Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system in which dendritic cells (DC) play an important role in the development of inflammatory responses. Recently it has been shown that Muc1, a membrane tethered glycoprotein, has an ability to suppress inflammatory responses in cultured DC. The objective of this study was to investigate the possible involvement of Muc1 in the development of MS using experimental encephalomyelitis (EAE) in mice, a widely used animal model of MS. Our results showed that: (1) Muc1−/− mice developed greater EAE severity compared with wild type (wt) mice, which correlated with increased numbers of Th1 and Th17 cells infiltrating into the CNS; (2) Upon stimulation, splenic DC from Muc1−/− mice produced greater amounts of IL-1β, IL-6, and IL-12 but less amounts of IL-10 compared with those from wt mice; and (3) The ability of splenic DC to differentiate antigen-specific CD4+ T cells into Th1 and Th17 cells was greater in Muc1−/− mice compared with wt mice. We conclude that Muc1 plays an anti-inflammatory role in EAE. This is the first report demonstrating the possible involvement of Muc1 in the development of MS and might provide a potential target for immunotherapy.
Keywords: Multiple sclerosis, Experimental autoimmune encephalomyelitis (EAE), Muc1 mucin, Th1/Th17 cells, Dendritic cells, anti-inflammatory
1. Introduction
Muc1 (MUC in human and Muc in animals) is a heavily glycosylated transmembrane glycoprotein expressed on the apical surface of mucosal epithelial cells, as well as the surface of hematopoietic cells (Hattrup and Gendler, 2008; Linden et al., 2008). MUC1 was originally cloned from epithelial-derived human breast and pancreatic cancer cells (Gendler et al., 1990; Lan et al., 1990), where it is uniformly expressed on the cell surface as well as in the cytosol and nucleus (Wen et al., 2003). The Muc1 protein consists of two non-covalently associated polypeptide subunits, an N-terminal extracellular and a C-terminal subunit. The Muc1 ectodomain is composed almost entirely of variable numbers of 20-amino acid tandem repeats, while the C-terminal subunit consists of a 58-amino acid extracellular juxtamembranous region, a transmembrane domain, and a highly conserved 72-amino acid cytoplasmic tail (CT) containing multiple phosphorylation sites (1). The phosphorylation status of the Muc1 CT has been associated with intracellular signal transduction and cancer progression and metastasis (Hollingsworth and Swanson, 2004; Kufe, 2009; Zrihan-Licht et al., 1994).
Muc1 is involved in controlling airway inflammation in mouse models of acute (Choi et al., 2011; Lu et al., 2006) and chronic (Kato et al., 2012) bacterial infection. Detailed mechanistic studies using cultured epithelial cells revealed that Muc1 is upregulated by inflammatory mediators (Koga et al., 2007; Kuwahara et al., 2007) and has the ability to suppress Toll-like receptor (TLR) signaling (Kato et al., 2012; Kato et al., 2007; Kyo et al., 2012; Li et al., 2010; Ueno et al., 2008). Thus, as reviewed recently (Kim and Lillehoj, 2008), during an airway infection, inflammation is controlled through a negative feedback loop comprising inhaled pathogens → activation of TLR signaling → release of inflammatory mediators → upregulation of Muc1 → suppression of TLR signaling (Kyo et al., 2012). The anti-inflammatory activity of Muc1 during airway infection is mediated primarily through intracellular interactions between Muc1 and TLRs and attributed mainly to its ability to suppress TLR signaling (Kim, 2012; Kim and Lillehoj, 2008).
Although airway epithelial cells are likely to be the major source of Muc1 during airway infection, the possible contribution of Muc1 in hematopoietic cells to its anti-inflammatory effects remains to be established. A few recent reports support the regulatory role of Muc1 in adaptive immunity, especially in T cell differentiation and function. Using pulmonary and splenic myeloid dendritic cells (DC) derived from Muc1−/− mice, Williams et al (2010) demonstrated that deletion of Muc1 promotes a heightened functional response of DC to TLR4 and TLR5 ligands, and could affect DC-induced T cell differentiation. A recent report using double Muc1/Rag1-deficient mice in a model of colitis showed that Muc1 functions as a negative feedback pathway preventing excessive Th17 differentiation and activity within the colon (Nishida et al., 2012). Other reports also suggest that Muc1 might play an important role in the generation and activity of regulatory Foxp3+ T cells, either directly or indirectly through the generation of regulatory DC (Monti et al., 2004; Konowalchuk and Agrawal, 2012;).
Multiple sclerosis (MS) is an autoimmune disorder of unknown etiology resulting in inflammatory demyelination and axonal degeneration in the central nervous system (CNS). As a widely used animal model for MS, experimental autoimmune encephalomyelitis (EAE) provided crucial evidence of autoimmune mechanisms that involve primarily, although not exclusively, encephalitogenic Th1 and Th17 CD4+ T cells (Frohman et al., 2006; Slavin et al., 2010). In EAE, DC play a double role. First, in peripheral lymphoid organs, they activate naïve antigen-specific CD4+ T cells and contribute to their differentiation into Th1 and Th17 effector cells. Second, in the perivascular space in the CNS, DC restimulate migrating encephalitogenic T cells and facilitate their infiltration into the CNS parenchyma (Bailey et al., 2007; Slavin et al., 2010). Although Muc1 has been shown to be involved in both innate and adaptive immunity (Agrawal et al., 1998; Konowalchuk and Agrawal, 2012; Monti et al., 2004; Nishida et al., 2012; Williams et al., 2010), its role in MS has not been studied.
In the present study, we showed for the first time that Muc1 plays an anti-inflammatory role in EAE. Muc1 deficiency increased EAE severity and resulted in increasing numbers of peripheral and CNS infiltrating Th1 and Th17 cells. Splenic Muc1-deficient DC expressed a different functional phenotype compared to wild type (wt) controls, with enhanced production of proinflammatory cytokines and reduced expression of the anti-inflammatory cytokine IL-10. Furthermore, the ability of Muc1-deficient splenic DC to drive antigen-specific CD4+ T cells to differentiate into Th1 and Th17 cells was enhanced compared with wt DC. These results suggest that Muc1 plays a protective role in EAE and might provide a potential target for MS immunotherapy.
