Abstract
Increased polyamine synthesis and inflammation have long been associated with intraepithelial neoplasia, which are risk factors for cancer development in humans. Targeting polyamine metabolism (by use of polyamine synthesis inhibitors or polyamine catabolism activators) and inflammation (by use of nonsteroidal anti-inflammatory drugs) has been studied for many cancers, including colon, prostate, and skin. Genetic epidemiology results indicate that a genetic variant associated with the expression of a polyamine biosynthetic gene is associated with risk of colon and prostate cancers. A clinical trial of difluoromethylornithine (DFMO), a selective inhibitor of polyamine synthesis, showed that the 1 year treatment duration reduced prostate volume and serum prostate-specific antigen doubling time in men with a family history of prostate cancer. A second, clinical trial of DFMO in combination with sulindac, a NSAID in patients with prior colon polyps found that the 3-year treatment was associated with a 70% reduction of all, and over a 90% reduction of advanced and/or multiple metachronous colon adenomas. In this chapter, we discuss that similar combination prevention strategies of targeting polyamines and inflammation can be effective in reducing risk factors associated with the development of human cancers.
4.1 Introduction
Inflammation plays an essential role in the initiation and progression of many types of human epithelial cancers. Chronic inflammation leads to activation of macrophages and other inflammatory cells that generate increased amounts of growth factors and cytokines, as well as reactive oxygen and nitrogen species that may cause DNA damage. Persistent activation of macrophages can lead to continuous tissue damage in a microenvironment that sustains proliferation of damaged cells, thus predisposing areas of chronic inflammation to neoplasia. Polyamines are aliphatic cations present in all cells, whose levels are intricately controlled by their transport and metabolic enzymes. Polyamines are essential for cell growth and proliferation, and have also been shown to play an important role in inflammation-induced carcinogenesis. Cells have developed complex regulatory machinery to finely control intracellular polyamine pools, as dysregulation of polyamine metabolism can have serious effects on cell growth. Increased polyamine synthesis has been detected as a product of inflammation, and elevated intracellular polyamine pools are frequently observed in actively proliferating cells, including tumor cells. This review will highlight the studies and research which is being done in targeting either inflammation or polyamines for the prevention of cancers. Further, we will provide rationale for designing clinical trials which should target both inflammation and polyamines for the increased chem-preventive actions.
4.2 Polyamines, Inflammation, and Cancer
4.2.1 Polyamine Metabolism
Polyamines (putrescine, spermidine and spermine) are aliphatic polycations present in all cells. They have pleiotropic effects on cell physiology and play a relevant role in cell proliferation (Thomas and Thomas 2001). Cells have developed complex regulatory machinery, which controls intracellular polyamine pool sizes in a fast and accurate manner by the combined action of de novo synthesis, uptake, catabolism, and uptake of polyamines. The regulatory machinery consists of finely regulated enzymatic steps. These include reactions catalyzed by the biosynthetic enzymes, ornithine decarboxylase (ODC), S-adenosylmethionine decarboxylase (SAMDC), and the spermidine and spermine synthases. Polyamine catabolism is catalyzed by the combined actions of spermidine/spermine N1-acetyltransferase (SAT1) and the FAD-dependent polyamine oxidase (APAO) or directly by spermine oxidase (SMO). Intracellular levels of polyamines are tightly controlled. The key enzymes involved in both metabolic pathways, in particular ODC, SAMDC, SAT1, and SMO, are present in low abundance, exhibit rapid turnover, and are under complex transcriptional and posttranscriptional regulation. As their half-lives are very short (Coleman et al. 1994), their expression levels can change by several orders of magnitude very quickly in response to different types of stimuli. ODC is typically induced by growth-promoting factors. It has been shown to be critical in cell transformation, and thus has been suggested to be a proto-oncogene. It undergoes complex regulation, mostly based on the induction of a unique, nonenzymatic, regulatory protein named ornithine decarboxylase antizyme (OAZ) (Hayashi et al. 1996).
Polyamine catabolism occurs through two distinct pathways, and uses a total of three enzymes: spermidine/spermine N1-acetyltransferase (SAT1), acetyl polyamine oxidase (APAO), and spermine oxidase (SMO). SAT1 acetylates both spermine and spermidine, thus providing acetylated polyamines as substrates for further back-conversion to spermidine or putrescine by APAO. Alternatively, spermine can be directly oxidized back to spermidine through SMO. Importantly, the oxidization reactions of both APAO and SMO result in the generation of the toxic byproduct, hydrogen peroxide (H2O2). This H2O2, a reactive oxygen species (ROS), has been shown to play an essential role in the DNA damage-associated changes observed following elevated levels of polyamine catabolism (Fig. 4.1). Furthermore, the depletion of intracellular polyamine pools that typically accompanies induction of polyamine catabolism can itself be growth inhibitory (Seiler 1987).
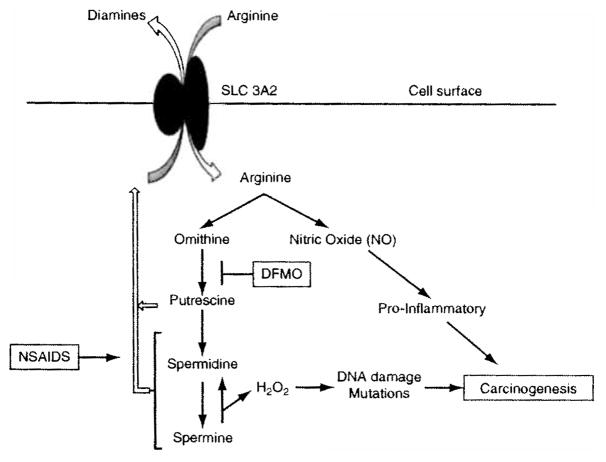
Fig. 4.1.
Polyamine Metabolism, Inflammation and Carcinogenesis. The processes of polyamine metabolism and inflammation are coupled, in part, by the solute carrier transporter SLC3A2, which imports the amino acid arginine using diamines as the antiport molecule (Uemura et al. 2008). SLC3A2 is physically associated with the spermidine/spermine N1-acetyltransferase (SAT1), which acetylates spermidine and spermine and target them for export by this transporter. As shown in this figure, the activity of the surface transporter to import aiginine can be enhanced by increasing polyamine synthesis and/or export, as occurs in human prostate carcinogenesis (Bettuzzi et al. 2000). Inhibition of polyamine synthesis, by agents such as DFMO and activation of polyamine catabolism and export by drugs such as the NSAIDS, reduce cell and tissue polyamines and subsequently arginine transport. The consequence of arginine pool size reduction is to reduce the levels of the pro-inflammatory molecule NO. Reduced NO, along with reduced polyamines, is associated with reduced carcinogenesis. Spermine can be oxidized to spermidine with the generation of H2O2 in the process, which can in turn lead to DNA damage which is pro-carcinogenic (Babbar and Casero 2006). Increased catabolism/export by NSAIDs leads to a decrease in spermine, thereby leading to decreased production of the DNA damaging H2O2
4.2.2 Polyamines and Cancer
For more than 25 years, it has been recognized that there is a strong association between high levels of polyamines and rapid proliferation (Russell and Snyder, 1968). In both rodent and human neoplastic cells and tissues, polyamine contents are often elevated when compared to normal cells and tissues. Polyamine metabolism is an integral component of the mechanism of carcinogenesis in epithelial tissues. Increases in ODC are often associated with initiation of normal cell growth and sustained neoplastic cell growth.
