Abstract
Existing biological models of post-traumatic-stress disorder (PTSD) posit that the amygdala plays a critical role in the development and expression of this disorder. However, increasing data indicate that the amygdalae are not functionally identical, raising the possibility that the two amygdalae may make differential contributions to the expression of PTSD. We present a unique patient who developed PTSD following a traffic accident that occurred two years after she had undergone removal of her left amygdala in order to treat pharmacologically intractable epilepsy. We propose that the right amygdala is preferentially involved in several processes related to the expression of PTSD symptoms, such that the disorder can occur even in the absence of the left amygdala.
Keywords: Emotion, Affect, Epilepsy, Anxiety disorders, Lateralization; Hemispheric asymmetries
Neurobiological models of post-traumatic stress disorder (PTSD) consistently highlight the role of the amygdala in the development and expression of this psychopathology (Rauch, Shin & Phelps, 2006; Rauch, Shin, & Wright, 2003). Such models focus on the critical role of the amygdala in fear conditioning (seeDavis, Walker & Lee, 1997; LeDoux, 2000, Maren, 2001, for reviews), and its involvement in modulating arousal and vigilance functions (Davis & Whalen, 2001). This structure also plays a key role modulating memory for emotional context (Rudy, Huff & Matus-Amat, 2004; Malin & McGaugh, 2006). Thus, the amygdala is in a position to directly mediate many of the symptoms of PTSD, such as the sustained elevations in arousal and startle sensitivity, and the ability of contextual cues related to the trauma (e.g., places, activities, and people) to trigger emotional distress.
To date, existing amygdalocentric theories of PTSD do not differentiate between the roles of the left and right amygdalae. This is not surprising given that much of the early work of fear conditioning in animals used bilateral preparations, and patients with medial temporal lobe lesions in either hemisphere have shown reductions in acquisition of conditioned fear responses (Labar et al., 1995). However, recent research suggests that the amygdalae are, in fact, structurally (Szabo et al., 2001) and functionally asymmetrical (see Baas, Aleman, & Kahn, 2004; Zald, 2003). For instance, Baker and Kim (2004) observed that in rats, post-training lesions to the right amygdala lead to a considerably larger impairment in conditioned contextual fear responses than lesions to the left amygdala. In humans, temporal lobectomy patients show asymmetries in their recall of unpleasant autobiographical memories, with patients with right temporal lobectomies showing a decreased ability to recall unpleasant emotional events, while left temporal lobectomy patients show normal levels of recall (Buchanan, Tranel, & Adolphs, 2006). Damage to the right amygdala also has been observed to produce a more global deficit in electrodermal responses than damage to the left amygdala (Gläscher & Adolphs, 2003; Weike et al., 2005), perhaps reflecting an asymmetry in global autonomic control.
Neuroimaging data have also pointed to functional asymmetries in amygdalar function. Several studies suggest that the right amygdala responds more to experientially learned or conditioned fearful stimuli, whereas, the left amygdala appears more active during the perception of innately fear-related items such as photographs of threatening stimuli or fearful faces (e.g., Büchel et al., 1998; Dolan and Morris, 2000; Morris et al., 1996, 1998). Similarly, Furmark and colleagues (Furmark et al., 1997) noted that rCBF in the right, but not the left, amygdala correlated with autonomic responses to aversively conditioned photographs. Finally, experiences of intense fear or panic more commonly arise during seizures involving the right medial temporal region than the left temporal lobe, a ratio that has been estimated at greater than 5:1 in favor of the right hemisphere (Sazgar, Carlen, & Wennberg, 2003).
Taken together, these animal-lesion, neuropsychological, and neuroimaging data suggest that many of the cognitive processes and physiological responses that are symptomatic of PTSD are preferentially mediated by the right amygdala. This viewpoint is supported by the work of Shin and colleagues (Shin et al., 2004) who reported that regional cerebral blood flow (rCBF) in the right amygdala, but not the left amygdala, positively correlated with PTSD symptom severity in veterans exposed to trauma scripts. From these findings, one would predict that there is an asymmetry in the importance of the right and left amygdala in the development of PTSD. While both amygdalae may contribute to the expression of fear conditioning, the right amygdala would be predicted to play a more critical role in mediated the larger constellation of symptoms associated with PTSD. As such, the symptoms of PTSD may arise in full even in the absence of the left amygdala. Consistent with this possibility, we present data from a patient who developed PTSD after being hit by a car two years after having undergone a resection of her left amygdala and anterior hippocampus.
