Summary
Autophagy is a stress response protecting cells from unfavorable conditions, such as nutrient starvation. The class III phosphatidylinositol-3 kinase, Vps34, forms multiple complexes and regulates both intracellular vesicle trafficking and autophagy induction. Here, we show that AMPK plays a key role in regulating different Vps34 complexes. AMPK inhibits the non-autophagy Vps34 complex by phosphorylating T163/S165 in Vps34, therefore suppresses overall PI(3)P production and protects cells from starvation. In parallel, AMPK activates the pro-autophagy Vps34 complex by phosphorylating S91/S94 in Beclin1 to induce autophagy. Atg14L, an autophagy essential gene present only in pro-autophagy Vps 34 complex, inhibits Vps34 phosphorylation but increases Beclin1 phosphorylation by AMPK. As such, Atg14L dictates the differential regulation (either inhibition or activation) of different Vps34 complexes in response to glucose starvation. Our study reveals an intricate molecular regulation of Vps34 complexes by AMPK in nutrient stress response and autophagy.
INTRODUCTION
Autophagy is a catabolic process to maintain cellular homeostasis in response to a wide spectrum of cellular stresses, such as nutrient starvation, infection, damaged organelles, and protein aggregates (Mizushima, 2007; Yang and Klionsky, 2010). These processes are coordinated by ATG proteins, among them, ULK1 and Vps34 complexes are well-known to function as important regulators in autophagy initiation and progression (Nakatogawa et al., 2009; Suzuki et al., 2007).
Vps34 is a class III PI3K that phosphorylates 3-position of phosphatidylinositol to produce phosphatidylinositol-(3)-phosphate (PI(3)P), which is a key membrane marker for both intracellular trafficking and autophagosome formation (Backer, 2008). PI(3)P functions to recruit proteins with PI(3)P binding domains, such as FYVE or PX domain, to modulate membrane activity (Obara and Ohsumi, 2011). Extensive yeast genetic and biochemical analyses have established that Vps34 forms multiple complexes that are responsible for its different cellular functions (Kametaka et al., 1998; Kihara et al., 2001; Obara et al., 2006). The autophagy-specific Vps34 complex consists of Vps34, Vps15, Atg6/Vps30, and Atg14 (Complex I). In parallel, Atg6/Vps30 interacts with Vps38 to form distinctive Vps34 complex II, which is required for endosome-to-Golgi retrograde trafficking.
The mammalian Vps34 also exists in different complexes and is involved in a variety of cellular functions, such as multivesicular body pathway, retrograde trafficking from endosomes to the Golgi, phagosome maturation, as well as autophagy (Backer, 2008). Core components of the Vps34 complexes include Vps34 and p150 (Vps15). Beclin1, a mammalian homologue of Atg6, participates in Vps34 complex formation and recruits additional proteins, such as Atg14L/Barkor and UVRAG. Recent reports demonstrated that Atg14L and UVRAG devote Vps34 complex to autophagy (Liang et al., 2006; Matsunaga et al., 2009; Sun et al., 2008). In addition, VMP1 (Ropolo et al., 2007), Ambra-1 (Fimia et al., 2007), Bif-1 (Takahashi et al., 2007), Rubicon (Matsunaga et al., 2009) are also reported as components of Vps34 complex, suggesting that many different Vps34 complexes are present and contribute to a wide range of cellular regulations.
The complexity of Vps34 biology indicates that multiple layers of regulation likely impinge on different complexes. Much effort in understanding Vps34 function has been centered on the interaction between Vps34 and Beclin1. The anti-apoptotic protein Bcl-2 may inhibit autophagy by disrupting Vps34-Beclin1 interaction due to its competitive binding to Beclin1 (Pattingre et al., 2005). JNK is suggested to phosphorylate Bcl-2 and dissociate its interaction from Beclin1 (Wei et al., 2008). DAP-kinase and CDK1/5 are also reported to regulate Vps34-Beclin1 interaction through phosphorylation (Furuya et al., 2010; Zalckvar et al., 2009). Although Vps34 complex is long-believed to have critical role in autophagy, it is not even clear whether Vps34 lipid kinase is activated upon the autophagy-inducing conditions. Also, it remains to be determined how the lipid kinase activity of different Vps34 complexes is regulated and if there is coordination of these different Vps34 complexes under starvation.
AMPK is a key cellular energy sensor and functions to maintain energy homeostasis upon nutrient starvation (Hardie, 2007). Lines of evidence support a role of AMPK in autophagy induction, including ULK1 activation, in response to nutrient starvation (Egan et al., 2011; Kim et al., 2011). In this study, we show molecular mechanism underlying differential regulations of pro- and non-autophagy Vps34 complexes by AMPK in nutrient stress and autophagy. These intricate regulations are accomplished by a direct phosphorylation of Vps34 T163/S165 and Beclin1 S91/S94 by AMPK under the control of autophagy-specific subunit, such as Atg14L.
RESULTS
Activity of different Vps34 complexes is differentially regulated by glucose starvation
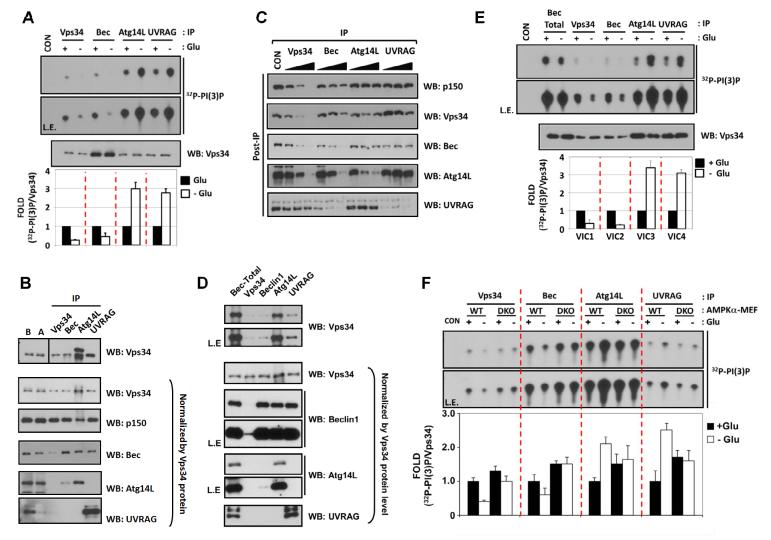
To study the regulation of different Vps34 complexes upon autophagy induction, we firstly measured the lipid kinase activity of four different Vps34 complex pools from glucose-starved cells. Four different Vps34 complexes were prepared by immunoprecipitation (IP) against Vps34, Beclin1, Atg14L, and UVRAG, respectively. Glucose starvation decreased the lipid kinase activity of IP isolated with antibody against Vps34 or Beclin1, whereas the lipid kinase activity of either Atg14L- or UVRAG-IPs was increased (Fig.1A). Notably, the basal Vps34 lipid kinase activity in the Atg14L- or UVRAG-IP was much higher than that of the Vps34- or Beclin1-IP. We performed western blot to determine the composition of the above IPs. When normalized to Vps34 protein levels, we found that Vps34-IP contained p150 (Vps15) and some Beclin1 but no detectable level of Atg14L or UVRAG (Fig.1B). The Beclin1-IP contained Vps34, p150, and some Atg14L, but little UVRAG. The Atg14L and UVRAG-IPs contained Vps34, p150, Beclin1, and Atg14L or UVRAG in a mutually exclusive manner as previously reported (Itakura et al., 2008). Notably, glucose starvation had almost no effect on Vps34 protein levels in each IP (Fig.1A), indicating the starvation-induced changes in Vps34 activity was not due to alteration of the composition of each Vps34 complex. Similar IP analyses in different cell lines suggest that the stoichiometry of Vps34 complex varies in the cell lines. For example, the majority of Vps34 exists as Atg14L-free complex in MEFs and NIH3T3, but significant amount of Atg14L was observed in Vps34-IP from HeLa cells, possibly due to different expression levels of Beclin1 and Atg14L (Fig.S1A).