Cancer development is a multistep process, during which genetic alterations confer specific growth advantages, thereby driving the progressive transformation from normal cells to malignant cancer cells. Malignant growth is characterized by several key changes: self-sufficiency of growth signals, insensitivity to antigrowth signals, escape from apoptosis, unregulated proliferation potential, enhanced angiogenesis, and metastasis (Hanahan and Weinberg 2000). Each of these shifts is complicated and accomplished by the combined efforts of various signaling processes. Inflammation is a physiologic response to tissue damage resulting from microbial pathogen infection, chemical irritation, and/or wounding. However, if inflammation resolution is dysregulated, the cellular response changes to a pattern of chronic inflammation, in which the inflammatory foci are dominated by macrophages and other inflammatory cells that generate even greater amounts of growth factors and cytokines, as well as reactive oxygen and nitrogen species that may cause DNA damage. Persistent activation of macrophages can lead to continuous tissue damage (Macarthur et al. 2004) in a microenvironment that sustains proliferation of damaged cells, thus predisposing areas of chronic inflammation to neoplasia (Balkwill and Mantovani 2001).
The association between inflammation and cancer has been illustrated by numerous epidemiologic and clinical studies (Balkwill and Mantovani 2001; Coussens and Werb 2002). Causes of inflammation that have been linked to cancer include bacterial and viral infection (Helicobacter, pylori for gastric adenocarcinoma (Coussens and Werb 2002; Macarthur et al. 2004), hepatitis B and C for hepatocellular carcinoma (Block et al. 2003), and human papillomavirus for penile cancers), or noninfective physical and/or chemical irritants. The risk of developing esophageal, pancreatic, and gallbladder cancers may be increased by certain inflammatory diseases, such as esophagitis, Barrett’s metaplasia, and chronic pancreatitis (Macarthur et al. 2004; Whitcomb 2004). Possible associations have also been described between Marjolin’s ulcer and skin carcinoma (Macarthur et al. 2004); asbestos and mesothelioma (Macarthur et al. 2004); silica, cigarette smoke, and bronchial cancer (Macarthur et al. 2004); chronic asthma and lung cancer (Vesterinen et al. 1993); and pelvic inflammatory disease or ovarian epithelial inflammation and ovarian cancer (Risch and Howe 1995; Macarthur et al. 2004).
Polyamines have been shown to play an important role in inflammation-induced carcinogenesis (Russell and Snyder 1968). Increased polyamine synthesis has been detected as a product of inflammation, and elevated intracellular polyamine pools are frequently observed in actively proliferating cells, including tumor cells. In one study, parasitic infection of the small intestine led to increased mucosal hyperplasia with elevated polyamine biosynthesis (Wang et al. 1991). Additionally, H. pylori infection has been demonstrated to increase expression and activity of polyamine biosynthetic enzymes (Gobert et al. 2002). Meanwhile, intracellular levels of the polyamines themselves are capable of affecting inflammation. The polyamine spermine has been shown to inhibit pro-inflammatory cytokine synthesis in human mononuclear cells (Zhang et al. 1997), as well as nitric oxide-mediated intestinal damage (ter Steege et al. 1999).
Polyamine and nitric oxide metabolism are linked by their common dependence on arginine, which is the substrate for nitric oxide synthases (NOS) and arginase. NOSs produce nitric oxide and arginases produce ornithine, the substrate for putrescine biosynthesis. One member of a di- and acetyl-polyamine exporter was recently shown to be the solute carrier family member SLC3A2 (Uemura et al. 2008). SLC3A2 was found to export di- and acetyl-polyamines via a mechanism involving arginine antiport. Deletion of NOS2 alleles in genetically engineered mouse models of inflammation and intestinal carcinogenesis suppress both these processes (Yerushalmi etal. 2006; Bemstemetal. 2007). Pharmacological depletion of tissue polyamine contents likewise suppresses aiginine-dependent colonic carcinogenesis in ApcMin/+ mice (Yerushalmi et al. 2006b). Thus, polyamines, acting via SLC3A2, might be an important component in the transport of arginine for the production of pro-inflammatory molecule nitric oxide (Fig. 4.1).
4.3 Roles of Inflammation and Polyamines in Prostate Cancer
4.3.1 Polyamines and Prostate Cancer
The prostate and prostatic secretions have played an important role in our current understanding of polyamines. The prostate has one of the highest polyamine concentrations of any tissue. The initial identification of one of the polyamines, i.e. spermine, was reported in human semen as early as the seventeenth century by Antonie van Leeuwenhoek. In rat prostate, spermidine is the predominant polyamine, whereas in the human prostate spermine is present in high amounts. The activities of the polyamine biosynthetic enzymes ODC, SAMDC, and spermidine synthase are induced by androgens in a coordinated way, and expression of these enzymes is primarily localized to the glandular epithelial cells of the prostate (Fjosne et al. 1992; Cyriac et al. 2002). Studies on the murine ODC gene revealed that the ODC promoter contains an androgen-responsive element-like sequence that can bind to the androgen receptor in vitro (Crozat et al. 1992). Studies on the rat and human prostate-derived tumors showed that ODC activity was substantially higher in the more malignant sublines (Schipper et al. 2000). Immunoblot analysis of tissue specimens of patients with prostate cancer showed a significantly elevated protein expression of ODC in the cancerous tissues as compared with the benign tissues (Mohan et al. 1999). Moreover, studies on the expression levels of the ODC gene in a series of 23 human prostate cancers dissected from radical prostatectomy specimens revealed significantly increased ODC expression.