2. Materials and methods
2.1. Animals
C57BL/6 Muc1 knockout (KO, Muc1−/−), C57BL/6 wild type (wt, Muc1+/+) mice and 2D2 C57BL/6-Tg (Tcra2D2, Tcrb2D2) 1kuch/J (MOG35-55 specific TCR) of 8-10 weeks of age were used for this study. Muc1 KO mice originally developed by Dr. Sandra Gendler (Mayo Clinic, Scottsdale, AZ) (Spicer et al., 1995) were bred and maintained in our animal facility, and both C57BL/6 and 2D2 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Offspring of Muc1+/− mice were genotyped using two sets of oligonucleotides specific for the Muc1 gene (Spicer et al., 1995) (sense: 5′-ACCTCACACACGGAGCG CCAG-3′, and antisense: 5′-TCCCCCCTGGCACATACTGGG-3′ or for the LacZ gene (sense: 5′-ACCTCACACACGGAGCGCCAG-3′, antisense: 5′-TTCTGGTGCCGGAAACCAG GC-3′). Mice were bred and housed in accordance with the guidelines of the Temple University Animal Care and Use Committee.
2.2. Reagents
Recombinant murine IL-1β, IL-6 and IL-12 were purchased from Peprotech Inc. (Rocky Hill, NJ). Lipopolysaccharide (LPS) (Escherichia coli O55:B5), phorbol myristate acetate (PMA) and ionomycin were purchased from Sigma–Aldrich (St. Louis, MO). CD4 MicroBeads were purchased from Miltenyi Biotec (Bergish-Gladbach, Germany). Capture and biotinylated anti-mouse IL-1β antibodies, phycoerythrin (PE)-conjugated anti-mouse T-bet, PE-conjugated anti-mouse RORγt, anti-mouse CD3 and anti-mouse CD28 antibodies were purchased from eBioscience (San Diego, CA). Capture and biotinylated anti-mouse IL-17 antibodies, recombinant mouse IL-17 and recombinant TGFβ were purchased from R&D Systems (Minneapolis, MN). Recombinant IFNγ, capture and biotinylated anti-mouse IFNγ , capture and biotinylated anti-mouse IL-6 , capture and biotinylated anti-mouse IL-12p40 and IL-12p70 , APC-conjugated anti-mouse CD4, PE-conjugated anti-mouse IFNγ, PE-conjugated anti-mouse IL-17, and Alexa-conjugated anti-mouse IL-17 antibodies, GolgiPlug, Cytofix/Cytoperm, Perm/Wash buffer and tetramethylbenzidine (TMB) Substrate Reagent Set were purchased from BD PharMingen (San Diego, CA). Carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit was purchased from Invitrogen (Carlsbad, CA).
2.3. EAE induction
C57BL/6 female Muc1−/− and wt mice (7-8 weeks old) were immunized with 200 μg MOG33-55 peptide emulsified in complete Freund’s adjuvant containing Mycobacterium tuberculosis H37 RA (final concentration 2 mg/ml) s.c. on day 0 and 200 ng pertussis toxin (PTX) was administered i.p. on day 0 and day 2. Clinical scores were as follows: 0, normal mouse, no overt signs of disease; 1, limp tail or hind limb weakness; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund state. At stage 5, animals were euthanized and removed from the clinical score calculation.
2.4. Isolation of inflammatory CD4+ T cells from CNS
C57BL/6 female Muc1−/− and wt mice (7-8 weeks old) were immunized as described. Isolation of mononuclear cells was performed at day 12. Mice were anesthetized with 20 μl of ketamine HCl and xylazine and perfused through the left cardiac ventricle with 30 ml of Hanks’ Balanced Salt Solution (HBSS) containing 2 mM EDTA. The brain was dissected and spinal cord was flushed out with HBSS. CNS tissue was digested with 10 ml HBSS containing DNase I and Liberase for 45 min at 37°C with shaking, followed by blocking solution (10% FBS, 10 mM EDTA in HBSS). The tissue was pelleted and resuspended in 10 ml of 30% isotonic Percoll (diluted with 10× HBSS and distilled water), underlayered with 5 ml of 70% isotonic Percoll. Mononuclear cells were isolated from the 30/70 interphase after gradient centrifugation. Cells were washed with RPMI 1640 medium. Mononuclear cells were cultured in the presence of PMA (50 ng/ml), ionomycin (750 ng/ml) and GolgiPlug (1 μl/ml) for 4 h. Cells were stained with APC-conjugated anti-CD4 antibody for 30 min, fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer according to the manufacturer’s instructions. Cells were stained with PE-conjugated anti-IFNγ and PE anti-IL-17 antibodies for 30 min prior to FACS analysis. T cells were identified by gating on CD4+ cells and the numbers of CD4+ T cells and cytokine producing cells were determined with 10,000 total cells.
2.5. Isolation of splenocytes and CD4+ T cells
Splenocytes were isolated from C57BL/6 Muc1−/− and wt mice, and CD4+ T cells were purified by positive immunomagnetic selection using anti-CD4 antibody conjugated to magnetic beads (Miltenyi Biotec). The purified T cells were 98% CD4+ as determined by FACS analysis. To purify naïve MOG-specific CD4+ T cells, cells from the spleens of 2D2 mice were harvested and naive T cells were isolated using the CD4+CD62L+ T cell isolation kit (Miltenyi Biotec). (>85% CD4+ CD62L+).
2.6. Isolation of splenic DC
Spleens from wt or Muc1−/− mice were cut into small pieces and incubated with 0.5 mg/ml Liberase and 1 mg/ml DNase1 for 45 min at 37oC. After enzymatic disaggregation, single-cell suspensions were prepared and incubated with anti-CD11c antibody conjugated to magnetic beads (Miltenyi Biotec). Cells were purified based on CD11c expression (>85% CD11c+).
2.7. T cell proliferation assay
T cell proliferation was measured in triplicate cultures in round-bottom 96-well microtiter plates. Splenic DC (1x104 cells/well) were treated with LPS for 14 h and pulsed with MOG35-55 (50 μg/ml) or proteolipid protein (PLP: nonspecific Ag, 50μg/ml) for 3 h. After extensive washing, splenic DC were co-cultured with 2D2 CD4+CD62L+ T cells (1x105 cells/well) labeled with CFSE, according to the manufacturer’s instructions. Cells were collected 72 h and 96 h later and subjected to FACS analysis.