Fig.1.
Different regulation of Vps34 complexes by glucose starvation in a manner dependent on AMPK.
(A) Regulation of Vps34 complexes by glucose starvation. Wild-type MEF cells were starved for glucose (3 hrs) as indicated and the four Vps34-IP complexes were assayed for PI(3)P lipid kinase activity (n=4).
(B) Subunit composition of Vps34 complexes. Vps34 protein level in each preparation (Fig.1A) was firstly examined (Top) and then normalized to determine subunit composition of each complexes (lower panels). IP efficiency of each antibody was examined by comparison of the target protein amount in the lysate before (B) and after (A) IP.
(C) Relative abundance of Vps34 complexes. A quantitative immuno-depletion assay was performed with increasing amount of the indicated antibodies. Supernatants of MEF lysates after immuno-depletion (Post-IP) were examined to determine the level of Vps34 complex proteins.
(D) Preparation of four different highly-enriched Vps34 complexes. Subunit composition was examined as described in Fig.1B.
(E) Glucose starvation inhibits VIC1 and VIC2 but activates VIC3 and VIC4. Four different VPS34 complexes in Fig.1D were subjected to Vps34 lipid kinase assay (n=3).
(F) AMPK is required for Vps34 regulation by glucose starvation. Endogenous Vps34 complexes were immuno-purified from glucose-starved MEFs as Fig.1A. The Vps34 kinase activity was normalized by the Vps34 protein levels (Fig.S1D) (n=4). Data are represented as mean ± S.D.; CON, mouse IgG-IP as a negative control. See also Figure S1.
To further explore the relative abundance of different Vps34 complexes in MEFs, we performed quantitative immuno-depletion with increasing amount of antibodies (Fig.1C). Quantitative removal of Vps34 resulted in an effective depletion of p150, Beclin1, Atg14L, and a significant decrease of UVRAG, indicating that most of Beclin1 and Atg14L are associated with Vps34. Quantitative removal of Beclin1 fully depleted Atg14L but significant amount of Vps34 and p150 was still remained in the post-IP supernatant, suggesting the existence of a large amount of Beclin1-free Vps34 complex in MEFs. Importantly, Atg14L immuno-depletion did not effectively reduce Vps34 or Beclin1, indicating that the majority of Vps34 or Beclin1 is not associated with Atg14L. Immunodepletion of Atg14L or UVRAG did not affect the levels of UVRAG or Atg14L, respectively, confirming a mutually exclusive association of Atg14L and UVRAG with Vps34 complex. The above data demonstrate the existence of four distinct VPS34 complexes and their relative abundance in MEFs.
The reduction in lipid kinase activity from Beclin1-IP is quite perplexing because it also contains Atg14L, although at a much reduced level. To further investigate the differential regulation of different Vps34 complexes, we attempted to obtain highly-enriched individual Vps34 population as outlined in Fig.S1B. For simplicity, we named them VIC1 (Vps34-containing PI3K-III Complex 1, labeled as Vps34), VIC2 (Beclin1), VIC3 (Atg14L), and VIC4 (UVRAG). The compositions of these four complexes were confirmed by western blots. As shown in Fig.1D, purification and fractionation successfully provided individual Vps34 complex as anticipated. Consistent with Fig.1A, the lipid kinase assay clearly showed that VIC3 and VIC4 were activated, but VIC1 and VIC2 were inhibited by glucose starvation (Fig.1E). The activities of VIC1 and VIC2 were low, but further decreased upon the starvation. Our data clearly demonstrate that different Vps34 complexes are differentially regulated in response to glucose starvation.
AMPK is required for glucose starvation-induced Vps34 regulation
As a primary energy source, glucose starvation results in cellular energy depletion and concomitantly activates AMPK, which is important for autophagy induction. Therefore, we tested whether AMPK is required for the regulation of Vps34 complexes in response to glucose starvation, in which AMPK was activated as evidenced by the increased phosphorylation of AMPK and its substrate, ACC (Fig.S1C). In this experimental setting, we determined Vps34 lipid kinase activity in AMPKα wild-type (WT) and α1/α2 double-knockout (DKO) MEFs. Notably, the regulation of Vps34 complex activity by glucose starvation was blunted in AMPKα-DKO MEFs for both VIC1 and VIC2 inhibition, and VIC3 and VIC4 activation (Fig.1F).
To further confirm a role of AMPK in glucose starvation-induced Vps34 regulation, we immuno-purified the four different Vps34 complexes from HEK293 cells co-transfected with a kinase-inactive AMPKα (AMPK-DN). Expression of AMPK-DN diminished the effect of glucose starvation on Vps34 regulation (Fig.S1E). Moreover, co-expression of wild type AMPKα inhibited VIC1 and VIC2, but activated VIC3 and VIC4 even in the glucose-rich condition (Fig.S1F). Notably, the non-autophagy Vps34 complex VIC1 and VIC2 showed quite low lipid kinase activity when normalized with Vps34 protein compared with VIC3 and VIC4, which is consistent with the reports that Atg14L and UVRAG activate Vps34 activity (Liang et al., 2006; Zhong et al., 2009). Consistently, AMPK activators, 2-DG or Metformin treatment, were sufficient to enhance VIC3 activity and suppress VIC2 activity (Fig.S1G). Collectively, these data demonstrate a critical role of AMPK in the differential regulation of pro-autophagy (VIC3 and VIC4) and non-autophagy (VIC1 and VIC2) Vps34 complexes in response to glucose starvation.
AMPK directly regulates Vps34 complex activity
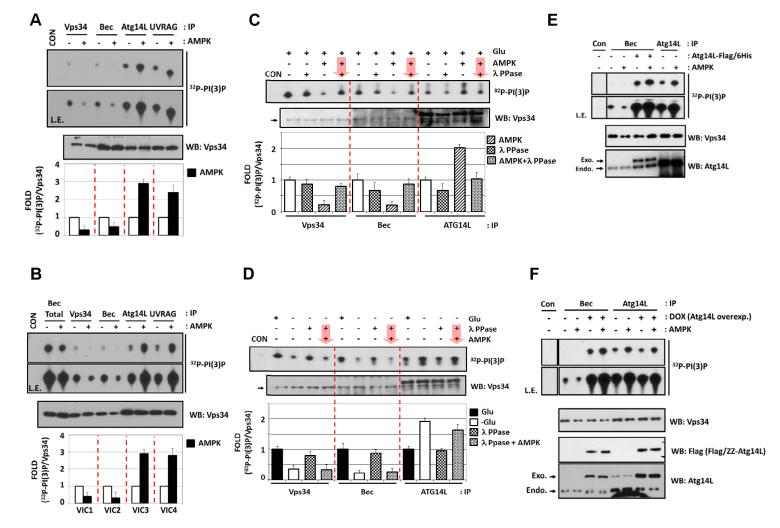
We asked whether AMPK could directly regulate Vps34 complex activity. Four different endogenous Vps34 immune-complexes were incubated with AMPK in vitro in the presence of cold ATP, and then were subjected to Vps34 lipid kinase assay. Similar to glucose starvation, in vitro AMPK treatment strongly inhibited VIC1 and VIC2, but activated VIC3 and VIC4 (Fig.2A). We also performed in vitro AMPK treatment on pure Vps34 populations prepared by the procedure outlined in Fig.S1B and obtained the same result as Fig.2A (Fig.2B), suggesting that AMPK plays a direct role in Vps34 regulation.