4.3.2 Polyamines, Inflammation, and Prostate Cancer
In the prostate, studies suggest widespread asymptomatic prostatitis arising from a multitude of stimuli, including microbial infection and dietary components. Emerging epidemiological, histopathological, and molecular evidence suggests that such inflammation may be associated with the development of putatively preneoplastic proliferative inflammatory atrophy (PIA), as well as prostatic intraepithelial neoplasia (PIN) and prostate cancer. The most compelling molecular evidence for a mechanistic association between inflammation and prostate cancer is the appearance of somatic epigenetic alterations of certain androgen-regulated differentiation genes and candidate oncogenes in preneoplastic PIA lesions that are reminiscent of those known to be present in prostate cancers (Bardia et al. 2009). Further PIA lesions consist of regions of atrophic epithelium exhibiting an elevated proportion of proliferating cells, association with inflammatory cells, and increased expression of the pro-inflammatory enzyme cyclooxygenase-2 (COX-2) (De Marzo et al. 2007a). In addition, various inflammatory stimuli, including sexually transmitted infections, dietary factors, exposure to estrogen, and urine reflux, have been implicated as potential environmental triggers of prostatic inflammation (De Marzo et al. 2007b). Even though the precise molecular mechanisms linking the inflammatory microenvironment to potentially carcinogenic events including DNA damage, tumor suppressor gene inactivation, and increased proliferation remain to be identified, until recently it was shown that PINs have an increased expression of SMO. SMO is induced in human gastric and lung epithelial cells by H. pylori infection and the proinflammatory cytokine tumor necrosis factor-a (TNF-a), respectively, and this increase in SMO activity produces sufficient H2O2 to cause oxidative DNA damage and apoptosis (Xu et al. 2004; Babbar and Casero 2006). This increased expression of SMO suggested the role SMO plays in the development of precursor PIN lesions and initiation events in prostate carcinogenesis (Goodwin et al. 2008) as it is known that in certain epithelial cells, increased SMO expression produces sufficient H2O2 to cause oxidative DNA damage and apoptosis (Xu et al. 2004; Babbar and Casero 2006).
4.3.3 Targeting Polyamines and Inflammation in Prostate Cancer
D,L-α-difluoromethylornithine (DFMO), the most widely studied inhibitor of ODC, has striking inhibitory effects on the growth of cultured prostatic cancer cells. Numerous in vitro and in vivo studies with DFMO in prostate models have shown its efficacy in decreasing prostate polyamine levels, tumor growth, prostate growth, and regrowth. DFMO-inhibited growth of PC-3, PC-82, and androgen-stimulated LNCaP cells could be reversed by exogenously added polyamines or their acetylated derivatives. Rodents were castrated with subsequent decrease in prostate size and polyamine content. With the return of exogenous androgens, the prostatic atrophy was readily reversed and polyamine content was normalized. DFMO markedly slowed prostatic weight gain from the androgens to half of the weight of the controls and blocked increases in putrescine and spermidine levels in the prostate (Danzin et al. 1979). Inhibition of polyamine synthesis by 1 % DFMO showed marked reduction in weight and volume of the prostate as well as metastasis (Gupta et al. 2000), while activation of polyamine catabolism by over-expression of SAT1 in the transgenic adenocarcinoma mouse prostate model had smaller weights of their prostates at 30 and 36 weeks and better histologic scores (Kee et al. 2004). In humans, a prostate study also showed no risk stratification for cancer based on the ODC polymorphism alone, but those with an A allele when linked with androgen receptor polymorphisms (CAG repeats <22) had an odds ratio of 2 for prostate cancer. Smoking has also been linked to prostate cancer risk in men with an A allele only (Visvanathan et al. 2004). A prospective, randomized double-blind, placebo-controlled clinical trial of DFMO showed that the 1 -year treatment duration reduced putrescine levels, prostate volume, and serum prostate-specific antigen (PSA) doubling time in men with a family history of prostate cancer (Simoneau et al. 2008). In addition, stratification by ODC genotype showed that men with the AA or AG genotype showed a reduction in putrescine level and decrease in total prostate volume, but no reduction in these variables were seen in men with the GG genotype. A promising alternative to the use of inhibitors of polyamine biosynthetic enzymes is the application of synthetic polyamine analogs such as BENSpm, DENSpm, BE-4-4-4-4, etc. which function by activating the polyamine catabolism. Multiple studies showed that these analogs have differential effects on cell growth and polyamine homeostasis in different prostatic carcinoma cells, i.e. the androgen-independent DU-145 cells were the most sensitive, whereas the well-differentiated androgen-dependent LNCaP cells were relatively insensitive. Treatment with these analogs evoked intracellular accumulation of the analog and various regulatory responses, for example, down-regulation of polyamine biosynthesis, the induction of the catabolic enzyme spermidine/spermine-N1-acetyltransferase (SAT1), and the depletion or decrease of the natural polyamines.
NSAIDs, as nonsteroidal anti-inflammatory drugs, have been extensively studied in relation to its chemopreventive actions in prostate cancer. Numerous preclinical studies using selective and nonselective COX inhibitors for prostate cancer prevention and treatment have been shown to promote inactivation of Bcl-2, inactivation of Akt, reduction of NF-kappaB p65, down-regulation of androgen receptor activity, up-regulation of the p75 NTR tumor suppressor, and increase in detoxification enzyme GSTP1, all of which could contribute to potential tumor inhibitory effects. Population-based studies of aspirin intake have hinted at a protective effect on prostate cancer risk (Jacobs et al. 2005; Mahmud et al. 2006). The inverse association between aspirin use and prostate cancer appears stronger for higher frequency use, for greater duration of use, among younger men, and for advanced prostate cancer. The association between nonaspirin NSAIDs and prostate cancer in population studies is more varied. The systematic review by Mahmud et al. found that nonaspirin NSAIDs were inversely associated with prostate cancer (OR = 0.87, 95% CI: 0.61, 1.23), although this finding was not statistically significant (P = 0.43), and substantial heterogeneity was evident between studies (P = 0.005). One major unresolved issue that might add clarity to the population studies concerns the fraction of ingested aspirin or of the different nonaspirin NSAIDs appearing in prostate tissues and/or the lumens of prostate glands (the location of many of the inflammatory cells). Careful pharmacological studies, using drugs like aspirin and desipramine as model agents, have suggested that factors such as hydrophobicity, pH–pKa partitioning, and metabolism, might affect the propensity of drugs to pass into the prostate and seminal vesicles. For protection against prostatic carcinogenesis, drugs that are incapable of accumulating in the prostate at sufficient concentrations to attenuate inflammation are unlikely to be of great benefit.
While there have been clinical trials evaluating the combined effects of polyamine modulators (DFMO) with aspirin or other nonaspirin NSAIDs on colorectal cancer incidence, there has been no randomized clinical trial testing the effect of aspirin in combination with polyamine modulators on prostate cancer incidence. When considered together, the basic, preclinical, epidemiological, and limited clinical trial evidence supports further study of the protective effects of aspirin and/or nonaspirin NSAIDs with DFMO against prostate cancer development. Identifying a subpopularion of patients that are most likely to respond to NSAIDs or DFMO, based on biomarker profile, and utilizing NSAIDs as targeted therapies are most likely to optimize success.
