Abstract
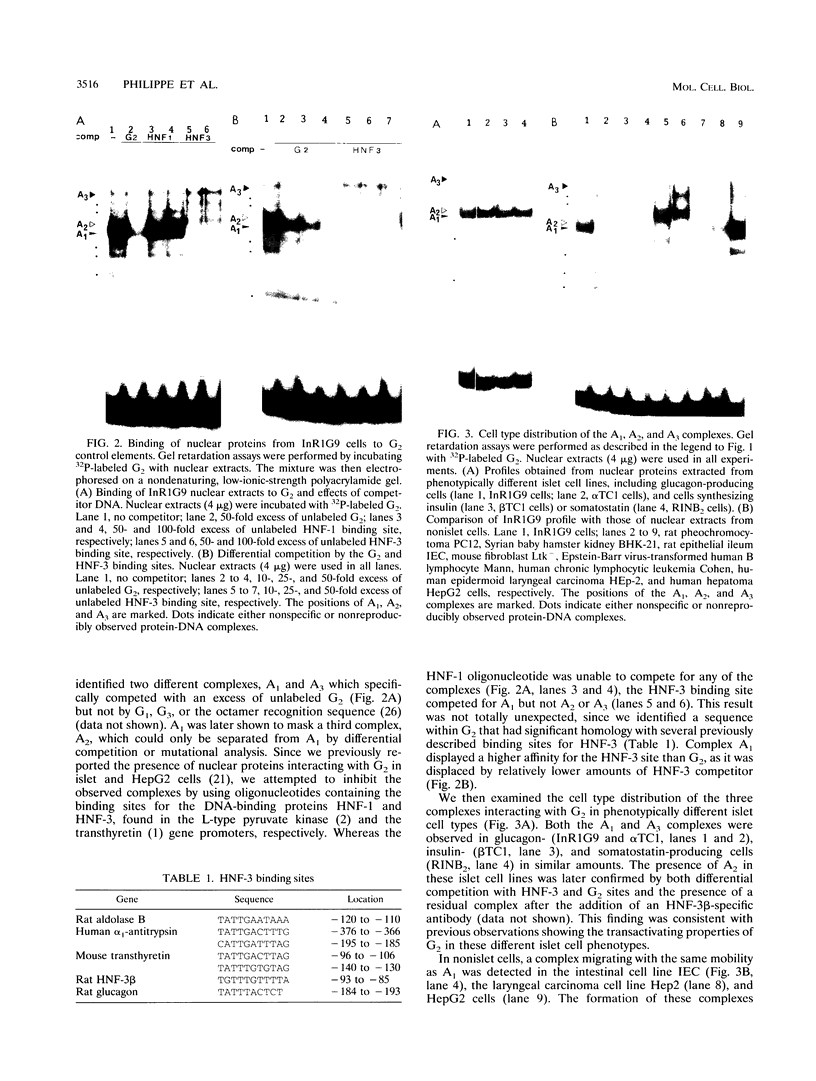
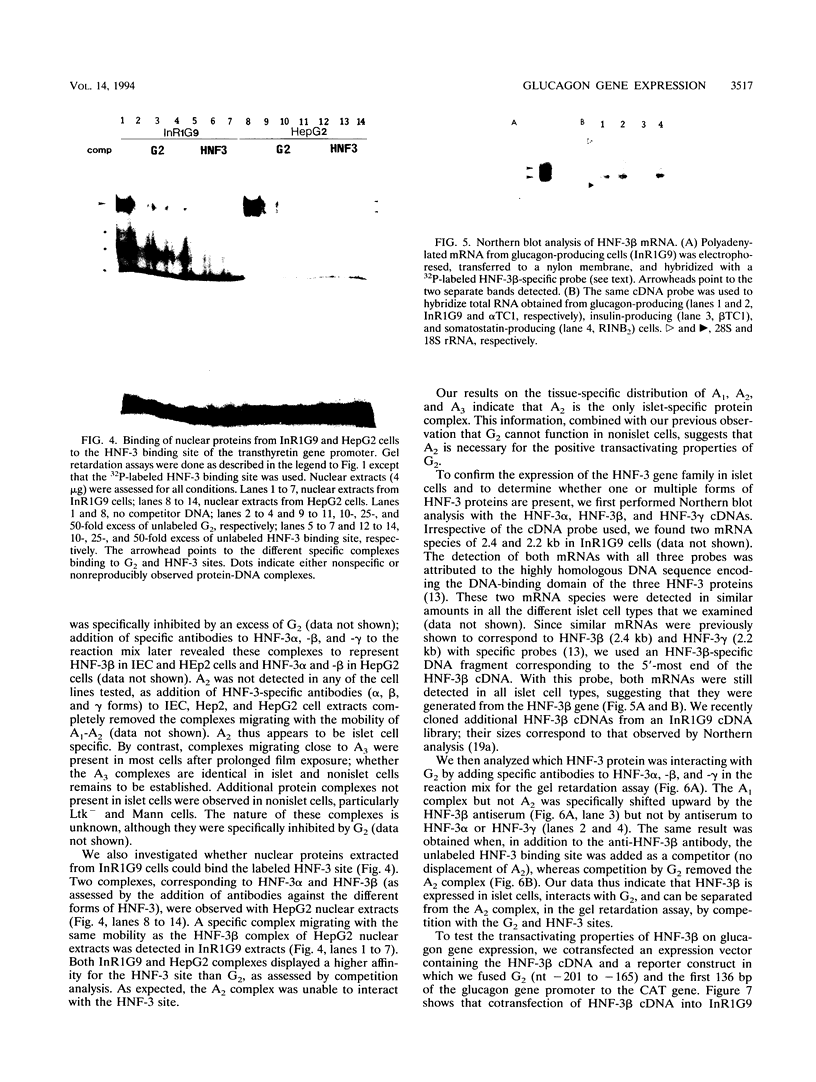
Pancreatic expression of the glucagon gene depends on multiple transcription factors interacting with at least three DNA control elements: G1, the upstream promoter element, and G2 and G3, two enhancer-like sequences. We report here that the major enhancer of the rat glucagon gene, G2, interacts with three protein complexes, A1, A2, and A3. A2 is detected only in islet cells, and impairment of its binding to mutant G2 causes a marked decrease in transcriptional activity. We identify A1 as hepatocyte nuclear factor 3 beta (HNF-3 beta), a member of the HNF-3 DNA-binding protein family found in abundance in the liver which has been proposed to play a role in the formation of gut-related organs. HNF-3 beta binds G2 on a site which overlaps A2 and acts as a repressor of glucagon gene expression, as demonstrated by mutational analyses of G2 and by cotransfection of HNF-3 beta cDNA along with reporter genes containing G2 into glucagon-producing cells. Our data implicate HNF-3 beta in the control of glucagon gene expression and strengthen the idea of endodermal origin of the islet cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaux J. F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989 Jul 15;264(20):11584–11590. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Philippe J., Jepeal L., Habener J. F. Glucagon gene 5'-flanking sequences promote islet cell-specific gene transcription. J Biol Chem. 1987 Nov 15;262(32):15659–15665. [PubMed] [Google Scholar]
- Efrat S., Linde S., Kofod H., Spector D., Delannoy M., Grant S., Hanahan D., Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Teitelman G., Anwar M., Ruggiero D., Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic alpha cells. Neuron. 1988 Sep;1(7):605–613. doi: 10.1016/0896-6273(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Emens L. A., Landers D. W., Moss L. G. Hepatocyte nuclear factor 1 alpha is expressed in a hamster insulinoma line and transactivates the rat insulin I gene. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7300–7304. doi: 10.1073/pnas.89.16.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Krasnow M. A. Differential regulation of transcription preinitiation complex assembly by activator and repressor homeo domain proteins. Genes Dev. 1992 Nov;6(11):2177–2189. doi: 10.1101/gad.6.11.2177. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Conley P. B., Chen L., Sladek F. M., Darnell J. E., Jr, Crabtree G. R. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992 Jan 30;355(6359):457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- Lai E., Darnell J. E., Jr Transcriptional control in hepatocytes: a window on development. Trends Biochem Sci. 1991 Nov;16(11):427–430. doi: 10.1016/0968-0004(91)90169-v. [DOI] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Tao W. F., Chen W. S., Darnell J. E., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991 Mar;5(3):416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Yamada K., Yamagata K., Takenaka M., Nakajima H., Imai E., Wang Z., Tanaka T. Expression of liver type pyruvate kinase in insulinoma cells: involvement of LF-B1 (HNF1). Biochem Biophys Res Commun. 1991 Nov 27;181(1):259–264. doi: 10.1016/s0006-291x(05)81411-1. [DOI] [PubMed] [Google Scholar]