Fig.2.
AMPK directly regulates Vps34 complex through phosphorylation.
(A) AMPK directly regulates Vps34 complex. Vps34 complex was immuno-purified from MEFs in glucose-rich medium and incubated with AMPK for 15 min in vitro as indicated (n=4).
(B) AMPK inhibits VIC1 and VIC2 but activates VIC3 and VIC4 in vitro. Four highly-enriched Vps34 complexes obtained as Fig.1D were treated with AMPK in vitro and assayed for the lipid kinase assay (n=3).
(C) AMPK regulates Vps34 complex via phosphorylation. The indicated Vps34 complexes were immuno-purified from MEFs in glucose-rich condition. The complexes were incubated with AMPK and λ PPase as indicated by arrow for order of the treatment (n=3).
(D) AMPK-mediated phosphorylation is necessary and sufficient for the regulation of Vps34 complexes by glucose starvation. The Vps34 complexes were immuno-purified from glucose starved MEFs and then, treated with AMPK and λ PPase (n=3).
(E) Atg14L converts Vps34-Beclin1 complex from inhibition to activation by AMPK. Vps34-Beclin1 complex was immuno-purified from U2OS cells, to which purified Atg14L-Flag/6His protein was added. Also, Atg14L containing Vps34 complex was directly prepared by Atg14L-IP from the cells as a control. The immune-complex was treated with AMPK in vitro and assayed for Vps34 lipid kinase assay (n=2).
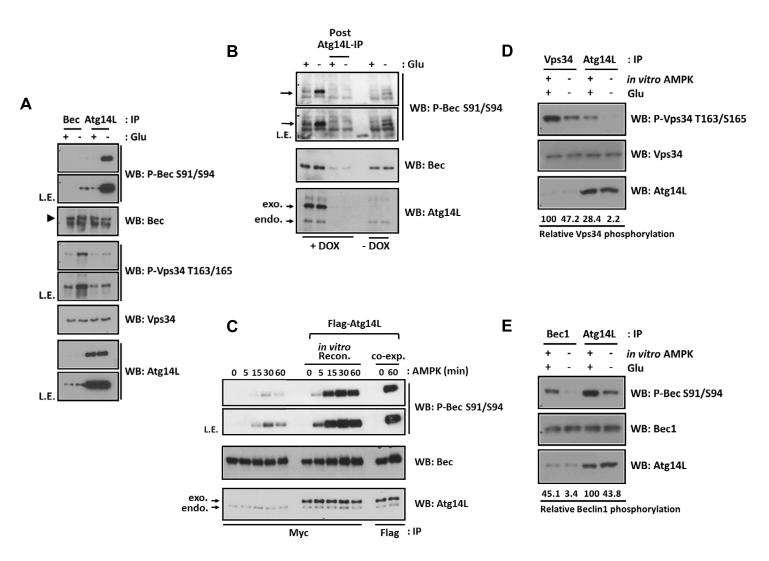
(F) Overexpression of Atg14L activates Vps34 kinase activity of Beclin1-IP in response to AMPK treatment. Vps34 complex were immuno-purified from the U2OS cell lines overexpressing Atg14L, which was induced by DOX, and then incubated with AMPK in vitro before the lipid kinase assay as indicated (n=2).
Data are represented as mean ± S.D., See also Figure S2.
To verify that AMPK regulates Vps34 by phosphorylation, Vps34 complex was treated with both AMPK and lambda phosphatase as outlined in Fig.S2A. We found that lambda phosphatase treatment reversed both the inhibitory effect of AMPK on VIC1 and VIC2, and the activating effect on VIC3 (Fig.2C), indicating that AMPK regulates Vps34 complexes by phosphorylation. Consistently, AMPK could not activate VIC3 in the absence of ATP during in vitro treatment (Fig.S2B). Next, we investigated whether phosphorylation by AMPK plays an important role in Vps34 regulation induced by glucose starvation (Fig.2D). Dephosphorylation by lambda phosphatase treatment activated VIC1 and VIC2 isolated from glucose starvation condition to levels similar to those isolated from un-starved cells. Moreover, dephosphorylation inhibited the lipid kinase activity of VIC3 isolated from glucose starvation. These results demonstrate that the phosphorylation is the major mechanism for Vps34 complex regulation by glucose starvation. Importantly, re-phosphorylation of lambda phosphatase-treated Vps34 complexes by AMPK was sufficient to inhibit VIC1 and VIC2, and to activate VIC3 (Fig.2D) to the level similar to the complexes from glucose-starved cells. A parallel experiment showed that lambda phosphatase or AMPK treatment affected the UVRAG-containing VIC4 in a manner similar to VIC3 regulation (Fig.S2C). Collectively, these data demonstrate that AMPK dependent phosphorylation is necessary and sufficient for Vps34 regulation (both inhibition of non-autophagy VIC1 and VIC2, and activation of pro-autophagy VIC3 and VIC4) in response to glucose starvation.
Atg14L converts Vps34 regulation from inhibition to activation by AMPK
The lipid kinase activities of VIC2 and VIC3 show opposite responses to glucose starvation or AMPK treatment. A key difference between VIC2 and VIC3 is the presence of autophagy-specific subunit Atg14L in VIC3. We hypothesized that Atg14L may play a critical role to switch Vps34 regulation from inhibition to activation by AMPK. To test this possibility, purified Atg14L was added into the endogenous Beclin1-IP (VIC2). In vitro addition of Atg14L to VIC2 increased the basal activity in a dose dependent manner (Fig.S2D). The purified Atg14L protein had no contaminating Vps34 activity (Fig.S2E). Importantly, addition of Atg14L switched VIC2 regulation by AMPK from inhibition to activation (Fig.2E). Therefore, Atg14L is a key factor to determine the effect (either inhibition or activation) of AMPK on Vps34 complexes. Experiment using several Atg14L deletion mutants further demonstrated that the N-terminal 72 residues and the C-terminal BATS domain of Atg14L are dispensable for Vps34 activation by AMPK (Fig.S2F).