Patient Details
CD is a 50-year-old, right-handed female who underwent a unilateral selective amygdalohippocampectomy (SAH) in order to alleviate pharmacologically intractable medial-temporal-lobe epilepsy. CD’s neurological history indicates that she had a prolonged febrile seizure at seven months of age, and likely began experiencing seizures at age four. She was diagnosed with epilepsy in her early 20’s. Her seizures were of the complex partial type with myoclonic jerking in the right hand. EEG data during this period indicated a left-temporal focus to her seizures. CD’s seizure frequency peaked in 1996, during which she recorded 115 seizures. She subsequently underwent an evaluation for neurosurgery. An MRI indicated left hippocampal sclerosis and FGD-PET scanning detected hypometabolism in the left inferior mesial-temporal lobe. WADA testing revealed right lateralization of memory, whereas language showed the typical left lateralization.
CD underwent a SAH utilizing the transcortical approach (Olivier, 2000) in 1999. As can be seen in Figure 1, her entire left amygdala has been removed, as has the anterior portion of her hippocampus to approximately 8 mm posterior to the posterior commissure. CD has been free of epileptiform activity since her surgery and is currently taking 1.5mg of clonazepam per day.
Figure 1.
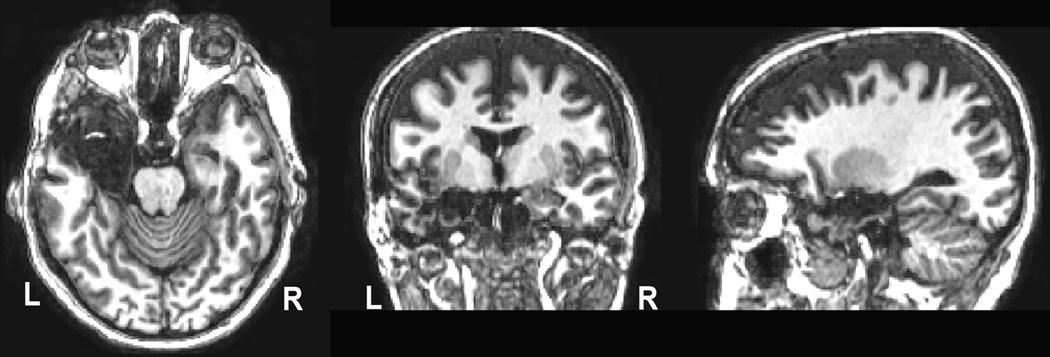
T1-weighted MRI performed 6 years post-surgery. The entire amygdala and anterior half of the hippocampus are absent in the left hemisphere. Data were collected on a 3T Phillips Intera Achieva scanner with a 3D Turbo Field Echo sequence, with 1 mm3 voxels: FOV= 256 × 256 × 170, flip = 5°, TE = 3.7 ms; TR = 8 ms).
Results of pre and post-surgical neuropsychological testing are presented in Table 1, with the post-surgery assessment occurring 6 years following her surgery. At both time points, her nonverbal intellectual abilities were better than verbal intellectual abilities, with the difference becoming more accentuated post-surgery (her 22-point difference represents a statistically significant difference in performance). The 36-point difference between her low average Verbal Comprehension Index and her high average Perceptual Organizational Index following surgery was also highly significant, and is consistent with intact right- relative to left-hemisphere functioning. As was predicted by her initial WADA testing, CD’s memory functioning was right lateralized, and removal of her left hippocampus does not appear to have produced additional memory impairment.
Table 1.