4.4 Roles of Inflammation and Polyamines in Colon Cancer
4.4.1 Polyamines and Colon Cancer
Colon carcinogenesis involves several intermediate stages. Normal colonic epithelium gives rise to small adenomas and then to large adenomas, which subsequently progress to metastatic carcinomas (Fearon and Vogelstein 1990). In colon cancer tissue, the activities of polyamine-synthesizing enzymes and polyamine content are increased 10- to 15-fold in comparison to normal colonic epithelium (Elitsur et al. 1992), and polyamines have therefore been considered even as specific markers for neoplastic proliferation in the colon (Higuchi and Wang 1995). ODC activity and expression have been among the first bio-markers of neoplastic proliferation: as early as 1984 it was shown that ODC activity correlates with risk for neoplastic transformation in patients with colonic adenomatous polyposis (Luk and Baylin 1984). ODC activity correlates also with a degree of dysplasia in Barrett’s esophagus (Gerner et al. 1994) and different stages of colonic carcinogenesis (Giardicllo et al. 1997). Two of the most commonly mutated genes in colon cancer found in this study include the adenomatous polyposis coli (APC) tumor suppressor gene and the K-RAS oncogene, both of which have been shown to regulate the expression of several polyamine metabolic genes (Erdman et al. 1999; Ignatenko et al. 2004). Polyamine synthesis and levels of individual polyamines increase in colorectal cancers, compared to adjacent apparently normal mucosal tissue (Hixson et al. 1993). One mechanism by which APC mutations promote tumorigenesis is to increase transcription of ODC, via a c-MYC dependent process, and polyamine synthesis in human cells and ApcMin/+ mice (Erdman et al. 1999; Fultz and Gerner 2002). ODC enzyme activity and polyamine contents are also elevated in the apparently normal colonic mucosa of presymptomatic, genotype-positive individuals with familial adenomatous polyposis (FAP), an inherited syndrome caused by mutation/deletions in the APC gene. K-RAS acts to increase cell and tissue polyamine contents by increasing ODC enzyme activity and by own-regulating expression of the SAT1 via a transcriptional mechanism involving the peroxisomal proliferator activated receptor g (PPARg) (Ignatenko ct al. 2004). Inactivation of PAO has been shown to impede colon carcinogenesis in dimethylhyclrazine treated rat model (Halline and Brasitus 1990). Derivatives of spermidine and spermine, acetylated at the N1 position by SAT1, have been shown to accumulate in neoplastic but not normal tissues during carcinogenesis in the rat model and in human cancer (Loser et al. 1990), probably reflecting high levels of these amines in neoplasia.
4.4.2 Polyamines, Inflammation, and Colon Cancer
Colonic bacteria provide sources of potential tumor-promoting polyamines and other luminal risk factors for colon cancer. These bacteria metabolize primary bile acids to secondary bile acids, which have been associated with colon cancers in humans and are capable of promoting colon carcinogenesis in rodent models. There have been multiple studies suggest a linkage between polyamines and inflammation, and polyamines, inflammation, and colon cancer (Bernstein et al. 2006, 2007; Yerushalmi et al. 2007a). This linkage may be more than simply an association. Microarray analysis of human colon cancer-derived cells identified SAT1 as a target of the nonsteroidal anti-inflammatory drug sulindac (Babbar et al. 2003). Sulindac and other nonsteroidal anti-inflammatory drug act by distinct transcriptional mechanisms to induce SAT1 and promote the export of diamines and acetylpolyamines in both human cell and mouse models (Babbar et al. 2006; Ignatenko et al. 2006). Polyamines are oxidized by several amine oxidases to produce reactive oxygen species and aldehydes (Babbar et al. 2007). Polyamines can also influence the expression of the pro-inflammatory gene cyclooxygenase 2 by a posttranscriptional mechanism (Parker and Gerner 2002).
4.4.3 Targeting Polyamines and Inflammation In Colon Cancer
NSAIDs have been used to treat arthritis since 1899, when the analgesic and anti-inflammatory effects of aspirin were first recognized. NSAIDs have been shown experimentally to stimulate apoptosis and to inhibit angiogenesis, two mechanisms that help to suppress malignant transformation and tumor growth. Numerous epidemiologic studies have found that long-term users of aspirin or other NSAIDs have a lower risk of colorectal adenomatous polyps and colorectal cancer than nonusers (Thun et al. 1991; Deutsch 1992; Paganini-Hill et al. 1992; Schreinemachers and Everson 1994; Goldberg 1996). Randomized clinical trials have confirmed that the NSAIDs, Sulindac (Giardiello et al. 1993; Stoner et al. 1999) and the selective COX-2 inhibitor celecoxib (Steinbach et al. 2000), effectively inhibit the growth of adenomatous polyps and cause regression of existing polyps in patients with FAP. Observations from our own studies and those of others indicate that several NSAIDs, which have the ability to suppress carcinogenesis in some tissues, activate SAT1 as part of their anticancer activity. These activation mechanisms are NSAID-specific (Babbar et al. 2006), but involve PPAR-gamma in the case of sulindac (Babbar et al. 2003).
Inhibitors of polyamine synthesis, such as DFMO, suppress intestinal and colon carcinogenesis in experimental murine models (Gerner et al. 2003). Further, there is evidence suggesting that diet and genetic host factors may distinguish between individuals who will and will not benefit from specific, high-priority colon cancer-preventive agents. Specifically, we have found that DFMO suppresses only the development of high-grade colon adenomas that form in the ApcMin/+ mouse as a consequence of dietary supplementation of arginine at levels corresponding to arginine consumption in humans (Yerushalmi et al. 2007b). These results showed that the major effect of DFMO was to reduce the number of high risk, as determined by pathological high grade, adenomas while having little effect on the number of total colon adenomas.
Due to the suppressive effects of both NSAIDs and polyamine inhibitors on colon carcinogenesis, it was logical to assume that combination of these types of agents might lead to increased anticarcinogenic results. Further, combinations of these drugs could offer the potential of efficacy at much lower concentrations than would have required if they were used independently. Actually, evidence for the efficacy of targeting inflammation and polyamine synthesis for cancer chemoprevention began to accumulate over 20 years ago with the work of Nigro and colleagues (Nigro et al. 1986), which proposed the strategy of combination chemoprevention following the success of combination chemotherapy for certain types of cancer. Combination chemoprevention offered the prospect of reduced toxicities by lowering doses of individual agents. Many experimental studies have shown that DFMO acts at least additively with a number of NSAIDS, including the COX1 selective agent aspirin (Li et al. 1999), the COX2 selective agent celecoxib, and nonselective inhibitors of both COX1 and COX2, including piroxicam and sulindac (Lawson et al. 2000). Clinical evidence for beneficial effects of many NSAIDs on colon polyp formation has also accumulated – sulindac in high-risk individuals such as those with FAP (Giardiello et al. 1993), aspirin, and colon adenomas (Greenberg et al. 1993; Sandler et al. 2003). We have described that aspirin is able to decrease the risk of colon adenoma recurrence in a statistically significant manner, especially in individuals who were found to have a single nucleotide polymorphism (SNP) in the ODC promoter (Martinez et al. 2003). The relationship between this ODC SNP and aspirin and risk of polyp recurrence has now been independently corroborated in participants of a prospective randomized trial of aspirin for colon polyp prevention (Barry et al. 2006; Hubner et al. 2008). It is speculated that the mechanism of this association involves the combined action of the ODC-A allele-specific suppression of ODC transcription, and aspirin activation of SAT1 and polyamine export.