One possible explanation for AMPK-induced inhibition of Beclin1-IP is that only a very small fraction of Beclin1-IP contains Atg14L (Fig.1B&1C). Therefore, inhibition of the Atg14L-free complex (VIC2) by AMPK overwhelmed the activating effect of the very small fraction of Atg14L-contacting complex (VIC3) in the Beclin-IP. If so, we reasoned that overexpression of Atg14L should increase the fraction of endogenous Beclin1 in complex with Atg14L. As predicted, Beclin1-IP from the Atg14L overexpressing cells contained higher level of the ectopic Atg14L protein, which was comparable to endogenous Atg14L in VIC3 when normalized to Vps34 protein level (Fig.2F). As a consequence of increased Atg14L, the lipid kinase activity of Beclin1-IP prepared from Atg14L overexpressing cells was indeed activated by AMPK. Collectively, these data demonstrate that Atg14L plays a key role in determining whether the Vps34 complex is inhibited (in the absence of Atg14L) or activated (in the presence of Atg14L) by AMPK.
AMPK phosphorylates Vps34 and Beclin1
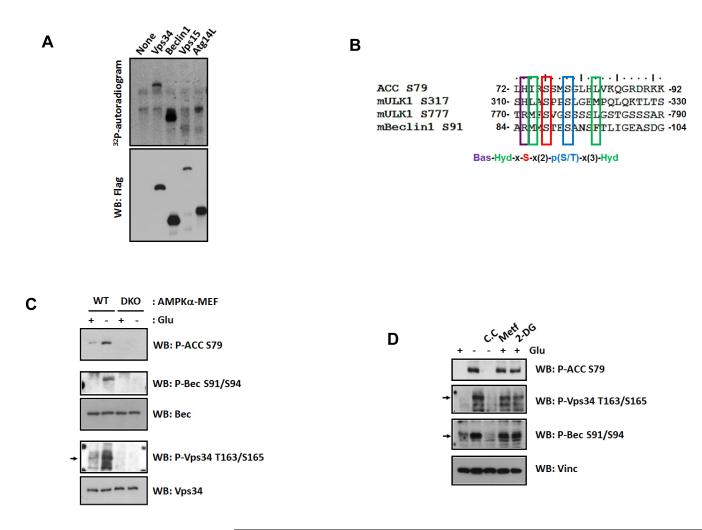
It is intriguing how AMPK exerts opposite effect on different Vps34 complexes that have the same catalytic subunit. Considering that AMPK regulates Vps34 complex by phosphorylation, we performed in vitro AMPK phosphorylation of individual Vps34 complex subunit. We observed that Vps34 and Beclin1 were directly phosphorylated by AMPK in vitro (Fig.3A&S3A). Based on mass spectrometry, systemic deletions, and site-directed mutation, we identified S91 and S94 in Beclin1 (Fig.S3B, S3C) and T163 and S165 in Vps34 (Fig.S3D, S3E) as the major AMPK phosphorylation sites. Notably, the Beclin1 phosphorylation sites do not fit the conventional AMPK consensus motif (Gwinn et al., 2008). However, they align with two AMPK phosphorylation sites (S317 and S777) in ULK1 (Fig.3B) (Kim et al., 2011). Also, the AMPK site S79 in ACC1 fits this sequence defined as Bas-Hyd-X-S-X-X-pS/T-X-X-X-Hyd (Bas and Hyd denote for basic and hydrophobic residues, respectively). This may represent a new AMPK phosphorylation consensus sequence.
Fig.3.
AMPK phosphorylates T163/S165 in Vps34 and S91/S94 in Beclin1.
(A) AMPK directly phosphorylates Vps34 and Beclin1 in vitro. The indicated Flag-tagged Vps34 complex proteins were purified from the transfected cells and tested as substrates for AMPK in vitro by 32P-autoradiogram.
(B) Sequence alignment of the AMPK phosphorylation sites in Beclin1 and other known AMPK substrates.
(C) AMPK is required for glucose starvation-induced phosphorylation of Beclin1 S91/S94 and Vps34 T163/S165.
(D) Activation of AMPK is sufficient to induce phosphorylation of Vps34 and Beclin1. 293A cells were incubated with compound C (C.C, 20 μM, 30 min), before glucose starvation. In parallel, 5 mM Metformin (Metf) or 25 mM 2-DG were added in glucose-rich medium for 3 hrs. See also Figure S3.
To confirm Vps34 and Beclin1 phosphorylations in vivo, we generated phospho-specific antibodies for Beclin1 and Vps34 and confirmed the antibodies preferentially recognizing the in vitro phosphorylated GST-Beclin1 and GST-Vps34 by AMPK (Fig.S3F). Using these phosphospecific antibodies, we observed that glucose starvation increased the phosphorylation of endogenous Beclin1 S91/S94 and Vps34 T163/S165 (Fig.3C). Importantly, these phosphorylations were abolished in AMPKα DKO MEFs, demonstrating that AMPK is required for Beclin1 and Vps34 phosphorylation. Consistently, AMPK activators, 2-DG or Metformin, also increased phosphorylation of Vps34 and Beclin1 even in glucose-rich condition, whereas AMPK inhibitor, compound C, blocked glucose-starvation induced phosphorylations (Fig.3D). Moreover, co-expression of wild-type AMPKα, but not the kinase inactive mutant, induced phosphorylation of Beclin1 (Fig.S3G) and Vps34 (Fig.S3H) even in glucose-rich condition. These data establish a physiological role for AMPK in the phosphorylation of Beclin1 S91/S94 and Vps34 T163/S165 in response to glucose starvation.
Beclin1 phosphorylation is required for activation of the pro-autophagy Vps34 complex
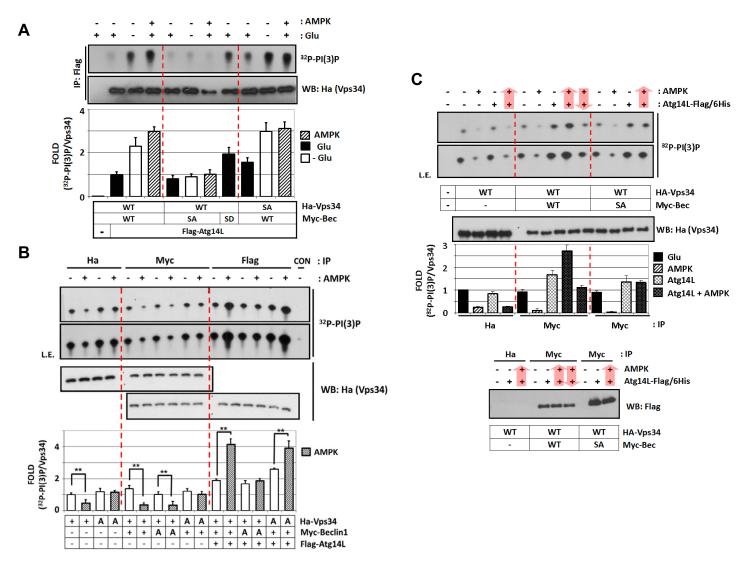
Next, we wished to determine the function of phosphorylation in the regulation of Vps34 complexes. Wild-type (WT) or phosphorylation defective mutants (SA) were co-expressed with Atg14L, and the Atg14L associated complex (VIC3) was immunoprecipitated to determine Vps34 lipid kinase activity. We found that VIC3 containing the Beclin1 S91/94A (SA) could not be activated by glucose starvation or AMPK co-expression in vivo (Fig.4A), whereas the complex containing Beclin1 WT was activated by both conditions. This result suggests that phosphorylation of Beclin1 S91/S94 is required for activation of the pro-autophagic Vps34 complex VIC3 by AMPK or glucose starvation. Consistently, the complex containing phosphorylation-mimetic mutant of Beclin1 S91/94D (SD) showed higher basal activity even in glucose-rich condition. In contrast, Vps34 phosphorylation was not required for VIC3 activation as the Vps34 T163/165A (SA) containing VIC3 was similarly activated upon either glucose starvation or AMPK co-transfection although it had a higher basal activity (Fig.4A).