2.8. Cytokine ELISA
Cytokine production was determined in culture supernatants by sandwich ELISA. ELISA plates were coated with capture antibodies against mouse IL-17, IFNγ, IL-1β, IL-6, and IL-10 at 4 μg/ml and incubated overnight at 4°C. Non-specific binding was blocked by adding 200 μl of blocking buffer per well at room temperature for 2 h. Recombinant mouse IL-17, IFNγ, IL-1β, IL-6, and IL-10 standards and samples were added to the plates and incubated overnight at 4°C. Biotinylated detection antibodies were added to the plates at 2 μg/ml and incubated at room temperature for 2 h, followed by peroxidase-labeled streptavidin and TMB substrate.
2.9. FACS analysis for intracellular cytokines and transcription factors
Intracellular cytokines (IFNγ and IL-17) were determined by FACS. Cells were stimulated with PMA (50 ng/ml), ionomycin (750 ng/ml) and GolgiPlug (1 μl/ml) for 4 h, stained with APC-conjugated anti-mouse CD4 antibody for 30 min at 4°C, and fixed with fixation/permeabilization solution for 30 min at 4°C. Cells were stained with PE-conjugated anti-mouse IFNγ and PE-conjugated anti-mouse IL-17 antibodies and analyzed by FACS in the CD4+ gated population. Transcription factors (T-bet and RORγt) were determined by FACS incells stained with PE-conjugated anti-mouse T-bet and PE-conjugated anti-mouse RORγt antibodies.
2.10. Real-time RT-PCR
Expression of IL-1β, IL-6, IL-12p35, IL-12p40, IL-17 and IFNγ was detected by real-time RT-PCR as previous described (Yen et al., 2010). The primers used were IL-1β: sense 5′-CC CTGCAGCTGGAGAGTGTGGA-3′ and antisense 5′-TGTGCTCTGCTTGTGAGGTGC TG-3′; IL-6: sense 5′-TCCTCTCTGCAAGAGACTTCCATCC-3′ and antisense 5′-GG GAAGGCCGTGGTTGTCACC-3′; IL-12p35: sense 5′-CTGTGCCTTGGTAGCATCT ATG-3′ and antisense 5′-GCAGAGTCTCGCCATTATGATTC-3′; IL-12p40: sense 5′-TGGTTTGCCATCGTTTTGCTG-3′ and antisense 5′-ACAGGTGAGGTTCACTG TTTCT-3′. IL-17A: sense 5′-CCTGGACTCTCCACCGCAAT-3′ and antisense 5′-ATGTGGTGGTCCAGCTTTCC-3′. IFNγ: sense 5′-AGCTCATCCGAGTGGTCCAC-3′ and antisense 5′-GCTTCCTGAGGCTGGATACC-3′.
2.11. Statistical Analysis
Results are given as means ± SE. Comparisons between two groups were done using the Student’s t-test, whereas comparisons between multiple groups were done by one way ANOVA followed by Bonferroni t-test.
3. Results
3.1. Muc1 deficiency increased EAE severity
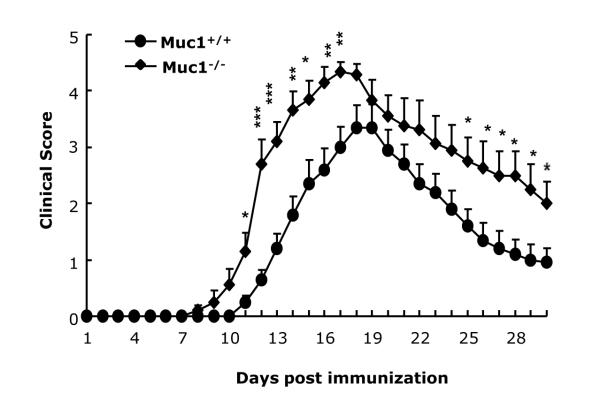
Although Muc1 has been shown to control inflammation in models of respiratory bacterial infection and in colitis, there is no information at the present time on its possible role in neuroinflammation. Therefore, we explored the role of Muc1 in EAE, a model for human MS. Muc1−/− mice and wt controls were immunized with MOG35-55/CFA as described in Methods. Both wt (n=10) and Muc1−/− mice (n=10) developed clinical disease. However, there was an earlier onset and a more severe clinical score in the Muc1−/− EAE mice (Fig. 1 and Table 1). In addition, the mortality rate was 20% in Muc1−/− EAE mice compared to 0% in wt mice (Table 1). More importantly, the cumulative disease scores were significantly higher in Muc1−/− EAE mice that in wt (67.9 vs. 37.9, Table 1). Collectively, these results suggest that Muc1 provides a protective effect in EAE.
Fig. 1.
Deficiency in Muc1 exacerbates EAE.
C57BL/6 wt mice (n = 10) and Muc1−/− mice (n = 10) were immunized with MOG35-55 as described in Materials and Methods. The EAE mice were followed for clinical scores for 30 days. Data represent mean ± SE. Statistical significance was determined as: * p<0.05, ** p<0.01 and *** p<0.001.
Table 1.
Summary of EAE clinical scores in Muc1+/+ and Muc1−/− mice.
| Muc1+/+ | Muc1−/− | |
|---|---|---|
| Incidence | 10/10 | 10/10 |
| Mortality | 0/10 | 2/10 |
| Onset of diseasea | 12.0 ± 0.39 | 10.4 ± 0.43* |
| Maximum scorea | 3.6 ± 0.37 | 4.5 ± 0.18* |
| Cumulative scorea | 37.9 ± 5.34 | 67.9 ± 6.8** |
Data represent the mean ± SE.