Neuropsychological Results
| Presurgery | Postsurgery | |||
|---|---|---|---|---|
| Test | Index Score |
Percentile | Index Score |
Percentile |
| IQ* | 95 | 37 | ||
| Verbal IQ | 107 | 68 | 88 | 21 |
| Performance IQ | 109 | 73 | ||
| Verbal Comprehension | 82 | 12 | ||
| Perceptual Organizational | 118 | 88 | ||
| Working Memory Index | 90 | 25 | ||
| Perceptual Speed Index | 91 | 27 | ||
| Memory** | 73 | 4 | ||
| Auditory Immediate | 117 | 87 | 86 | 18 |
| Auditory Delayed | 86 | 18 | ||
| Visual Immediate | 115 | 84 | ||
| Visual Delayed | 106 | 66 | ||
Presurgery - Wechsler Adult Intelligence Revised (Wechsler, 1981), Postsurgery -Wechsler Adult Intelligence Scale III (Wechsler, 1997).
Presurgery - Wechsler Memory Scale-Revised (Wechsler, 1987), Postsurgery -Wechsler Memory Scale III (Wechsler, 1997).
PTSD Symptoms
Two years following her neurosurgery, CD was struck by a red, Chevrolet Ventura van while walking down a sidewalk. The impact resulted in broken ribs, a punctured lung, and permanent damage to her left leg. Although she hit her head upon impact with the ground, there is no specific evidence that she sustained a closed-head injury. She quickly regained consciousness after being hit, and has vivid memories of the emergency responders that arrived at the scene of the accident.
The DSM-IV diagnosis of PTSD includes three classes of symptoms: recurrent re-experiencing of the trauma, persistent symptoms of arousal, and avoidance of stimuli associated with the trauma (American Psychiatric Association, 1994). CD displays pronounced symptoms in each of these three categories. She consistently experiences intense distress when exposed to situations and stimuli that are linked to the event. Paramount among these emotional reactions is the feeling of fear or distress when exposed to motor vehicles that resembled the van that hit her. She remains apprehensive whenever cars approach a car she is riding in. She similarly experiences severe distress when seeing flashing EMS lights (e.g., as on ambulances or police cars) or hearing sirens that she associates with the emergency vehicles that arrived at the accident scene. She describes several incidents in which hearing a siren forced her to stop whatever she was doing and caused her to scream and cry. The patient additionally reports recurrent intrusive, distressing recollections of the event, as well as nightmares related to the trauma. In all of these situations, she demonstrates marked physiological reactivity including perceived difficulty breathing, tachycardia, and sweating. On multiple occasions, these fear responses were observed by the authors, and the patient’s self-reports indicates that they occur with substantial regularity.
Persistent symptoms of increased arousal were also evident. These included difficulty breathing, irritability, difficulty concentrating, hypervigilance, and an exaggerated startle response. There was no evidence of emotional detachment or a general numbing of responsiveness; if anything, her affect was more labile, rather than blunted, since the trauma. The patient indicated that this increased arousal represented a substantial change from her pre-trauma state.
Symptoms of avoidance were also notable, as she avoided numerous activities that would require exposure to cars or stimuli associated with the accident. CD also demonstrates severe apprehension when crossing streets. Complete avoidance of these situations is not possible due to the necessity of traveling in cars to obtain medical care and to purchase food and the need to cross streets when walking. However, she demonstrates extreme caution while walking, and for a period of time wore a bright orange work vest with the words “LIVE PEDESTRIAN” written on it.