- Pani L., Overdier D. G., Porcella A., Qian X., Lai E., Costa R. H. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Mol Cell Biol. 1992 Sep;12(9):3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L., Quian X. B., Clevidence D., Costa R. H. The restricted promoter activity of the liver transcription factor hepatocyte nuclear factor 3 beta involves a cell-specific factor and positive autoactivation. Mol Cell Biol. 1992 Feb;12(2):552–562. doi: 10.1128/mcb.12.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Chick W. L., Habener J. F. Multipotential phenotypic expression of genes encoding peptide hormones in rat insulinoma cell lines. J Clin Invest. 1987 Feb;79(2):351–358. doi: 10.1172/JCI112819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Knepel W., Jepeal L., Misulovin Z., Habener J. F. Alpha-cell-specific expression of the glucagon gene is conferred to the glucagon promoter element by the interactions of DNA-binding proteins. Mol Cell Biol. 1988 Nov;8(11):4877–4888. doi: 10.1128/mcb.8.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7224–7227. doi: 10.1073/pnas.88.16.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Missotten M. Functional characterization of a cAMP-responsive element of the rat insulin I gene. J Biol Chem. 1990 Jan 25;265(3):1465–1469. [PubMed] [Google Scholar]
- Philippe J., Rochat S. Strict distance requirement for transcriptional activation by two regulatory elements of the glucagon gene. DNA Cell Biol. 1991 Mar;10(2):119–124. doi: 10.1089/dna.1991.10.119. [DOI] [PubMed] [Google Scholar]
- Philippe J. Structure and pancreatic expression of the insulin and glucagon genes. Endocr Rev. 1991 Aug;12(3):252–271. doi: 10.1210/edrv-12-3-252. [DOI] [PubMed] [Google Scholar]
- Powers A. C., Efrat S., Mojsov S., Spector D., Habener J. F., Hanahan D. Proglucagon processing similar to normal islets in pancreatic alpha-like cell line derived from transgenic mouse tumor. Diabetes. 1990 Apr;39(4):406–414. doi: 10.2337/diab.39.4.406. [DOI] [PubMed] [Google Scholar]
- Prost E., Moore D. D. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45(1):107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G. POU-domain transcription factors: pou-er-ful developmental regulators. Genes Dev. 1991 Jun;5(6):897–907. doi: 10.1101/gad.5.6.897. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Takaki R., Ono J., Nakamura M., Yokogawa Y., Kumae S., Hiraoka T., Yamaguchi K., Hamaguchi K., Uchida S. Isolation of glucagon-secreting cell lines by cloning insulinoma cells. In Vitro Cell Dev Biol. 1986 Mar;22(3 Pt 1):120–126. doi: 10.1007/BF02623498. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E., Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989 May 19;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]










