscience. 2003;117:681–695. doi: 10.1016/s0306-4522(02)00769-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JC, Crepel F. Blockade of NMDA receptors unmasks a long-term depression in synaptic efficacy in rat prefrontal neurons in vitro. Exp Brain Res. 1991;85:621–624. doi: 10.1007/BF00231747. [DOI] [PubMed] [Google Scholar]
- Jay TM, Burette F, Laroche S. Plasticity of the hippocampal-prefrontal cortex synapses. J Physiol Paris. 1996;90:361–366. doi: 10.1016/s0928-4257(97)87920-x. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30:673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301504. E-pub ahead of print, July 11, 2007 (doi: 10.1038/sj.npp.1301504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Differential effect of ethanol on NMDA EPSCs in pyramidal cells in the posterior cingulate cortex of juvenile and adult rats. J Neurophysiol. 2002;87:705–711. doi: 10.1152/jn.00433.2001. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. John Wiley; New York: 2002. [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Yeh HH. Ethanol modulates Ampa-induced current responses of primary somatosensory cortical neurons. Neurochem Int. 1999;35:175–183. doi: 10.1016/s0197-0186(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalec W, Aistrup GL, Narahashi T. Ethanol modulation of excitatory and inhibitory synaptic interactions in cultured cortical neurons. Alcohol Clin Exper Res. 1998;22:1516–1524. [PubMed] [Google Scholar]
- Mihic SJ, Wu PH, Kalant H. Potentiation of gamma-aminobutyric acid-mediated chloride flux by pentobarbital and diazepam but not ethanol. J Neurochem. 1992;58:745–751. doi: 10.1111/j.1471-4159.1992.tb09781.x. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: Effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Mody I, Glykys J, Wei W. A new meaning for “Gin & Tonic”: tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Soldo BL, Allan AM, Dunwiddie TV. Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Res. 1992;595:220–227. doi: 10.1016/0006-8993(92)91053-h. [DOI] [PubMed] [Google Scholar]
- Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47:704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira C, Ben-Ari Y. Developmental study of miniature IPSCs of CA3 hippocampal cells: modulation by midazolam. Brain Res Dev Brain Res. 1999;114:79–88. doi: 10.1016/s0165-3806(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Nogueira L, Lavin A. Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb Cortex. 2003;13:1242–1250. doi: 10.1093/cercor/bhg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Signore AP, Yeh HH. Chronic exposure to ethanol alters GABA(A) receptor-mediated responses of layer II pyramidal cells in adult rat piriform cortex. J Neurophysiol. 2000;84:247–254. doi: 10.1152/jn.2000.84.1.247. [DOI] [PubMed] [Google Scholar]
- Soldo BL, Proctor WR, Dunwiddie TV. Ethanol selectively enhances the hyperpolarizing component of neocortical neuronal responses to locally applied GABA. Brain Res. 1998;800:187–197. doi: 10.1016/s0006-8993(98)00455-7. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci. 2007;27:4765–4775. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci U S A. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]