p < 0.05;
p < 0.01 compared with data from Muc1+/+ mice
3.2. Increased pathogenic T cell infiltration in the CNS of Muc1-deficient EAE mice
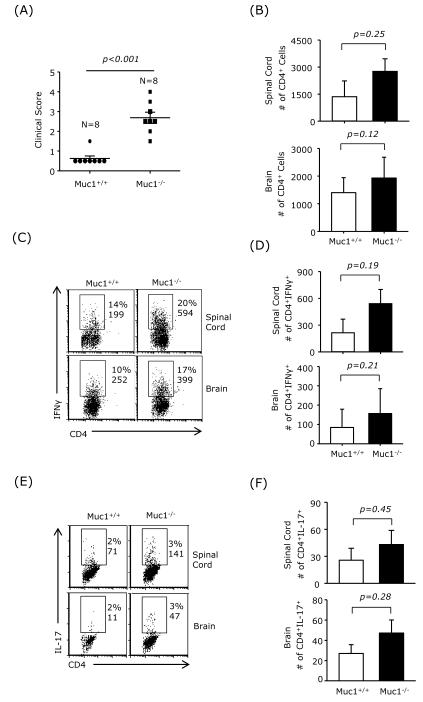
CNS infiltrating CD4+ Th1 and Th17 cells play a crucial pathological role in EAE. To investigate whether the increased disease score observed in Muc1−/− EAE mice was associated with increased CNS infiltration of Th1 and Th17 cells, mononuclear cells were isolated from the brains and spinal cords of wt and Muc1−/− EAE mice on day 12 post-immunization. The clinical score was significantly higher in Muc1−/− EAE mice (Fig. 2A). There was also a trend towards higher numbers of infiltrating CD4+ T cells in brain and spinal cord of Muc1−/− EAE mice (Fig. 2B). Moreover, the frequency of IFNγ-producing CD4+ T cells in brain and spinal cord was elevated in Muc1−/− EAE mice compared with wt mice (Fig. 2C and D). Although the numbers of IL-17-producing CD4+ T cells in the CNS were smaller than the IFNγ-producing T cells, there were more IL-17+ CD4 T cells in the CNS of Muc1−/− EAE mice compared to wt controls (Fig. 2E and F). These results suggest that the higher clinical score observed in Muc1−/− EAE mice correlates with elevated CNS infiltration of pathogenic Th1 ant Th17 cells.
Fig. 2.
Increased Th1 and Th17 infiltration in the CNS of Muc1-deficient EAE mice.
C57BL/6 wt mice (n = 8) and Muc1−/− mice (n = 8) were immunized with MOG35-55 for EAE induction. (A) Clinical scores were assessed on day 12. (B) Mononuclear cells were isolated from spinal cord and brain on day 12 post-immunization and the numbers of CD4+ T cells were determined. (C) and (D) Percentage and numbers of intracellular IFNγ+ CD4 T cells determined by FACS. (E) and (F) Percentage and numbers of intracellular IL-17+ CD4 T cells determined by FACS.
3.3. Increased Th1 and Th17 differentiation in the spleens of Muc1-deficient EAE mice
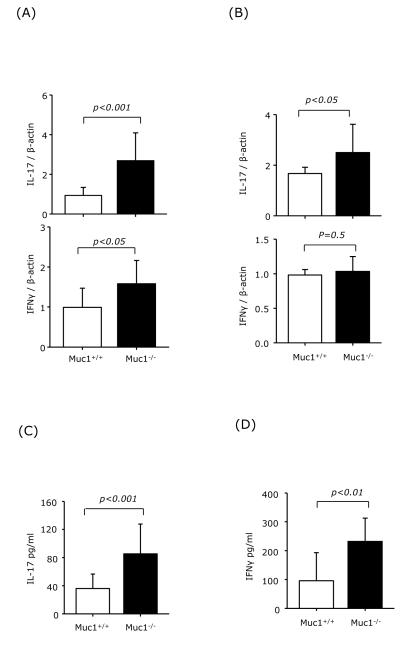
To study whether increased pathogenic T cell infiltration in the CNS of Muc1−/− EAE mice is due to an increase in Th1 and Th17 differentiation in the periphery, cells were isolated from spleen and draining lymph nodes (dLNs) of wt and Muc1−/− EAE mice on day 12 post-immunization and analyzed for IL-17 and IFNγ by real-time RT-PCR. Significantly increased IL-17 expression was observed in cells isolated from spleen and dLNs of Muc1−/− EAE mice compared to wt. We also observed an increase in IFNγ expression in splenocytes from Muc1−/− EAE mice (Fig. 3A and B). When splenocytes from wt and Muc1−/− EAE mice were restimulated ex vivo with MOG35-55, the recall response in terms of IL-17 and IFNγ secretion was significantly higher in Muc1−/− splenocytes (Fig. 3C and D). Taken altogether, these results suggest that lack of Muc1 expression results in augmented peripheral Th1 and Th17 differentiation in EAE.
Fig. 3.
Increased Th1 and Th17 differentiation in the spleens of Muc1-deficient EAE mice.
C57BL/6 wt mice (n = 3) and Muc1−/− mice (n = 4) were immunized with MOG35-55. On day 7 post-immunization, cells isolated from spleens (A), and axillary and brachial lymph nodes (B) were subjected to mRNA extraction followed by real-time RT-PCR for IFNγ and IL-17 expression. (C) and (D) Recall response. Splenocytes were restimulated ex vivo with 25 μg/ml of MOG35-55. Three days later, supernatants were collected and analyzed for IL-17 and IFNγ production. Data represent the combined results from wt EAE (n=3) and Muc1−/− EAE mice (n = 4).
3.4. Muc1 deficiency in splenocytes promotes Th1 and Th17 differentiation
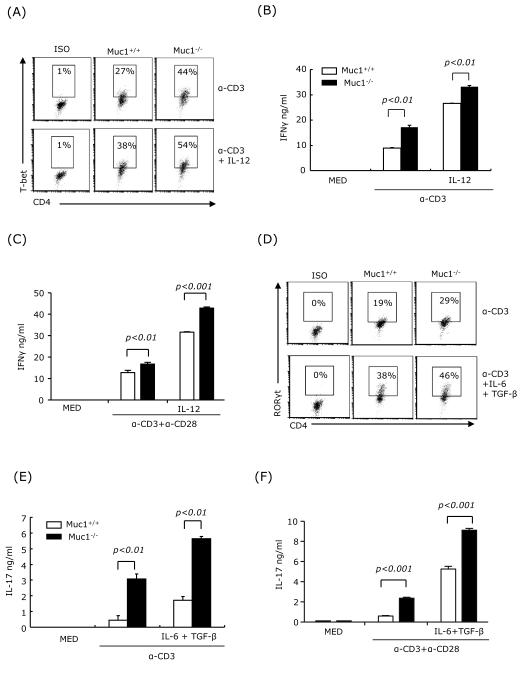
Next, we investigated whether Muc1 plays a role in the in vitro differentiation of Th1 and Th17 cells. Splenocytes harvested from spleens of wt and Muc1−/− mice were activated with anti-CD3 or anti-CD3 and anti-CD28 under non-polarizing or polarizing Th1 and Th17 conditions, i.e. IL-12 for Th1 and IL-6+TGFβ for Th17. We used flow cytometry to analyze the expression of the Th1 and Th17 signature transcription factors T-bet and RORγt, and ELISA to measure the production of IFNγ and IL-17. Muc1−/− splenocytes activated in both polarizing and non-polarizing conditions expressed higher levels of T-bet and RORγt than wt splenocytes (Fig. 4A and D), and produced higher levels of IFNγ and IL-17 than wt splenocytes (Fig. 4B, C, E and F). Taken altogether, these results suggest that Muc1 plays a role in regulating Th1 and Th17 cell differentiation in splenocytes.