Meyskens et al. recently showed the dramatic efficacy of a combination of DFMO and the sulindac in a randomized double-blind, placebo-controlled phase III trial for colorectal adenoma prevention (Meyskens et al. 2008). This combination was markedly more effective in preventing metachronous colorectal adenomas when compared to other interventions, as highlighted in Table 4.1. In the Meyskens’ trial, patients with a genetic risk of colon cancer or with current or prior colon or other cancers were excluded. Participants received the combination of DFMO (two 250-mg pills daily) and sulindac (one 150 mg pill daily), or placebo pills, for 3 years. Sulindac was used at a relatively low concentration to avoid any adverse cardiovascular (CV) events, which have been shown to occur in several randomized trials with cyclooxygenase-2-selective NSAIDs (Bertagnolli et al. 2006; Solomon et al. 2006) and assumed to occur with COX-2 nonselective NSAIDs including sulindac. The primary end points included adenoma recurrence and toxicity assessment. Treatment with DFMO and sulindac produced a 70% reduction in total polyps, and 91.5% reduction in both advanced adenomas and in patients with multiple recurrent adenomas, at the end of 3 years. Treatment-associated toxicities were rare but the risk of adverse CV event associated with DFMO/sulindac increased with a high, but not with a low, baseline CV risk score (Zell et al. 2009). These findings were consistent with data from larger studies, but due to small numbers, the study did not have sufficient power to study the statistical interaction between baseline CV risk and the intervention. This combination clinical trial of DFMO and sulindac provides as an important proof of principle that targeting polyamine metabolism and inflammation can be an effective strategy for reducing risk factors, such as colon adenomas, that are closely associated with the development of colon cancers in humans. This strategy may be applicable for reduction of risk factors in other human cancers such as breast, lung, and prostate.
Table 4.1.
Comparison of recent colorectal polyp prevention trails. This table summarizes studies reported over the past decade that have evaluated a number of interventions for their potential to reduce metachronous colorectal polyps. These trials all accrued similar patient populations (i.e. patients with prior sporadic colorectal polyps) and employed similar intervention intervals (approximately 3 years in all cases). The numbers of patients in each trial were different and give an indication of the ability to detect differences between treatment and placebo groups. The metachronous adenoma rate in the placebo arms of these trials is listed, to show that the populations were similar in this regard
| Intervention | Investigator (year) | Participants | Metachronous adenoma rate (%) | Intervention effect (ralative risk or fraction placebo) |
|---|---|---|---|---|
| Calcium | (Baron et al. 1999) | 832 | 38 | 0.85 (P = 0.03) |
| Fiber | (Alberts et al. 2000) | 1303 | 51 | 0.88 (P = 0.28) |
| High Vegetable & Fruit/Low Fat | (Schatzkin et al. 2000) | 1905 | 40 | 1.00 |
| Aspirin | (Baron et al. 2003) | 1084 | 47 | 0.81 (any) 0.59 (advanced) |
| Celebrex | (Bertagnolli et al. 2006) | 2035 | 61 (any) 17 (advanced) |
0.67 (P<0.001) 0.55 (P<0.001) |
| Celebrex | (Arber et al. 2006) | 1561 | 49 (any) 10 (advanced) |
0.64 (P<0.001) 0.49 (P<0.001) |
| Vioxx | (Baron et al. 2006) | 2587 | 55 | 0.76 (P<0.01) |
| DFMO+Sulindac | (Meyskens et al. 2008) | 375 | 42 (any) 11 (advanced) |
0.31 (P<0.001) 0.08 (P<0.001) |
4.5 Roles of Inflammation and Polyamines in Other Cancers
Besides prostate and colon cancer, polyamine metabolism (by use of polyamine synthesis inhibitors or polyamine catabolism activators) and inflammation (by use of NSAIDs) have been studied for many cancers, including skin, lung, bladder (Messing et al. 2006; Herr 2007), cervical (Vlastos et al. 2005), esophageal (Fong et al. 2001), gastric (Takahashi et al. 2000), pancreatic, and breast (Xu et al. 2008). These agents have been used independent of each other and have shown varying results due to the lack of a consensus for the dose and the length of the study. There is compelling evidence that both polyamines and inflammation play a crucial role in these cancers. For example, inflammation has been postulated to play a key role in lung carcinogenesis. There is a growing body of evidence to suggest that smoking induced pulmonary inflammation increases lung cancer development in smokers (Brody and Spira 2006; Smith et al. 2006) and in the regular use of aspirin and other nonsteroidal anti-inflammatory drugs is associated with reduced risk of developing lung cancer in animal models and in smokers (Moysich et al. 2002; Smith et al. 2006). It has been shown that induction of spermine oxidase by a pro-inflammatory cytokine like TNFalpha which is generated due to chronic inflammation, can lead to the continuous production of reactive oxygen species (ROS) which in turn can lead to DNA damage thereby leading to mutations (Babbar and Casero 2006). Further, drugs that decrease the concentrations of intracellular polyamines have been shown to have preventive effects in lung cancer cells, as is the case with polyamine analogs, which increase the polyamine catabolism (Huang et al. 2005) or DFMO which inhibits polyamine synthesis.