Fig.4.
Phosphorylation of Beclin1 and Vps34 are required for the regulation of Vps34 complexes by glucose starvation or AMPK.
(A) Beclin1 phosphorylation, but not Vps34 phosphorylation, is required for Atg14L-associated Vps34 complex activation in response to glucose starvation. Wild-type (WT), phosphorylation defective (SA), -mimetic (SD) Ha-Vps34, Myc-Beclin1 and Flag-Atg14L were co-transfected with AMPK into HEK293 cells as indicated. The cells were glucose-starved for 3 hrs (n=3).
(B) Phosphorylation of Vps34 and Beclin1 is required for Vps34 regulation by AMPK. Vps34 complexes were immuno-purified by HA (Vps34), Myc (Vps34-Beclin1), or Flag (Vps34-Beclin1-Atg14L) and then incubated with AMPK for 15 min in vitro before Vps34 lipid assay (n=3). +, wild-type; A, a phosphorylation-defective mutant.
(C) Atg14L determines whether Vps34-Beclin1 complex is activated or inhibited by AMPK. Vps34 complex was immuno-purified by either HA (Vps34) or Myc (Vps34-Beclin1) from the transfected HEK293 cells, to which purified Atg14L was added, and then treated with AMPK as indicated by arrow for order of the treatment. Atg14L protein level in the complexes was determined by western blot (bottom panel) (n=2).
Data are represented as mean ± S.D.
To further characterize the role of AMPK-induced phosphorylation in the Vps34 complex regulation, we have prepared VIC1, VIC2, and VIC3 from the transfected cells and incubated them with AMPK in vitro (Fig.4B). Consistent with the in vivo data in Fig.4A, Beclin1 phosphorylation, but not Vps34 phosphorylation, was required for VIC3 activation by AMPK in vitro. Interestingly, Vps34 phosphorylation, but not Beclin1 phosphorylation, was required for inhibition of the non-autophagy VIC1 and VIC2 by AMPK in vitro (Fig.4B). These data indicate that phosphorylation of Beclin1 and Vps34 protein play differential roles in the regulation of different Vps34 complexes by AMPK.
Atg14L appears to play a critical role in determining the effect, either activation or inhibition, of AMPK on Vps34 complex regulation. To test this possibility, Vps34 complexes were immunoprecipitated from the transfected cells, in which purified Atg14L was added to reconstitute Atg14L containing Vps34 complex (Fig.4C). Vps34 without Beclin1 (VIC1) was inhibited by AMPK and addition of Atg14L did not affect this inhibition (Fig.4C). This is an expected result because Atg14L participates in Vps34 complex via interaction with Beclin1. AMPK suppressed Vps34-Beclin1 complex (VIC2) activity, but in vitro Atg14L reconstitution reversed the effect of AMPK to activation as shown in Fig.2E. However, the Atg14L reconstituted Vps34 complex containing Beclin1 S91/94A was not activated by AMPK, demonstrating that Beclin1 phosphorylation activates the pro-autophagy Vps34 complex (VIC3) by AMPK.
Next, we questioned whether association of Atg14L in the Vps34 complex is a prerequisite for activation by AMPK. When Vps34-Beclin1 complex was incubated with AMPK first, then followed by Atg14L addition, AMPK pretreatment did not activate Vps34 lipid kinase activity (Fig.4C), indicating that Atg14L must be present in the Vps34 complex in order for AMPK to activate the complex. Notably, AMPK treatment did not induce any change on Beclin1-Atg14L interaction (Fig.4C bottom panel). Together, our data demonstrate that AMPK inhibits VIC2 via Vps34 phosphorylation and activates VIC3 via Beclin1 phosphorylation. Moreover, preassembly of Atg14L on Vps34 complex is required for VIC3 activation by AMPK, suggesting that binding of Atg14L may affect phosphorylation of Vps34 and/or Beclin1 by AMPK.
Atg14L enhances the activating phosphorylation of Beclin1 and suppresses the inhibitory phosphorylation of Vps34 by AMPK
We investigated the mechanism underlying Beclin1 and Vps34 differential phosphorylation in VIC2 and VIC3 despite both proteins being present in these complexes. To address this question, the phosphorylation status of endogenous Vps34 and Beclin1 in VIC2 and VIC3 were determined. Interestingly, we found that the phosphorylation level of Beclin1 S91/S94 was significantly higher in VIC3 (Atg14L-IP) than that in VIC2 (Beclin1-IP) and the opposite was observed for Vps34 T164/S165 phosphorylation (Fig.5A). Phosphorylation of Vps34 was low in VIC3 and unaffected by glucose starvation whereas phosphorylation of Vps34 in VIC2 was high and was robustly induced. In line with endogenous data, ectopic expression of Atg14L strongly promoted Beclin1 phosphorylation (Fig.S4A) and inhibited Vps34 phosphorylation (Fig.S4B) upon AMPK co-expression.
Fig.5.
Atg14L stimulates the phosphorylation of Beclin1, but suppresses the phosphorylation of Vps34 by AMPK.
(A) Phosphorylation of Beclin1 and Vps34 is oppositely regulated by glucose starvation in Atg14L-dependent manner. Endogenous Beclin1 and Atg14L were immunoprecipitated from glucose-starved MEFs (3 hrs) as indicated.
(B) Overexpression of Atg14L increases endogenous Beclin1 phosphorylation. Overexpression of Atg14L was induced in U2OS as Fig.2F. Post Atg14L-IP denotes the lysate after Atg14L immunoprecipitation, representing the Atg14L (both endogenous and overexpressed)-free fraction.
(C) Atg14L directly enhances Beclin1 phosphorylation by AMPK in vitro. Beclin1 was immuno-purified from the transfected HEK293 cells, to which purified Flag-Atg14L was added. In parallel, Beclin1-Atg14L was obtained from the transfected cells for comparison (co-exp.). The complexes were treated with AMPK for the indicated time in vitro and the Beclin1 phosphorylation was examined.
(D,E) Determination of the relative phosphorylation of endogenous Vps34 (D) and Beclin1 (E) proteins. The endogenous Vps34 complexes were immuno-purified from MEFs with or without glucose as indicated. Relative phosphorylation of Vps34 and Beclin1 was compared with in vitro AMPK-phosphorylated Vps34 complexes.
See also Figure S4.