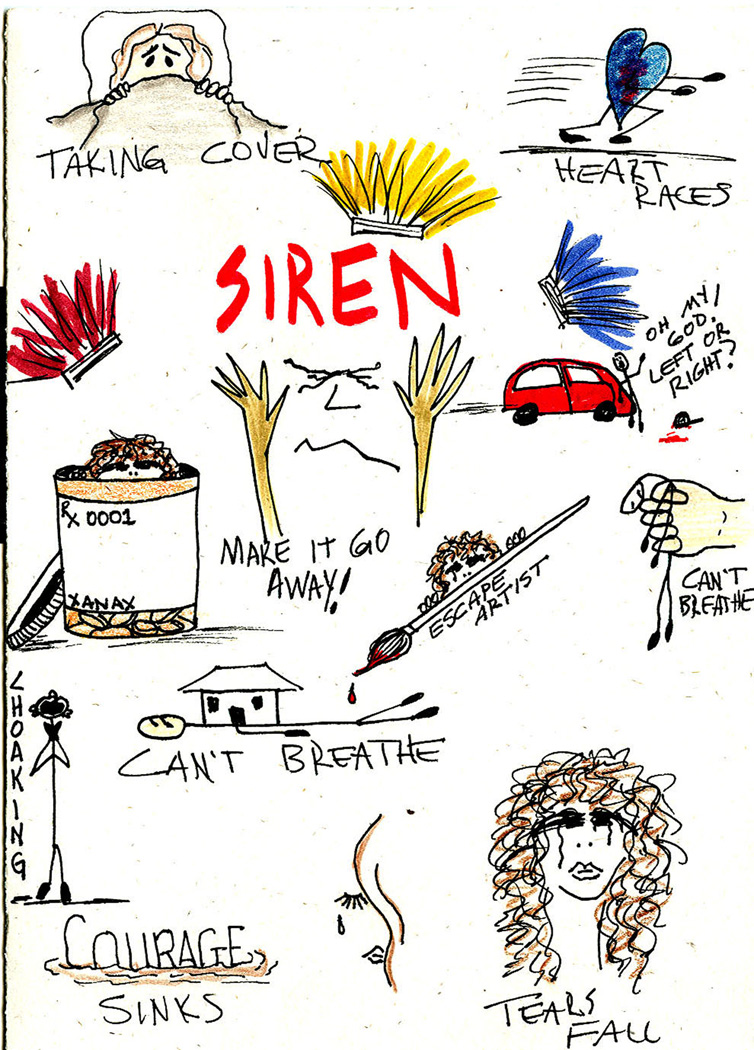
Whether or not she experiences a sense of foreshortened future, as described in the DSM-IV criteria, is not clear, but she indicated that she does not expect to be able to return to having a career, and seemed doubtful about romantic possibilities. At the time of assessment she was receiving disability payments, and did not plan on returning to work. Instead, she focuses on seeking treatment of her symptoms and artwork. An example of her art expressing the terror that she experiences is shown in Figure 2.
Figure 2.
A drawing by the patient expressing her anxiety symptoms. Reprinted with permission of the patient.
Quantitative assessment of CD’s PTSD symptoms were performed using the Revised Civilian Mississippi Scales, a 30-item scale designed to measure PTSD symptoms (Norris & Perilla, 1996). CD scored 107 on this scale (mean item score = 3.56 / 5), which is substantially greater than scores for non-PTSD individuals (mean item score = 1.68) and is consistent with a severe level of PTSD symptoms.
Experimental Tasks
Observation of CD’s behavior and her own self report suggest a high degree of attention and emotional reactivity to stimuli associated with her trauma. To empirically assess this reactivity, we asked CD and a group of 9 age- and education-matched controls (M =50.9, SD = 4.9 years) to complete two experimental tasks that measure the level of attentional disruption caused by exposure to emotional stimuli.
The emotional-Stroop task provides an index of the degree to which emotion interferes with the ability to focus attention on specific characteristics of linguistic stimuli (Williams, Mathews & MacLeod., 1996). Previous research has shown that emotionally arousing words slow participants’ ability to state the color in which the word is printed. Research also indicates that PTSD patients show enhanced distraction when exposed to trauma-related material (McNally, 1998; McNeil et al., 1999; Beck, 2001).
In the current study, CD and 6 of the control participants completed two emotional-Stroop tasks involving emotionally positive or emotionally negative words (data from 3 of the control participants were unfortunately not available for this task). Both of the Stroop tasks consisted of eight blocks, each consisting of 16 experimental trials. Four of the blocks consisted of emotionally neutral words from the same general category (e.g., evening, morning), two blocks of highly arousing words (e.g., pain, fear), and two blocks of mildly arousing words (e.g., lonely, gloom). On each trial, participants were instructed to state the color in which the word was printed as quickly and as accurately as possible. Reaction times and accuracy data were recorded for each participant.