The nonmelanoma skin cancers (NMSC), basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are the most common malignancies in whites worldwide. The vast majority of NMSCs are caused by excessive exposure to UV radiation. BCC and SCC prevention has focused mainly on limiting the amount of UV radiation that reaches the skin. Research into the pathogenesis of BCC and SCC has stimulated interest in chemopreventive agents that inhibit the activity of COX-2 and ODC, both of which contribute to UVB-induced skin cancer development. In murine models as well as in humans, UVB enhances the expression of COX-2-dependent prostaglandin production, particularly of PGE2 (Athar et al. 2001; An et al. 2002; Rundhaug and Fischer 2008). COX-2 and PGE2 have important roles in skin carcinogenesis, stimulating the proliferation of preneoplastic cells and promoting inflammation during the promotion stage. They also facilitate the epitheiial-to-mesenchymal transition, suppress host antitumor defense mechanisms, inhibit tumor cell apoptosis, and stimulate angiogenesis during the progression stage of skin carcinogenesis. Not expressed in undamaged normal epidermis, COX-2 can be induced in such tissue by acute and chronic exposure to UVB (Rundhaug and Fischer 2008) and is overexpressed in the epithelia of actinic keratoses and SCCs (An et al. 2002). COX-2 is also found in BCC, where its distribution differs from that of SCCs. COX-2 is found in the stroma surrounding islands of epithelial BCC cells in mice and primarily in the tissue adjacent to BCC islands in humans (An et al. 2002). ODC activity and polyamine levels are dramatically elevated in human squamous cell carcinomas compared to adjacent normal skin tissue. ODC is also transiently induced in the skin by a variety of stimuli including mitogens, tumor promoters such as 12-O-tetradecanoylphorbol ester (TPA), and hormones which has been confirmed by the use of transgenic mouse models for skin tumorigenesis. Several transgenic mouse model studies have shown the essential role of polyamines in the early promotion of skin tumorigenesis. Elevated levels of ODC activity are sufficient to promote skin tumor formation, without the addition of tumor-promoting agents, in the carcinogen-exposed skin of K6/ODC transgenic mice, where ODC is constitutively targeted to the skin with a keratin 6 or keratin 5 promoters (Tang et al. 2004). In addition, doses of UVB radiation that are insufficient to produce tumors in SKH-1 hairless mice will induce both premalignant papillomas and SCCs in K6/ODC transgenic mice. Numerous preclinical studies provide further evidence of the contribution of ODC to the development of BCC is convincing (Tang et al. 2004). DFMO has been shown to have an inhibitory effect on skin carcinogenesis by depleting polyamine levels in murine models of skin cancer (Gensler 1991; Ahmad et al. 2001; Megosh et al. 2002). As recently reported by Bailey et al. a randomized, double-blind, placebo-controlled phase III trial of DFMO (0.5 g/m2/day) versus placebo for up to 5 years produced promising results in men and women with a previous history of skin cancer (Bailey et al. 2010). Although DFMO did not achieve the primary objective of a statistically significant reduction in new NMSC or inhibit the development of SCC, it decreased BCCs by 30%. The BCC result was significant not only from a statistical standpoint (P = 0.03), but also because it is the first time that a chemopreventive agent other than sunscreens has prevented BCCs in subjects who do not have conditions that predispose them to develop this cancer. This study provided evidence that ODC inhibition represents an important molecular target-based approach for preventing skin cancer and validates the utility of DFMO as a cancer chemopreventive agent in another human organ system besides the colon. Fischer et al. showed that treatment with a combination of DFMO and the NSAID celecoxib was more effective in diminishing UVB-induced SCCs in murine skin than the treatment with either agent alone (Fischer et al. 2001). These findings coincide nicely with the findings of Meyskens et al. in colorectal adenomatous polyps. Therefore, adding an oral COX inhibitor to oral DFMO may have additive or synergistic effects in BCC and may allow chemopreventive efficacy with a lower dose of DFMO and/or the COX inhibitor, thus potentially reducing the toxicity either single agent might cause.
4.6 Conclusions
Recent clinical trials suggest that targeting polyamine metabolism alone or in combination with anti-inflammatory drugs (NSAIDS) is an effective and relatively safe method of reducing risk factors (e.g., colon adenomas for colon cancer, PSA doubling time for prostate cancer) for specific cancers. Experimental evidence suggests that polyamine metabolism may influence inflammatory processes and that certain NSAIDS act on polyamine metabolism by activating polyamine export mechanisms. The strategy of combining polyamine synthesis inhibitors with NSAIDS may be applicable for reduction of risk factors for several major types of human cancer.
Acknowledgments
This work was supported in part by grants from the USPHS National Institutes of Health CA95060 and CA123065.
Abbreviations
- APAO
FAD-dependent polyamine oxidase
- DFMO
D,L-α-difluoromethylornithine
- NSAID
Non-steroidal anti-inflammatory drug
- ODC
Ornithine decarboxylase
- SAMDC
S-adenosylmethionine Decarboxylase
- SAT1
Spermidine/Spermine N1-Acetyltransferase
- APC
Adenomatous polyposis coli
- SMO
Spermine oxidase
- OAZ
Ornithine decarboxylase antizyme
- NOS
Nitric oxide synthases
- PPAR
Gamma: Peroxisomal proliferator activated receptor γ
- COX-2
Cyclooxygenase-2
- TNFalpha
Tumor necrosis factor-α
- BCC
Basal cell carcinoma
- FAP
Familial adenomatous polyposis
- PIA
Proliferative inflammatory atrophy
- PIN
Prostatic intraepithelial neoplasia
- PSA
Prostate specific antigen
- NMSC
Nonmelanoma skin cancers
- SCC
Squamous cell carcinoma
Footnotes
Disclaimer The views and opinions presented by Babbar, N. are solely the author’s and do not necessarily reflect the views and opinions of his employer, Osmetech Molecular Diagnostics.
Contributor Information
Naveen Babbar, Email: naveen.babbar@osmetech.com, Research and Development, Osmetech Molecular Diagnostic, 757 S Raymond Avenue, Pasadena, CA 91105, USA.