The above data demonstrate that in the absence of Atg14L, Vps34, but not Beclin1, is preferentially phosphorylated by AMPK. In contrast, when Atg14L is in the complex, Beclin1 is preferentially phosphorylated by AMPK. The majority of endogenous Beclin1 is not associated with Atg14L because Atg14L is limiting (Fig.1B-1D). Therefore, we reasoned that increased Atg14L expression should enhance Beclin1 phosphorylation by AMPK in vivo because more Beclin1 would be in complex with Atg14L. When Atg14L was overexpressed, glucose starvation induced a much stronger phosphorylation in Beclin1 (Fig.5B). It should be noted that endogenous Beclin1 protein level was slightly increased upon Atg14L overexpression. Two mechanisms may explain these observations: Atg14L makes Beclin1 a better substrate for AMPK or poorer substrate for a phosphatase. To this end, we determined the effect of Atg14L on Beclin1 phosphorylation by AMPK in vitro. Vps34-Beclin1 complex, VIC2, was immuno-purified from the transfected cells, to which purified Atg14L was added to reconstitute VIC3 in vitro, and then both complexes were phosphorylated by AMPK to examine Beclin1 phosphorylation (Fig.5C). Atg14L dramatically increased the rate and degree of Beclin1 phosphorylation by AMPK. Similarly, Beclin1 was more potently phosphorylated by AMPK in VIC3 than VIC2 obtained from transfected cells co-expressing Atg14L (Fig.S4C). These data support a model in which Atg14L makes Beclin1 a better substrate for phosphorylation by AMPK.
To determine the stoichiometry of endogenous Vps34 and Beclin1 phosphorylation, we phosphorylated Vps34 and Beclin1 in vitro under maximum AMPK phosphorylation condition, which possibly resulted in complete phosphorylation of Vps34 on T163/S165 and Beclin1 on S91/S94. Comparing the relative phosphorylation of endogenous Vps34 in VIC1 with the in vitro phosphorylated Vps34, we estimated that approximately 47.2% of endogenous Vps34 is phosphorylated in VIC1 under glucose starvation (Fig.5D). Similar experiments with Beclin1 showed that approximately 43.8% of Beclin1 was phosphorylated in VIC3 under glucose starvation (Fig.5E). As expected, AMPK phosphorylated Vps34 more efficiently in VIC1 than VIC3 whereas it phosphorylated Beclin1 more efficiently in VIC3 than VIC1. These data indicate that a substantial fraction of Vps34 and Beclin1 in specific Vps34 complexes can be phosphorylated upon glucose starvation.
Beclin1 phosphorylation is required for autophagy induction
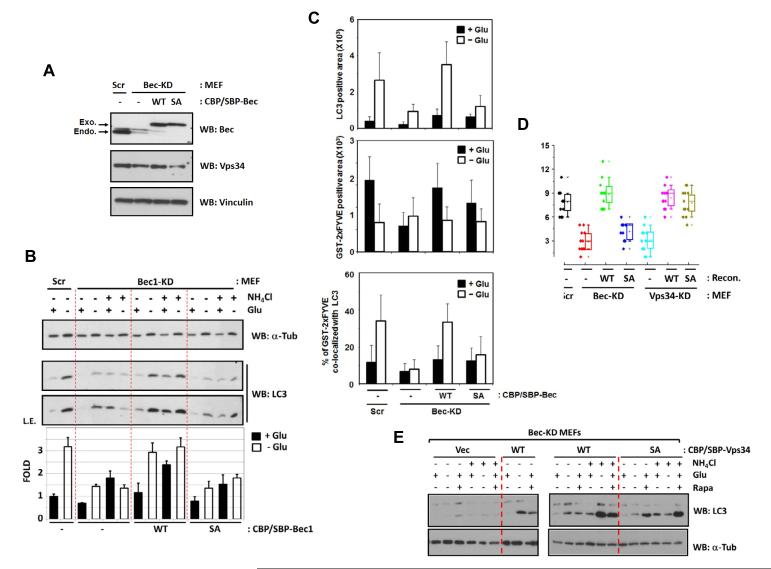
To determine the biological function of Beclin1 phosphorylation by AMPK, Beclin1-WT and S91/94A mutant (SA) were introduced into the Beclin1 knockdown (Bec-KD) MEFs (Fig.6A). It is worth noting that the knockdown of endogenous Beclin1 was more efficient in ectopic Beclin1 expressing cells. Consistent with a critical role of Beclin1 in autophagy, glucose starvation could not induce autophagy in Bec-KD MEFs, as evidenced by LC3-II accumulation (Fig.6B&S5A). Re-expression of wild-type Beclin1 (Bec-KD/WT) rescued autophagy defects of Bec-KD MEFs, as indicated by LC3 lipidation (Fig.6B), p62 degradation (Fig.S5B), LC3-positive puncta formation (Fig.6C), and autophagosome/autolysosome-like vacuole formation (Fig.6D). Importantly, all autophagic markers were significantly compromised in Bec-KD MEFs expressing Beclin1 S91/94A (Bec-KD/SA), indicating the functional importance of Beclin1 S91/94 phosphorylation in autophagy in response to glucose starvation.
Fig.6.
Beclin1 S91/S94 phosphorylation is required for autophagy induction.
(A) Beclin1 expression level in Beclin1 knockdown (KD) and reconstituted MEFs. Scr denotes scramble shRNA control.
(B) Cells expressing the Beclin1 S91/94A mutant are compromised in LC3 lipidation in response to glucose starvation (n=3).
(C) Beclin1 S91/S94A mutant is defective in autophagosome formation. The indicated MEFs were starved with glucose (3 hrs) and the number of LC3 puncta was measured by endogenous LC3 staining (top). Also, PI(3)P level was determined by counting the spots of immunostaining using GST-2xFYVE protein as a probe (middle). The number of LC3 and PI(3)P double-positive puncta was counted by the overlap of LC3 and GST-2xFYVE staining and % was shown (bottom). (10-15 randomly selected images of the cells, n=3). See Fig.S5C for confocal images.
(D) Beclin1 S91/84A mutant is defective in autophagy vacuole formation. The indicated MEFs were starved with glucose for 3 hrs and the autophagy vacuoles were examined on electron microscopy (EM). The numbers of autophagosome/autolysosome-like structures (AV) from 15-20 randomly selected cells were counted (See the representative images in Fig.S5D and S6C).
(E) Phosphorylation of Beclin1 S91/S94 is specifically involved in glucose starvation-induced autophagy. The indicated Beclin1-MEFs were incubated with either glucose-free or 50 nM rapamycin-containing culture medium for 3 hrs. Autophagy flux was examined in the presence of 10 mM NH4Cl.
Data are represented as mean ± S.D., See also Figure S5.
Notably, PI(3)P staining, using GST-2xFYVE as a probe, showed that overall PI(3)P level was decreased upon glucose starvation (Fig.6C middle, a quantitative ELISA analysis in 7D), which is consistent with the previous reports (Gulati et al., 2008) and our in vitro Vps34 kinase assay that VIC1 and VIC2, major Vps34 complexes in MEFs, were inhibited by glucose starvation. However, PI(3)P that co-localized with LC3 puncta, which was likely generated by pro-autophagy Vps34 complexes, was increased upon the starvation (Fig.6C bottom). To directly monitor autophagy-relevant PI(3)P, we examined the localization of DFCP1 (an ER-associated FYVE domain-containing protein that translocates to initiate autophagosome structure, omegasomes, upon the starvation condition) using MEFs stably expressing GFP-DFCP1. Consistently, GFP-DFCP1 puncta was increased in response to glucose starvation, whereas overall GST-2xFYVE staining was decreased (Fig.S5E), indicating that the autophagy-relevant PI(3)P is specifically increased upon the starvation. These results are consistent with our observation that only pro-autophagic Vps34 complexes, such as Atg14L containing VIC3, are activated upon the starvation.