Fig. 4.
Muc1 deficiency in splenocytes promotes Th1 and Th17 differentiation.
Splenocytes (2×106 cells/ml) from wt and Muc1−/− mice were stimulated under non-polarizing condition, i.e. soluble anti-CD3 (3 μg/ml) or soluble anti-CD3 (3 μg/ml) plus anti-CD28 (2 μg/ml), Th1 polarizing conditions in the presence of IL-12 (10 ng/ml), or Th17 polarizing conditions in the presence of IL-6 (20 ng/ml), TGF-β (2.5 ng/ml), anti-IL-4 (10 μg/ml) and anti-IFNγ (10 μg/ml). Intracellular expression of T-bet (A) and RORγt (D) were determined by FACS at 24 h. Supernatants were analyzed at 72 h for IFNγ (B and C) and IL-17 (E and F) by ELISA. One representative experiment of three is shown.
3.5. Muc1 does not affect CD4+ T cell differentiation in purified T cell cultures
To determine whether Muc1 plays a direct role in T cell differentiation, we purified CD4+ T cells from the spleen of wt and Muc1−/− mice, and activated them with anti-CD3 and anti-CD28 under non-polarizing and polarizing Th1/Th17 conditions. Supernatants were subjected to IFNγ and IL-17 ELISAs. Similar levels of IFNγ and IL-17 were obtained from both wt and Muc1-deficient T cells, in non-polarizing as well as in Th1/Th17 polarizing conditions (Figs. 5A and 5B). These results suggest that Muc1 might not play an essential role in regulating the differentiation of Th1 and Th17 cells.
Fig. 5.
Muc1 deficiency in CD4+ T cells does not promote Th1 and Th17 differentiation.
CD4+ T cells (2×106 cells/ml) were purified from the spleens of wt and Muc1−/− mice. (A) CD4+ T cells were cultured for 72 h in the presence of plate-bound anti-CD3 antibody (5 μg/ml), soluble anti-CD28 antibody (1 μg/ml) plus IL-12 for Th1 differentiation, or with IL-6, TGF-β, and anti-IL-4 and anti-IFNγ antibodies for Th17 differentiation. Supernatants were collected and IFNγ (A) and IL-17 (B) production was determined by ELISA. Data represent mean ± SE (n = 4). One representative experiment of three is shown.
3.6. Muc1 deficiency in splenic DC contributes to increased Th1 and Th17 differentiation
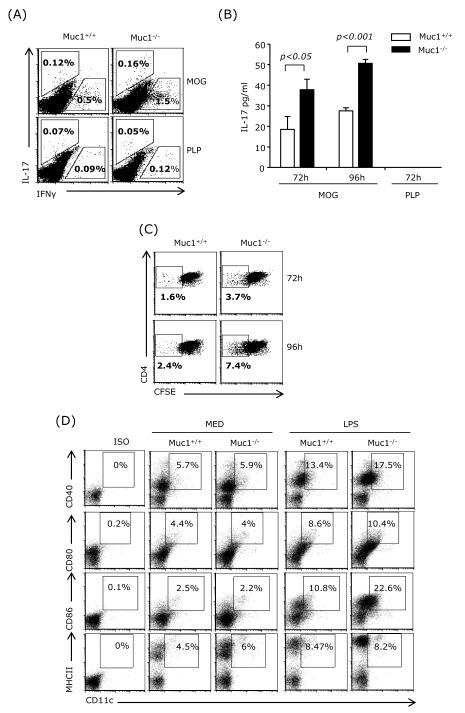
Since we observed augmented Th1 and Th17 differentiation in Muc1−/− splenocytes but not in purified Muc1−/− CD4+ T cells, we concluded that the Muc1−/− antigen-presenting cells might be the determining factor. Splenic conventional DC (cDC) are recognized as the major cell type that activates splenic naïve CD4+ T cells and contribute to their differentiation into various effector T cell subsets. Cytokines such as IL-1β and IL-6 are required for Th17 differentiation, whereas IL-12 is required for Th1 differentiation. DC were reported to express Muc1 and we also observed Muc1 expression in splenic DC (Supplemental Fig. 1). To examine the role of DC Muc1 on the activation of antigen-specific CD4+ T cells in a model relevant to EAE, splenic CD11c+ DC from Muc1−/− and wt mice stimulated with LPS and pulsed with MOG35-55 were co-cultured with MOG35-55-specific naïve CD4+ T cells purified from the spleen of TCR transgenic 2D2 mice.
The frequency of IFNγ- and IL-17-producing T cells was determined by flow cytometry using intracellular staining. Muc1−/− splenic DCs co-cultured with 2D2 naïve CD4+ T cells induced a higher percentage of IFNγ- and IL-17-expressing CD4+ T cells compared with wt DC (Fig. 6A). Although the frequency of IL-17-producing CD4+ T cells was low in co-cultures with either wt or Muc1−/− DC, the levels of secreted IL-17 as measured by ELISA was significantly higher in co-cultures containing Muc1−/− splenic DC (Fig. 6B).
Fig. 6.
Muc1 deficiency in splenic DC augments Th1 and Th17 differentiation.
CD11c+ cells were purified from the spleens of wt and Muc1−/− mice, stimulated with LPS (1 μg/ml) for 14 h and pulsed with MOG (50 μg/ml; specific antigen) or PLP (50 μg/ml; nonspecific antigen-control) for 3 h. After extensive washing, CD11c+ cells were cocultured with MOG-specific naïve CD4+ T cells purified from the spleens of 2D2 mice (2×106cells/ml, DC:T cell=1:10). After 72 h, cells were subjected to FACS for intracellular IFNγ and IL-17 staining (A). Supernatants were collected for IL-17 ELISA after 72 h and 96 h coculture (B). Proliferation of CFSE-labeled MOG-specific CD4+ T cells was measured at 72 h or 96 h (C). Splenocytes from wt or Muc1−/− mice were harvested and treated with LPS for 24 h. Cells were subjected to surface staining for CD11c, CD40, CD80, CD86 and MHCII. One representative experiment of three (A-C) or of two (D) is shown.