CD demonstrated an exaggerated response to highly arousing negative words, showing a dramatically slowed response (1107.6ms), which is 3.4 standard deviations higher than that showed by age and education matched controls (M = 732.3 ms, SD = 110.5). Her responses to highly arousing positive words were slower than those of controls, although to a much less extent than found for negative words (CD = 961.1 ms; Controls = 838.7ms, SD = 122.6ms; 1 standard deviation difference). In contrast, her reaction time for neutral stimuli was within normal limits (792.5 ms) relative to the controls (M = 734.2, SD = 97.3). Her responses to low arousing positive and negative words were not dramatically slowed compared to controls (both < 1 SD slower). These results indicate that CD was particularly sensitive to negative emotional words; she explained this effect by stating that these words reminded her of the fact that she has PTSD.
CD and 9 healthy controls also completed an emotional-attentional-blink paradigm (Most et al., 2005). In this task, participants view a series of 17 photographs presented in a rapid-serial-visual-presentation (RSVP) stream. Participants are instructed to detect a target image (a rotated photograph) amongst this RSVP stream, and to indicate whether the image was rotated 90° to the left or to the right. Critically, distracting emotional photographs can appear either 200ms (Lag 2) or 800ms (Lag 8) before the target; previous studies have shown that these distractors impair accuracy (correct detection of the target) at Lag 2, suggesting a transient, emotion-induced ‘blink’ of attention.
We have recently shown that aversively conditioned photographs can also elicit an attentional blink (Smith et al., 2006). In this study, photographs of either cars or birds were paired with a loud burst of white noise during a conditioning paradigm. Following these aversive exposures, participants completed an emotional-attentional-blink study in which the critical distractors were cars, birds, or landscapes (which were similar to the 16 other items in the RSVP display). We found that conditioned class of stimuli impaired target detection at Lag 2 relative to the unconditioned class of stimuli.
In the current study, we assumed that CD’s PTSD would serve as conditioning for photographs of cars. We therefore had her complete the same experiment as mentioned above, without the conditioning phase. Our hypothesis was that her learned, intense reactions to cars would impair her performance in this task. Consistent with our predictions, CD’s accuracy was dramatically impaired when cars served as the critical distractors. Her accuracy on these trials was 21% lower than on neutral trials; in contrast, controls were only 9.5% worse on car-related trials (SD = 5.05). CD was therefore more than 2 standard deviations below the norm, suggesting that she had an exaggerated sensitivity to these stimuli. Interestingly, CD’s accuracy on trials in which car photographs were presented was nearly identical to the accuracy of undergraduates who performed this task after being conditioned to fear cars or birds (Smith et al., 2006). Taken together with her emotional Stroop results, these data indicate that CD shows a significant attentional bias for processing threat-related stimuli that generalizes across at least two distinct types of attentional tasks.
Discussion
The current case study demonstrates that PTSD can develop in the absence of a left amygdala. The patient displayed the cardinal triad of PTSD symptoms including reexperiencing of the trauma, persistent avoidance of stimuli associated with the trauma, and persistent symptoms of increased arousal. In addition to these classic symptoms, CD also shows attentional biases towards stimuli that are related to her PTSD; her responses on two attentional tasks clearly demonstrated that these stimuli modulated her attention more than they affected healthy controls. If we assume that amygdalar functions are indeed necessary for the expression of PTSD, this case supports an asymmetric model of the amygdala’s influence on PTSD symptoms. The case is consistent with the hypothesis that due to functional asymmetries in amygdalar functioning, the right amygdala plays a more critical role than the left amygdala in the expression of PTSD. Indeed, the present data suggests that the asymmetry may be great enough that the disorder can occur even in the absence of the left amygdala.
However, several caveats must be considered regarding the generalizability of conclusions drawn from this case. First, CD’s history of seizures may have influenced the functioning of both her healthy right temporal lobe and closely connected neural structures. Due to the pre-surgical dysfunction of her left amygdala, CD’s right amygdala may have become exceptionally efficient at establishing or maintaining fear-related connections (i.e., the right amygdala was performing the functions of both amygdalae). This adaptation may have primed the right amygdala to respond to fear, thus making it more sensitive to fear learning, and less sensitive to extinction processes than the amygdalae of healthy brains. Similarly, the history of seizures may have altered other closely connected areas, such as the ventromedial frontal regions, that typically play a role in extinction (Quirk, Garcia, & Gonzalez-Lima, 2006). Thus, it might be speculated that CD’s continued hyper-responsivity to cars and sirens, despite years of re-exposure, might primarily reflect seizure-induced dysfunction of her extinction mechanisms.