Eugene W. Gerner, Email: egerner@azcc.arizona.edu, Arizona Cancer Center, University of Arizona, 1515 North Campbell Avenue, Tucson, AZ 85724, USA
References
- Ahmad N, Gilliam AC, Katiyar SK, et al. A definitive role of ornithine decarboxylase in photocarcinogenesis. Am J Pathol. 2001;159(3):885–892. doi: 10.1016/S0002-9440(10)61764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342(16):1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76(1):73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- Athar M, An KP, Morel KD, et al. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280(4):1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66(23):11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- Babbar N, Ignatenko NA, Casero RA, Jr, et al. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278(48):47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394(Pt 1):317–324. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbar N, Murray-Stewart T, Casero RA., Jr Inflammation and polyamine catabolism: the good, the bad and the ugly. Biochem Soc Trans. 2007;35(Pt 2):300–304. doi: 10.1042/BST0350300. [DOI] [PubMed] [Google Scholar]
- Bailey HH, Kim K, Verma AK, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila Pa) 2010;3(1):35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bardia A, Platz EA, Yegnasubramanian S, et al. Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol. 2009;9(4):419–426. doi: 10.1016/j.coph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- Barry EL, Baron JA, Bhat S, et al. Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention. J Natl Cancer Inst. 2006;98(20):1494–1500. doi: 10.1093/jnci/djj398. [DOI] [PubMed] [Google Scholar]
- Bernstein H, Holubec H, Bernstein C, et al. Unique dietary-related mouse model of colitis. Inflamm Bowel Dis. 2006;12(4):278–293. doi: 10.1097/01.MIB.0000209789.14114.63. [DOI] [PubMed] [Google Scholar]
- Bernstein H, Holubec H, Bernstein C, et al. Deoxycholate-induced colitis is markedly attenuated in Nos2 knockout mice in association with modulation of gene expression profiles. Dig Dis Sci. 2007;52(3):628–642. doi: 10.1007/s10620-006-9608-0. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Davalli P, Astancolle S, et al. Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res. 2000;60(1):28–34. [PubMed] [Google Scholar]
- Block TM, Mehta AS, Fimmel CJ, et al. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22(33):5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc. 2006;3(6):535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- Coleman CS, Stanley BA, Viswanath R, et al. Rapid exchange of subunits of mammalian ornithine decarboxylase. J Biol Chem. 1994;269(5):3155–3158. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat A, Palvimo JJ, Julkunen M, et al. Comparison of androgen regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase gene expression in rodent kidney and accessory sex organs. Endocrinology. 1992;130(3):1131–1144. doi: 10.1210/endo.130.3.1537280. [DOI] [PubMed] [Google Scholar]
- Cyriac J, Haleem R, Cai X, et al. Androgen regulation of spermidine synthase expression in the rat prostate. Prostate. 2002;50(4):252–261. doi: 10.1002/pros.10052. [DOI] [PubMed] [Google Scholar]
- Danzin C, Jung MJ, Claverie N, et al. Effects of alpha-difluoromethylomithine, an enzyme-activated irreversible inhibitor or ornithine decarboxylase, on testosterone-induced regeneration of prostate and seminal vesicle in castrated rats. Biochem J. 1979;180(3):507–513. doi: 10.1042/bj1800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol Oncol. 2007a;25(5):398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007b;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch ME. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1992;326(19):1289. doi: 10.1056/NEJM199205073261912. discussion 1290–1281. [DOI] [PubMed] [Google Scholar]
- Elitsur Y, Moshier JA, Murthy R, et al. Polyamine levels, ornithine decarboxylase (ODC) activity, and ODC-mRNA expression in normal and cancerous human colonoeytes. Life Sci. 1992;50(19):1417–1424. doi: 10.1016/0024-3205(92)90260-v. [DOI] [PubMed] [Google Scholar]
- Erdman SH, Ignatenko NA, Powell MB, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis. 1999;20(9):1709–1713. doi: 10.1093/carcin/20.9.1709. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fischer SM, Lee M, Lubet RA. Difluoro-methylornithine is effective as both a preventive and therapeutic agent against the development of UV carcinogenesis in SKH hairless mice. Carcinogenesis. 2001;22(1):83–88. doi: 10.1093/carcin/22.1.83. [DOI] [PubMed] [Google Scholar]
- Fjosne HE, Strand H, Sunde A. Dose-dependent induction of ornithine decarboxylase and S-adenosyl-methionine decarboxylase activity by testosterone in the accessory sex organs of male rats. Prostate. 1992;21(3):239–245. doi: 10.1002/pros.2990210307. [DOI] [PubMed] [Google Scholar]
- Fong LY, Nguyen VT, Pegg AE, et al. Alpha-difluoromethylornithine induction of apoptosis: a mechanism which reverses pre-established cell proliferation and cancer initiation in esophageal carcinogenesis in zinc-deficient rats. Cancer Epidemiol Biomarkers Prev. 2001;10(3):191–199. [PubMed] [Google Scholar]
- Fultz KE, Gerner EW. APC-dependent regulation of ornithine decarboxylase in human colon tumor cells. Mol Carcinog. 2002;34(1):10–18. doi: 10.1002/mc.10043. [DOI] [PubMed] [Google Scholar]
- Gensler HL. Prevention by alpha-difluoromethylornithine of skin carcinogenesis and immunosuppression induced by ultraviolet irradiation. J Cancer Res Clin Oncol. 1991;117(4):345–350. doi: 10.1007/BF01630718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner EW, Garewal HS, Emerson SS, et al. Gastrointestinal tissue polyamine contents of patients with Barrett’s esophagus treated with alpha-difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 1994;3(4):325–330. [PubMed] [Google Scholar]
- Gerner EW, Ignatenko NA, Besselsen DG. Preclinical models for chemoprevention of colon cancer. Recent Results Cancer Res. 2003;163:58–71. doi: 10.1007/978-3-642-55647-0_6. discussion 264–266. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Hylind LM, et al. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57(2):199–201. [PubMed] [Google Scholar]
- Gobert AP, Cheng Y, Wang JY, et al. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168(9):4692–4700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. Aspirin and colon cancer. N Engl J Med. 1996;334(12):800–801. [PubMed] [Google Scholar]
- Goodwin AC, Jadallah S, Toubaji A, et al. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68(7):766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Freeman DH, Jr, et al. Reduced risk of large-bowel adenomas among aspirin users. The Polyp Prevention Study Group. J Natl Cancer Inst. 1993;85(11):912–916. doi: 10.1093/jnci/85.11.912. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ahmad N, Marengo SR, et al. Chemoprevention of prostate carcinogenesis by alpha-difluoromethylornithine in TRAMP mice. Cancer Res. 2000;60(18):5125–5133. [PubMed] [Google Scholar]
- Halline AG, Brasitus TA. Effect of PAO inhibition on the colonic malignant transformation process induced by 1, 2-dimethylhydrazine. Carcinogenesis. 1990;11:2127–2132. doi: 10.1093/carcin/11.12.2127. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Murakami Y, Matsufuji S. Ornithine decarboxylase antizyme: a novel type of regulatory protein. Trends Biochem Sci. 1996;21 (1):27–30. [PubMed] [Google Scholar]
- Herr HW. Does difluoromethylornithine prevent recurrence in low-risk superficial bladder cancer? Nat Clin Pract Urol. 2007;4(5):240–241. doi: 10.1038/ncpuro0753. [DOI] [PubMed] [Google Scholar]
- Higuchi CM, Wang W. Comodulation of cellular polyamines and proliferation: biomarker application to colorectal mucosa. J Cell Biochem. 1995;57(2):256–261. doi: 10.1002/jcb.240570209. [DOI] [PubMed] [Google Scholar]
- Hixson LJ, Garewal HS, McGee DL, et al. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev. 1993;2(4):369–374. [PubMed] [Google Scholar]