To examine whether Beclin1 S91/S94 phosphorylation is specifically involved in AMPK-dependent autophagy induction, we examined the effect of rapamycin, an mTORC1 inhibitor, on LC-II accumulation. As shown in Fig.6E, both glucose starvation (AMPK-dependent) and rapamycin treatment (mTORC1-dependent) induced LC3-II accumulation in Beclin1-WT rescued MEFs. However, rapamycin could still induced autophagy in the Beclin1 S91/94A rescued cells whereas glucose starvation did not induce LC3-II accumulation. These data indicate that Beclin1 S91/S94 phosphorylation is particularly important for glucose starvation and AMPK-dependent autophagy.
Vsp34 phosphorylation is required for cell survival but not for autophagy in response to starvation
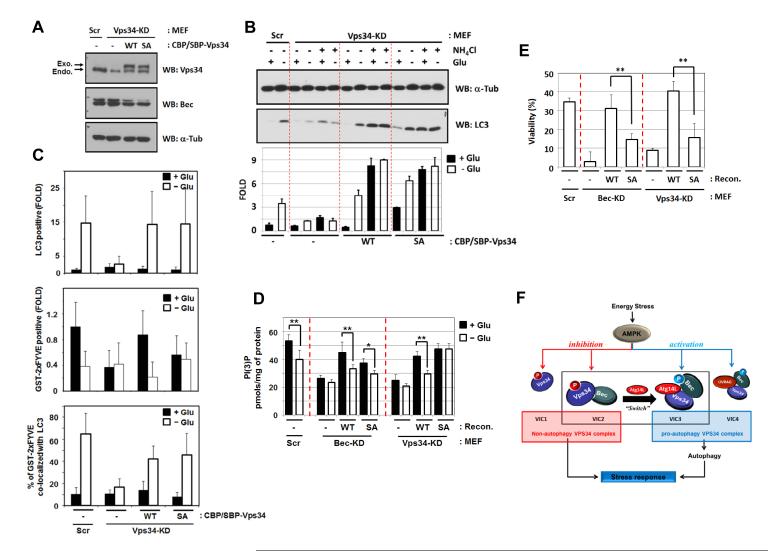
To determine the biological function of Vps34 phosphorylation by AMPK, Vps34 wild-type (WT) and T163/165A mutant (SA) were introduced into the Vps34 knockdown (Vps34-KD) MEFs. Although Vps34 knockdown was not as efficient as Beclin1 knockdown (Fig.7A), it was sufficient to decrease autophagy (Fig.S5A). Expression of either wild-type or T163/165A mutant rescued autophagy defects in Vps34-KD MEFs, as measured by LC3-II accumulation (Fig.7B), p62 degradation (Fig.S6A), LC3-positive autophagosome formation (Fig.7C), and autophagosome/autolysosome-like structure formation (Fig.6D). These results are consistent with our Vps34 lipid kinase assays, in which Vps34 T163/165A mutant did not affect activation of the pro-autophagy Vps34 complex, by glucose starvation (Fig.4A&4B). Our data indicate that phosphorylation of Vps34 by AMPK is not directly involved in autophagy regulation.
Fig.7.
Vsp34 phosphorylation is required for cell survival but not autophagy in response to starvation
(A) Vps34 expression level in Vps34-MEFs.
(B,C) The Vps34 T163/S165A mutant does not compromise autophagy induction in response to glucose starvation as evidenced by LC3 lipidation (B) and by LC3-positive puncta formation (C, See Fig.S6B for confocal images).
(D) Vps34 phosphorylation is required for PI(3)P reduction in response to glucose starvation. PI(3)P level in glucose-starved MEFs was determined by quantitative PI(3)P ELISA assay. The PI(3)P level was normalized by the amount of proteins used in the assay (n=6; *, p<0.05; **, p<0.01).
(E) Vps34 phosphorylation is required for cell survival in response to glucose starvation. Beclin1 or Vps34-MEF cells were starved with glucose for 24 hrs and then the viability was measured by FACS analysis using AnexinV and propidium iodide (PI) double-staining (n=4; **, p<0.01).
(F) A schematic model of Vps34 regulation by AMPK in response to energy starvation. Data are represented as mean ± S.D., See also Figure S6.
Although not required for autophagy induction, Vps34 phosphorylation by AMPK did play a role in overall PI(3)P production as determined by immunostaining with GST-2xFYVE (Fig.7C). We found that upon starvation PI(3)P levels decreased in control cells or Vps34 WT reconstituted cells, but not decreased in the Vps34 SA mutant reconstituted MEFs. Quantitative PI(3)P ELISA assays confirmed that total cellular PI(3)P level was decreased by glucose starvation in the wild type cells (Fig.7D). As expected, knockdown of Beclin1 or Vps34 reduced basal PI(3)P and abolished the glucose effect on PI(3)P. Re-expression of either wild-type Beclin1 or SA mutant restored the glucose-induced regulation of PI(3)P, indicating that Beclin1 phosphorylation is not required for the decrease of total PI(3)P by glucose starvation. In contrast, expression of the phosphorylation mutant Vps34 T163/165A could not restore PI(3)P regulation by glucose starvation (Fig.7C, 7D). These experiments support an important role of Vps34 phosphorylation in the inhibition of non-autophagy Vps34 complex and decrease of total cellular PI(3)P production in response to glucose starvation.
We next tested whether inhibition of non-autophagy VPS34 plays a role in stress response by measuring cell survival under glucose starvation. Knockdown of either Beclin1 or Vps34 significantly decreased cell viability (Fig.7E & Fig.S6D) upon glucose starvation, consistent with a protective role of autophagy in nutrient starvation. Beclin1 S91/94A reconstituted cells were sensitive to glucose starvation, similar to Beclin1-KD MEFs. Notably, re-expression of Vps34 T163/165A could not rescue cell death in Vps34-KD cells, even though it rescued the autophagy defects. Therefore, our data suggest that AMPK-induced phosphorylation inhibits the non-autophagy Vps34 complexes to protect cells from starvation. These observations support an important role of inhibition of non-autophagy Vps34 complexes mediated by AMPK-dependent Vps34 T163/S165 phosphorylation in cellular starvation response. It is conceivable that some cellular activity, such as general vesicle trafficking, should be suppressed upon starvation to preserve energy for survival.
Discussion
Multiple Vps34 complexes are reported to control distinct cellular processes, such as intracellular vesicle trafficking and autophagy (Backer, 2008; Obara and Ohsumi, 2011; Vergne and Deretic, 2010). However, the regulation and coordination of various Vps34 complexes in response to changing cellular conditions remain largely unknown. In this study, we provide a molecular mechanism underlying a dynamic regulation of different Vps34 complexes in response to cellular energy depletion via the action of AMPK.