Second, it is possible that the removal of the left amygdala actually accentuated right amygdalar processing through a release of interhemispheric inhibition. According to this view, in a normal brain the left and right amygdalae achieve a functional balance, with each amygdala ensuring that the other amygdala does not become hyper-responsive. Damage to one amygdala would therefore release the other amygdala from this inhibition, thereby allowing its functions to be expressed to a greater degree than usual. In CD’s case, this release would manifest itself as an enhanced propensity to develop learned fearful associations such as those experienced in PTSD. However, this explanation of CD’s condition appears unlikely for several reasons. The primate brain, as opposed to the rodent brain, has very sparse connections between the left and right amygdalae. Indeed, the primate brain sends almost no cross-hemisphere projections to the other amygdala through the anterior commissure or corpus callosum, with the exception of some sparse labeling of the dorsal cortical nuclei (Demeter, Rosene, & Hoesen, 1990; Di Virgilio et al., 1999; Pandya & Rosene, 1985). Thus, if the left amygdala influences the functioning of the right amygdala in a healthy brain, the pathway underlying this influence has yet to be discovered. The absence of a dense, direct interhemispheric connection suggests that the two amygdalae work in a parallel rather than a coordinated fashion. Therefore, lesioning of the left amygdala would have a minimal effect on processes that are primarily dependent upon right-amygdala activity. Consistent with this interpretation is the fact that we know of no studies in which fear conditioning or memory for emotional context were enhanced following unilateral amygdala lesions.
A final issue that must be addressed is the possibility that events prior to CD’s left amygdala resection predisposed her to suffer PTSD at a later date. During interviews, CD described being sexually abused at age 5. She also reported a history of physical abuse from her second husband. Both of these traumas occurred while she had both amygdalae (although in the latter instance her left amygdala would undoubtedly have been damaged by years of seizures). While there is no evidence that she had PTSD associated with these events, it is possible that these traumatic experiences altered her amygdalar functioning in such as way as to make her more susceptible to developing PTSD symptoms. Consistent with this view, Bremner and colleagues (Bremner et al. 1993) found that in combat-exposed veterans, rates of PTSD are more than 3.5 times higher in individuals who suffered childhood abuse. The precise biological mechanisms through which prior abuse creates a vulnerability for developing later PTSD are not clear. To the extent that the amygdala plays a specific role in creating this vulnerability, either of CD’s amygdalae could have participated, since her left amygdala was removed long after these events had occurred.
The current research complements one other case in the literature involving a patient who showed signs of PTSD after a resection of the amygdala. In this case (Adami et al., 2006), a 19-year-old girl with a history of childhood abuse started showing symptoms of PTSD, as well as hysterical symptoms, two weeks following a left-amygdala resection for intractable epilepsy. This case differs from CD in that the PTSD-like symptoms were associated with a pre-surgical trauma (childhood abuse) rather than a post-surgical trauma (CD being hit by a car). Indeed, CD appears to be the first reported case of an individual developing PTSD for a traumatic event that occurred after one amygdala was removed.
CD’s unique case makes clear that PTSD can develop in the absence of one amygdala, and the observed laterality appears consistent with the hypothesis that the right amygdala plays a greater role than the left amygdala in the development of PTSD. It must be noted, however, that the current study represents a single individual. Given possible individual differences in the lateralization of amygdalar function (see Cahill et al., 2001, 2004; Canli et al., 2002; Zald, 2003), we must be cautious in the degree to which we generalize conclusions regarding laterality to larger populations. This is an unavoidable limitation: in order to study responses to trauma in lesion patients, one needs to have the conjunction of neurological patients with specific brain lesions and exposure to traumatic events. Given the infeasibility of recruiting large numbers of individuals with this conjunction of neuropathology and subsequent trauma, singular examples, such as the case of CD, provide the best available evidence of the effects of lateralized lesions on trauma-related disorders.