- Huang Y, Pledgie A, Casero RA, Jr, et al. Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs. 2005;16(3):229–241. doi: 10.1097/00001813-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Hubner RA, Muir KR, Liu JF, et al. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res. 2008;14(8):2303–2309. doi: 10.1158/1078-0432.CCR-07-4599. [DOI] [PubMed] [Google Scholar]
- Ignatenko NA, Babbar N, Mehta D, et al. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol Carcinog. 2004;39(2):91–102. doi: 10.1002/mc.10166. [DOI] [PubMed] [Google Scholar]
- Ignatenko NA, Besselsen DG, Roy UK, et al. Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer. 2006;56(2):172–181. doi: 10.1207/s15327914nc5602_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EJ, Rodriguez C, Mondul AM, et al. A large cohort study of aspirin and other nonsteroidal anti-Inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97(13):975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- Kee K, Foster BA, Merali S, et al. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279(38):40076–40083. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- Lawson KR, Ignatenko NA, Piazza GA, et al. Influence of K-ras activation on the survival responses of Caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1155–1162. [PubMed] [Google Scholar]
- Li H, Schut HA, Conran P, et al. Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999;20(3):425–430. doi: 10.1093/carcin/20.3.425. [DOI] [PubMed] [Google Scholar]
- Loser C, Folsch UR, Paprotny C, et al. Polyamines in colorectal cancer. Evaluation of polyamine concentrations in the colon tissue, serum, and urine of 50 patients with colorectal cancer. Cancer. 1990;65(4):958–966. doi: 10.1002/1097-0142(19900215)65:4<958::aid-cncr2820650423>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Luk GD, Baylin SB. Ornithine decarboxylase as a biologic marker in familial colonic polyposis. N Engl J Med. 1984;311(2):80–83. doi: 10.1056/NEJM198407123110202. [DOI] [PubMed] [Google Scholar]
- Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G515–520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- Mahmud SM, Tanguay S, Begin LR, et al. Non-steroidal anti-Inflammatory drug use and prostate cancer in a high-risk population. Eur J Cancer Prev. 2006;15(2):158–164. doi: 10.1097/01.cej.0000197451.02604.25. [DOI] [PubMed] [Google Scholar]
- Martinez ME, O’Brien TG, Fultz KE, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100(13):7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megosh LC, Hu J, George K, et al. Generic control of polyamine-dependent susceptibility to skin tumorigenesis. Genomics. 2002;79(4):505–512. doi: 10.1006/geno.2002.6736. [DOI] [PubMed] [Google Scholar]
- Messing E, Kim KM, Sharkey F, et al. Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006;176(2):500–504. doi: 10.1016/j.juro.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Challa A, Gupta S, et al. Overexpression of ornithine decarboxylase in prostate cancer and prostatic fluid in humans. Clin Cancer Res. 1999;5(1):143–147. [PubMed] [Google Scholar]
- Moysich KB, Menezes RJ, Ronsani A, et al. Regular aspirin use and lung cancer risk. BMC Cancer. 2002;2:31. doi: 10.1186/1471-2407-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro ND, Bull AW, Boyd ME. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J Natl Cancer Inst. 1986;77(6):1309–1313. [PubMed] [Google Scholar]
- Paganini-Hill A, Hsu G, Ross RK, et al. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1992;326(19):1290. discussion 1290–1291. [PubMed] [Google Scholar]
- Parker MT, Gerner EW. Polyamine-mediated post-transcriptional regulation of COX-2. Bio-chimie. 2002;84(8):815–819. doi: 10.1016/s0300-9084(02)01439-6. [DOI] [PubMed] [Google Scholar]
- Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1995;4(5):447–451. [PubMed] [Google Scholar]
- Rundhaug JE, Fischer SM. Cyclooxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84(2):322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Russell D, Snyder SH. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- Schipper RG, Deli G, Deloyer P, et al. Antitumor activity of the polyamine analog N(1), N(11)-diethylnorspermine against human prostate carcinoma cells. Prostate. 2000;44(4):313–321. doi: 10.1002/1097-0045(20000901)44:4<313::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5(2):138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Sintoneau AR, Gerner EW, Nagle R, et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008;17(2):292–299. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18(9):667–677. doi: 10.1080/08958370600742821. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Budd GT, Ganapathi R, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Mai M, Nishioka K. alpha-difluoromethylornithine induces apoptosis as well as anti-angiogenesis in the inhibition of tumor growth and metastasis in a human gastric cancer model. Int J Cancer. 2000;85(2):243–247. [PubMed] [Google Scholar]
- Tang X, Kim AL, Feith DJ, et al. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1 +/− mice. J Clin Invest. 2004;113(6):867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Steege JC, Forget PP, Buurman WA. Oral spermine administration inhibits nitric oxide-mediated intestinal damage and levels of systemic inflammatory mediators in a mouse endotoxin model. Shock. 1999;11(2):115–119. doi: 10.1097/00024382-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58(2):244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Namboodtri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Uemura T, Yerushalmi HF, Tsaprailis G, et al. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem. 2008;283(39):26428–26435. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen E, Pukkala E, Timonen T, et al. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol. 1993;22(6):976–982. doi: 10.1093/ije/22.6.976. [DOI] [PubMed] [Google Scholar]
- Visvanathan K, Helzlsouer KJ, Boorman DW, et al. Association among an ornithine decarboxylase polymorphism, androgen receptor gene (CAG) repeat length and prostate cancer risk. J Urol. 2004;171(2 Pt 1):652–655. doi: 10.1097/01.ju.0000108384.74718.73. [DOI] [PubMed] [Google Scholar]
- Vlastos AT, West LA, Atkinson EN, et al. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin Cancer Res. 2005;11(1):390–396. [PubMed] [Google Scholar]
- Wang JY, Johnson LR, Tsai YH, et al. Mucosal ornithine decarboxylase, polyamines, and hyperplasia in infected intestine. Am J Physiol. 1991;260(1 Pt 1):G45–51. doi: 10.1152/ajpgi.1991.260.1.G45. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G315–319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- Xu H, Charurvedi R, Cheng Y, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64(23):8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- Xu H, Washington S, Verderame MF, et al. Role of non-receptor and receptor tyrosine kinases (TKs) in the antitumor action of alpha-difluoromethylornithine (DFMO) in breast cancer cells. Breast Cancer Res Treat. 2008;112(2):255–261. doi: 10.1007/s10549-007-9866-3. [DOI] [PubMed] [Google Scholar]
- Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. The role of NO synthases in arginine-dependent small intestinal and colonic carcinogenesis. Mol Carcinog. 2006a;45(2):93–105. doi: 10.1002/mc.20168. [DOI] [PubMed] [Google Scholar]
- Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. Role of polyamines in arginine-dependent colon carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog. 2006b;45(10):764–773. doi: 10.1002/mc.20246. [DOI] [PubMed] [Google Scholar]
- Zell AJ, Pelot D, Chen WP, et al. Risk of cardiovascular events in a randomized placebo-controlled, double blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila Pa) 2009;2(3):209–212. doi: 10.1158/1940-6207.CAPR-08-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185(10):1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]