We also investigated whether Muc1 deficiency in splenic DCs affected T cell proliferation. Splenic DC from wt or Muc1−/− mice were co-cultured with CFSE-labeled 2D2 T cells for 3 to 4 days, and T cell proliferation was determined by FACS. The results indicate that Muc1−/− DC induced a higher level of T cell proliferation (Fig. 6C). These experiments suggest that the presence of Muc1 on DC reduces both antigen-specific proliferation and differentiation of naïve T cells into Th1 and Th17 effectors.
To further dissect the molecular mechanisms by which Muc1−/− splenic DCs induced higher levels of T cell activation compared to wt DC, we determined the expression levels of MHCII and of the co-stimulatory molecules CD40, CD80 and CD86. Since DC purification may activate splenic DC to a certain degree, we analyzed total spleen cell suspensions. Immature CD11c-gated DC from either wt or Muc1−/− mice had similar levels of CD40, CD80, CD86 and MHCII, However, following LPS stimulation, the expression levels of CD40 and CD86 were increased in the Muc1−/− CD11c+ splenocytes when compared to wt (Fig. 6D).
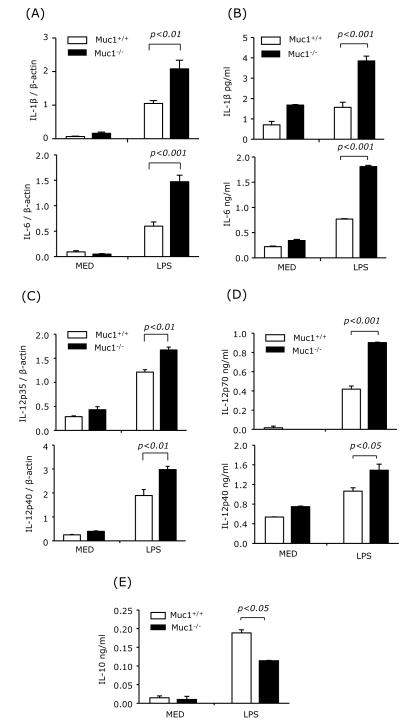
3.7. Muc1 regulates LPS-induced cytokine production in splenocytes and splenic DC
CD4 T cell differentiation into various effectors depends to a large degree on the cytokine microenvironment. IL-12 generated by APCs promotes Th1 differentiation, whereas IL-1β and especially IL-6 are required for Th17 differentiation. We first analyzed the mRNA and protein expression levels of IL-1β, IL-6 and IL-12 in wt and Muc1−/− LPS-activated splenocytes. LPS induced higher levels of IL-1β, IL-6, IL-12p35 and IL-12p40 mRNA in Muc1−/− splenocytes compared with wt cells (Figs. 7A and 7C). In addition, the levels of secreted IL-1β, IL-6, IL-12p70, and IL-12p40 were also significantly higher in LPS-treated Muc1−/− than in wt splenocytes (Figs. 7B and 7D). In contrast, IL-10 levels were lower in Muc1−/− splenocytes (Fig. 7E).
Fig. 7.
Muc1 deficiency enhances the expression and production of IL-1β, IL-6 and IL-12 and diminishes the production of IL-10 in splenocytes.
Splenocytes from wt or Muc1−/− mice were harvested and treated with LPS. After 3 h, mRNA expression of IL-1β, IL-6, IL-12p35 and IL-12p40 was measured by real-time RT-PCR (A and C). The production of IL-1β, IL-6, IL-12p70, IL-12p40 and IL-10 was measured by ELISA at 24 h after LPS stimulation (B, D and E). Data are representative of two independent experiments.
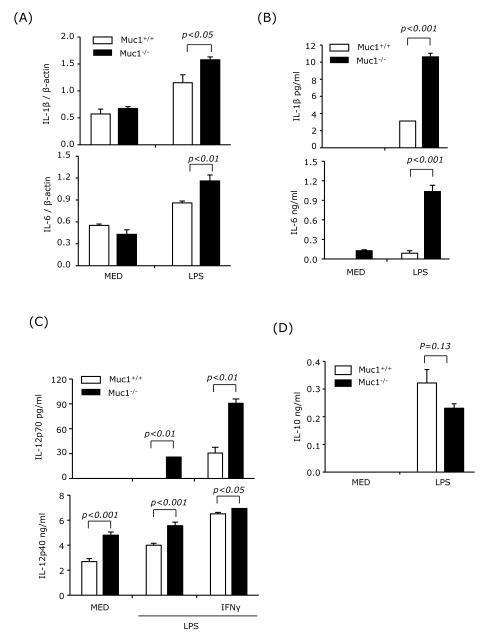
Next, we analyzed expression of the same cytokines in purified CD11c+ splenic DC treated with LPS or LPS plus IFNγ. Both IL-1β and IL-6 mRNA expression and cytokine production were significantly elevated in Muc1−/− splenic DC (Figs. 8A and 8B). In addition, Muc1−/− splenic DC treated with LPS or LPS+IFNγ produced significantly higher levels of IL-12p70 and IL-12p40 than wt DC (Fig. 8C), and lower levels of IL-10 (Fig. 8D). These results suggest that Muc1 plays a regulatory role in the production of cytokines required for Th1 and Th17 differentiation. Thus, Muc1 deficiency in splenic DCs could result in overproduction of cytokines leading to increased differentiation of pathogenic Th1 and Th17 cells.
Fig. 8.
Muc1 deficiency in splenic DC results in overexpression of IL-1β, IL-6 and IL-12 but underexpression of IL-10.
CD11c+ cells were purified from the spleens of wt and Muc1−/− mice and treated with LPS in the presence or absence of IFNγ (100 ng/ml). After 3 h, mRNA expression of IL-1β and IL-6 was measured by real-time RT-PCR (A). Production of IL-1β, IL-6, IL-12p70 and IL-10 was measured by ELISA at 24 h, and of IL-12p40 at 36 h after LPS stimulation (B-D). Data are representative of two independent experiments.