Acknowledgements
This work was supported by a grant from NIH (1R01 MH074567). The authors wish to thank Amy Cooter and Evan Shelby for their assistance with data collection. The authors also wish to thank patient CD for her patience, generosity, and intellectual curiosity.
References
- Adami P, Konig P, Vetter Z, Hausmann A, Conca A. Posttraumatic stress disorder and amygdala-hippocampectomy. Acta Psychiatrica Scandinavia. 2006;113:360–363. doi: 10.1111/j.1600-0447.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: Evidence for greater involvements of the right amygdala. Behavioral Neuroscience. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Beck JG, Freeman JB, Shipherd JC, Hamblen JL, Lackner JM. Specificity of Stroop interference in patients with pain and PTSD. Journal of Abnormal Psychology. 2001;100:536–543. doi: 10.1037//0021-843x.110.4.536. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behavioral Neuroscience. 2004;118:429–437. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain. 2006;129:115–127. doi: 10.1093/brain/awh672. [DOI] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related differences in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sexrelated hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learning & Memory. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Annals of the New York Academy of Sciences. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Demeter S, Rosene DL, Hoesen GWV. Fields of origin and pathways of the interhemispheric commissures in the temporal lobe of macaques. Journal of Comparative Neurology. 1990;302:29–53. doi: 10.1002/cne.903020104. [DOI] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. Cortical regions contributing to the anterior commissure in man. Experimental Brain Research. 1999;124:1–7. doi: 10.1007/s002210050593. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Morris JS. The functional anatomy of innate and acquired fear: Perspectives from neuroimaging. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 225–241. [Google Scholar]
- Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M. The amygdala and individual differences in human fear conditioning. NeuroReport. 1997;8:3957–3960. doi: 10.1097/00001756-199712220-00021. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal stimuli by the human amygdala. Journal of Neuroscience. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Malin ME, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences USA. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Experimental approaches to cognitive abnormality in posttraumatic stress disorder. Clinical Psychology Review. 1998;18:971–982. doi: 10.1016/s0272-7358(98)00036-1. [DOI] [PubMed] [Google Scholar]
- McNeil DW, Tucker P, Miranda R, Lewin M, Nordgren JC. Response to depression and anxiety Stroop stimuli in posttraumatic stress disorder, obsessive-compulsive disorder and major depressive disorder. Journal of Nervous and Mental Disease. 1999;187:512–516. doi: 10.1097/00005053-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin and Review. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Norris FH, Perilla JL. The revised Civilian Mississippi Scale for PTSD: Reliability, validity, and cross-language stability. Journal of Traumatic Stress. 1996;9:285–298. doi: 10.1007/BF02110661. [DOI] [PubMed] [Google Scholar]
- Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Canadian Journal of Neurological Science. 2000;27:68–76. doi: 10.1017/s031716710000069x. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL. Some observations on trajectories and topography of commissural fibers. In: Reeves AG, editor. Epilepsy and the Corpus Callosum. New York: Plenum; 1985. pp. 21–39. [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sazgar M, Carlen PL, Wennberg R. Panic attack semiology in right temporal lobe epilepsy. Epileptic Disorders. 2003;5:93–100. [PubMed] [Google Scholar]
- Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 6:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome L, Zald DH. An "emotional blink" of attention elicited by aversively conditioned stimuli. Emotion. 2006;6:523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: Differences regarding handedness. AJNR American Journal of Neuroradiology. 2001;22:1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Wechsler DA. WAIS-R Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler DA. Wechsler Memory Scale-Revised Manual. San Antonio TX: The Psychological Corporation; 1987. [Google Scholar]
- Wechsler DA. Wechsler Adult Intelligence Scale-III Manual. San Antonio TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler DA. Wechsler Memory Scale-III Manual. San Antonio TX: The Psychological Corporation; 1997. [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Weike AI, et al. Fear conditioning following unilateral temporal lobectomy: Dissociation of conditioned startle potentiation and autonomic learning. Journal of Neuroscience. 2005;25:11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]