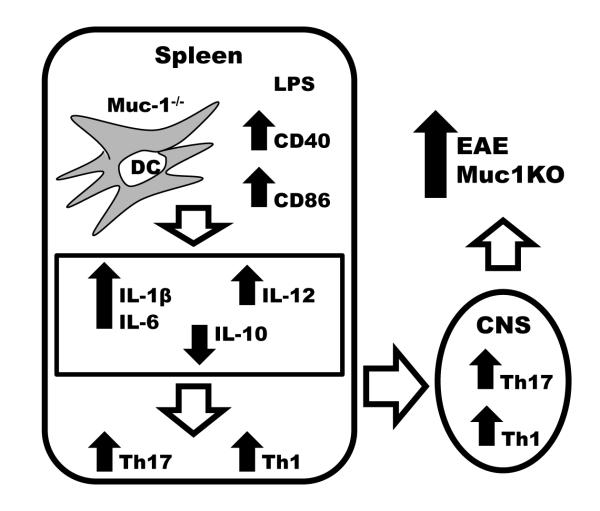
Taken together, our results suggest that lack of Muc1 in DC results in stronger activation of T cells through increased expression of co-stimulatory molecules, and in the upregulation of proinflammatory cytokines leading to preferential differentiation of proinflammatory subsets of effector T cells. In the context of autoimmune/inflammatory diseases such as EAE, the generation of higher numbers of pathogenic Th1, and especially Th17 cells, in the absence of Muc1 may lead to disease exacerbation (Fig. 9).
Fig. 9.
Model for the role of Muc1 in EAE.
Lack of Muc1 in DC results in stronger activation of T cells through increased expression of costimulatory molecules, and in the upregulation of proinflammatory cytokines leading to preferential differentiation of proinflammatory subsets of effector Th1 and Th17 cells. The generation of higher numbers of pathogenic Th1 and Th17 cells in the absence of Muc1 leads to EAE disease exacerbation.
4. Discussion
In the present study we report that mice deficient in Muc1 exhibit increased disease severity and mortality in a model of EAE. Higher EAE susceptibility in Muc1-deficient mice was associated with increased numbers of Th1 and Th17 cells infiltrating into the CNS, and with increased differentiation of splenic Th1 and Th17 cells, most likely attributable to Muc1 deficiency in DC. This is the first report demonstrating an anti-inflammatory role for Muc1-expressing splenic DC in EAE.
Recently, Muc1 has been described as an immunomodulator, acting primarily on antigen presenting cells including DC and T lymphocytes. In models of Th1 and Th2 colitis, the absence of Muc1 was shown to exacerbate disease by promoting IL-17 production from Th17 and innate lymphoid cells. This led to the proposal that during immune activation, Muc1, which is upregulated by Th17 signaling, may act as a negative feedback to prevent excessive production of the potent proinflammatory cytokine IL-17 (Nishida et al., 2012). These observations raise the possibility that, in addition to its normal physiological role in protecting and lubricating the epithelial layers, Muc1 may also act as an endogenous anti-inflammatory agent.
Although EAE was considered to be a Th1-type disease, the discovery of Th17, IL-17 Tγδ, and IL17-producing innate lymphoid cells (ILC17) and the emerging concept of the plasticity of T cell subsets have led to a more complicated picture for T cell involvement in EAE (Petermann and Korn, 2011; Pollinger, 2012). More recently, the distinction between Th1 and Th17 in EAE and in models of colitis has became less distinct, with the discovery that a new type of Th17 cells, which transiently co-express RORγt and T-bet and therefore are IL-17+IFNγ+, accumulate in the intestine and CNS, respectively (Ahern et al., 2010; Ghoreschi et al., 2010). Moreover, most of the IFNγ-producing T cells that migrated to the spinal cord of EAE mice were shown to have originated from T cells that produced IL-17 before their conversion to “classical” Th1 cells (Hirota et al., 2011). In this context, the fact that Muc1-deficient EAE mice show increased numbers of both Th1 and Th17 cells in spinal cord strongly suggests that Muc1 affects Th1/Th17 differentiation.
Indeed, Muc1-deficient splenocytes generated more T-bet- and RORγt-expressing T cells and higher levels of secreted IFNγ and IL-17 both in non-polarizing and Th1/Th17 polarizing conditions. T cell activation and differentiation requires stimulatory and co-stimulatory interactions with antigen-presenting DC. Since both DC and T cells express Muc1 (Agrawal et al., 1998; Cloosen et al., 2004), the difference in T cell differentiation could be due to lack of Muc1 expression on either cell type. In our results we observed purified Muc1−/− and wt T cells differentiate at similar levels into IFNγ- or IL-17-producing cells suggesting Muc1 might not play an essential role in regulating Th1 and Th17 differentiation. In contrast, Muc1-deficient splenic DC are more efficient than wt DC in activating T cell proliferation and Th1/Th17 differentiation when co-cultured with antigen-specific naïve CD4+ T cells. Although Williams et al. (2010) did not test IL-17 production, they reported results similar to ours for IFNγ and T cell proliferation in co-cultures of Muc1-deficient splenic DC with allogeneic T cells.
Since Muc1 has been reported to interfere with TLR signaling blocking the interaction with MyD88 (Ueno et al., 2008) and leading to reduction in NF-kB activation and subsequently in the expression of most proinflammatory cytokine genes, we expected Muc1-deficient DC to be better responders to TLR ligands. Indeed, purified splenic Muc1-deficient DC produced higher levels of IL-12p35 and p40, IL-6, and IL-1β. This is relevant especially since IL-12 is required for Th1 and IL-6 and IL1β are required for Th17 differentiation.
Our results suggest that exacerbation of EAE in Muc1-deficient mice is mediated through increased Th1/Th17 differentiation due to the higher proinflammatory phenotype of Muc1-deficient DC. Splenic DC consist of four different subpopulations, i.e. plasmacytoid DC, and conventional CD8α+CD4-; CD8α+CD4+ and CD8α-CD4-DC (Neuenhahn and Busch, 2007; Sathe and Shortman, 2008). It remains to be established whether all splenic DC subpopulations express Muc1 and whether Muc1 modulates their maturation and function in response to various stimuli.
Muc1 regulation of the immune response could be also mediated through additional mechanisms, such as induction of regulatory T cells (Treg). Recently, the majority of CD4+CD25+Foxp3+ Treg were found to express Muc1, and CD3/Muc1 co-stimulation has been reported to increase the number of Treg (Konowalchuk and Agrawal, 2012). Tumor cells express high levels of Muc1 that could affect the activation and function of tumor-associated immune cells. Indeed, tumor-derived Muc1 was shown to maintain monocyte-derived DC in an immature state, and to drive their differentiation into IL-10highIL-12low regulatory/tolerogenic DC which, in turn, induce suppressor/regulatory T cells (Monti et al., 2004). This is in agreement with our observation that Muc1-deficient splenic DC express more IL-12 and less IL-10 compared with wt DC, indicating that Muc1 promotes IL-10 and reduces IL-12 expression. In this respect, Muc1 might prove to be an endogenous anti-inflammatory factor acting through the induction of tolerogenic/regulatory DC. Other such endogenous factors have been described, including retinoic acid, vasoactive intestinal peptide (VIP), and α-melanocyte stimulating hormone (α-MSH) (Auriemma et al., 2012; Delgado et al., 2005; Sela et al., 2011).
In summary, in this study, we found that Muc1-deficient mice developed EAE at an earlier time point and exhibited increased mortality and more severe clinical disease scores, suggesting that Muc1 might play a protective role in autoimmune diseases such as EAE/MS and might become a target for therapeutic intervention.
Supplementary Material
Highlights.
This report describes, for the first time, the immunomodulatory role of Muc1 in an animal model of multiple sclerosis, providing a potential target for immunotherapy.
Acknowledgments
We thank Dr. Erik Lillehoj (University of Maryland School of Medicine) for editing the manuscript. This work was supported by grants from National Institutes of Health (R21 AI073988 and RO1 HL47125 for KCK and RO1 AI084065 for DG) and American Heart Association (12SDG8170005 for JY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement All authors declare that there are no conflicts of interest.
References
- Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–4081. [PubMed] [Google Scholar]
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriemma M, Brzoska T, Klenner L, Kupas V, Goerge T, Voskort M, Zhao Z, Sparwasser T, Luger TA, Loser K. alpha-MSH-Stimulated Tolerogenic Dendritic Cells Induce Functional Regulatory T Cells and Ameliorate Ongoing Skin Inflammation. The Journal of investigative dermatology. 2012;132:1814–1824. doi: 10.1038/jid.2012.59. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nature immunology. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. American journal of respiratory cell and molecular biology. 2011;44:255–260. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloosen S, Thio M, Vanclee A, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, Germeraad WT, Bos GM. Mucin-1 is expressed on dendritic cells, both in vitro and in vivo. Int Immunol. 2004;16:1561–1571. doi: 10.1093/intimm/dxh157. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. The Journal of biological chemistry. 1990;265:15286–15293. [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annual review of physiology. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature reviews. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, Kim KC. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J Immunol. 2012;188:2014–2022. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L686–692. doi: 10.1152/ajplung.00423.2006. [DOI] [PubMed] [Google Scholar]
- Kim KC. Role of epithelial mucins during airway infection. Pulmonary pharmacology & therapeutics. 2012;25:415–419. doi: 10.1016/j.pupt.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39:644–647. doi: 10.1165/rcmb.2008-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, Kim KC. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L693–701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- Konowalchuk JD, Agrawal B. MUC1 mucin is expressed on human T-regulatory cells: function in both co-stimulation and co-inhibition. Cellular immunology. 2012;272:193–199. doi: 10.1016/j.cellimm.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nature reviews. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. American journal of respiratory cell and molecular biology. 2007;37:691–698. doi: 10.1165/rcmb.2007-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo Y, Kato K, Park YS, Gajghate S, Umehara T, Lillehoj EP, Suzaki H, Kim KC. Antiinflammatory role of MUC1 mucin during infection with nontypeable Haemophilus influenzae. American journal of respiratory cell and molecular biology. 2012;46:149–156. doi: 10.1165/rcmb.2011-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. The Journal of biological chemistry. 1990;265:15294–15299. [PubMed] [Google Scholar]
- Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. American journal of physiology. Lung cellular and molecular physiology. 2010;298:L558–563. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal immunology. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, Kim KC. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176:3890–3894. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena P, Piemonti L. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol. 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- Neuenhahn M, Busch DH. Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens. Immunology letters. 2007;114:66–72. doi: 10.1016/j.imlet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Nishida A, Lau CW, Zhang M, Andoh A, Shi HN, Mizoguchi E, Mizoguchi A. The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice. Gastroenterology. 2012;142:865–874. e862. doi: 10.1053/j.gastro.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F, Korn T. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS letters. 2011;585:3747–3757. doi: 10.1016/j.febslet.2011.03.064. [DOI] [PubMed] [Google Scholar]
- Pollinger B. IL-17 producing T cells in mouse models of multiple sclerosis and rheumatoid arthritis. J Mol Med (Berl) 2012;90:613–624. doi: 10.1007/s00109-011-0841-4. [DOI] [PubMed] [Google Scholar]
- Sathe P, Shortman K. The steady-state development of splenic dendritic cells. Mucosal immunology. 2008;1:425–431. doi: 10.1038/mi.2008.56. [DOI] [PubMed] [Google Scholar]
- Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. The Journal of experimental medicine. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin A, Kelly-Modis L, Labadia M, Ryan K, Brown ML. Pathogenic mechanisms and experimental models of multiple sclerosis. Autoimmunity. 2010;43:504–513. doi: 10.3109/08916931003674733. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38:263–268. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. The Journal of biological chemistry. 2003;278:38029–38039. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bauer S, Lu W, Guo J, Walter S, Bushnell TP, Lillehoj EP, Georas SN. Deletion of the mucin-like molecule muc1 enhances dendritic cell activation in response to toll-like receptor ligands. Journal of innate immunity. 2010;2:123–143. doi: 10.1159/000254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JH, Kong W, Ganea D. IFN-beta inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J Immunol. 2010;184:3478–3486. doi: 10.4049/jimmunol.0902542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrihan-Licht S, Baruch A, Elroy-Stein O, Keydar I, Wreschner DH. Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins. Cytokine receptor-like molecules. FEBS letters. 1994;356:130–136. doi: 10.1016/0014-5793(94)01251-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.