AMPK-mediated differential regulation of Vps34 complexes
Although PI(3)P, a product of Vps34, is believed to recruit autophagy components on the autophagosome membrane, several studies have documented that Vps34 activity is inhibited and PI(3)P level is reduced upon the starvation (Byfield et al., 2005; Gulati et al., 2008; Nobukuni et al., 2005). Our study has provided a mechanistic interpretation for these paradoxical observations. We have prepared four different Vps34 complexes by immunoprecipitation against characteristic Vps34 complex subunits or by fractionation of highly-enriched individual Vps34 complex (VIC1-VIC4). Within the linear Vps34 lipid kinase assay condition (Fig.S7A), we show that Vps34 complexes that are free of autophagy-specific subunits, Atg14L and UVRAG, are inhibited by glucose starvation. This is accomplished by the inhibitory phosphorylation of Vps34 T163/S165 by AMPK. In contrast, the pro-autophagy Vps34 containing Atg14L is activated via phosphorylation of Beclin1 S91/S94 by AMPK.
Both inhibitory and activating effects of glucose starvation on non- and pro-autophagy Vps34 complexes were blunted in AMPK-DKO MEFs, clearly demonstrating role of AMPK in these regulation. We may consider the complication that the autophagy defect in AMPK-DKO MEFs is due to the metabolic changes caused by the loss of AMPK, which results in decrease of glucose usage and lactate formation at 24 hrs (Fig.S7B). However, these differences should not affect our analysis because cells were harvested at 3 hrs. Moreover, AMPK phosphorylation defective Vps34 (or Beclin1) mutant was no longer regulated by the starvation, similarly to Vps34 complex in AMPK-DKO MEFs, indicating that the Vps34 regulation observed in our study is directly linked to AMPK activity.
Notably, most of Vps34 is not in complex with Atg14L or UVRAG, suggesting that majority of Vps34 is in non-autophagy complexes, particularly in MEFs. Similar result was obtained from the analysis of Vps34 preparation by streptavidin pull-down (Fig.S7C), which was also inhibited by glucose starvation as Vps34 immunoprecipiates (Fig.S7D). Also, streptavidin pull-down of Beclin1 shows similar complex composition and regulation of lipid kinase activity in response to glucose starvation as Beclin1-immunoprecipitates (Fig.S7E). These data demonstrate that results from the non-autophagy Vps34 complexes (Vps34-IP and Beclin-IP) in this study were not due to an artifact in the kinase assay caused by antibodies.
Autophagy can be induced by multiple stresses via different signaling mechanisms. Several kinases, such as JNK, DAPK, and CDK1/5, have been demonstrated to regulate Vps34 complex by modulating the Vps34-Beclin1 interaction in response to the stresses, including nutrient starvation (Furuya et al., 2010; Wei et al., 2008; Zalckvar et al., 2009). However, we did not observe any significant change in the interaction between Vps34 and Beclin1 upon glucose starvation, consistent with a previous report (Byfield et al., 2005). It was recently reported that CCCP, a mitochondrial uncoupler activating AMPK, induces autophagy in a manner dependent on mTORC1, but not of AMPK (Kwon et al., 2011), suggesting that mammalian autophagy is controlled by multiple layers of regulation, in which AMPK and mTORC1 may cooperatively (for example, for ULK1 regulation) or differently (such as CCCP) function depending on the stress. In case of nutrient starvation, we observed that amino acid starvation, which relies on mTORC1 to activate autophagy, also differentially regulates Vps34 complexes as glucose starvation did (data not shown). Therefore, activation of autophagy-specific Vps34 complex and inhibition of non-autophagy complex seem to be a common mechanism in autophagy in response to nutrient starvation. However, phosphorylation of Beclin1 S91/S94 is selectively required for glucose starvation and AMPK dependent, but not rapamycin-induced, autophagy (Fig.6E).
Atg14L determines whether a Vps34 complex is activated by AMPK
The autophagy-specific subunit Atg14L functions to target the Vps34 complex to endoplasmic reticulum for autophagy (Matsunaga et al., 2010). Our study reveals another critical function for Atg14L as a switch converting the inhibitory effect of AMPK (or glucose starvation) on Vps34 complex to activation. Atg14L functions not only to prevent the inhibitory phosphorylation in Vps34, but also to promote the activating phosphorylation in Beclin1 by AMPK. As a result, we found that ectopic expression of Atg14L increased Beclin1 phosphorylation (Fig.5B) as well as autophagy level (Fig.S7F), possibly due to the increase of pro-autophagy VIC3 population. We speculate that Atg14L binding may induce conformational changes in the Vps34 complex to interfere with the availability of T163/S165 in Vps34 and promote availability of S91/S94 in Beclin1 for phosphorylation by AMPK. We also found that another pro-autophagy Vps34 complex containing UVRAG is similarly regulated by AMPK-dependent phosphorylation as Atg14L containing complex (Fig.S2C). UVRAG may act in a manner similar to Atg14L in Vps34 regulation. Indeed, UVRAG has been proposed to induce Beclin1 conformational changes, thus activating Vps34 complex (Noble et al., 2008). However, overexpression of UVRAG could not rescue the loss of Atg14L in autophagy induction in response to glucose starvation (Fig.S7G), which may support the current understanding that Atg14L and UVRAG function in initiation and maturation of autophagy, respectively.
Biology of differential regulation of Vps34 complexes
We showed that glucose starvation decreases overall PI(3)P levels, but the pool of autophagy-related PI(3)P is increased, indicating that there are discrete functions for the different PI(3)P pools produced by different Vps34 complexes in cellular adaptation to stress. Local activation of pro-autophagy Vps34 complex labels the membrane with PI(3)P for initiation and growth of autophagosome membrane, where LC3 protein is localized. Supporting a role of the local PI(3)P in autophagy, LC3/GFP-2xFYVE double-positive puncta is not increased by starvation in Beclin1 S91/94A reconstituted MEFs, which are defective in autophagy induction. The decrease in total PI(3)P levels appears to be a consequence of inhibition of the non-autophagy Vps34 complex because cells expressing the non-phosphorylatable Vps34 T163/S165A mutant could not decrease PI(3)P in response to starvation. Therefore, distinct Vps34 complexes appear to be responsible for production of different cellular pools of PI(3)P. Our data also show that the regulation of non-autophagy Vps34 complexes by AMPK is also important for stress response, as cells expressing the Vps34 T163/165A mutant are sensitive to starvation although autophagy remains normal. Knockdown of Vps34 in MEF did not show significant defects in endocytosis/trafficking, such as EGFR degradation, and Cathepsin D processing, but resulted in dramatic cell growth/proliferation retardation (data not shown), similar to the previous study in U-251 glioblastoma (Johnson et al., 2006). We observe that the defect in cell proliferation is significantly restored by expression of either wild-type or the phosphorylation mutant Vps34 (data not shown). One may speculate that decrease of non-autophagy PI(3)P reduces cellular activities associated with cell growth/proliferation to maintain cell viability upon starvation. In fact, accumulating evidence supports the function of Vps34 in cell cycle, growth, and cytokinesis (Furuya et al., 2010; Gulati et al., 2008; Sagona et al., 2010). Therefore, inhibition of non-autophagy Vps34 represents an important, although little understood aspect of the cellular stress response. In this study, we have not tested different localization of these different Vps34 complexes in cells due to the lack of antibodies suitable for immunostaining. GFP-fusion proteins have been widely used to examine localization of Vps34 complex. However, we should consider the possibility of its artifact due to the expression levels of the target proteins. Especially, stoichiometry of distinct Vps34 is very sensitive to the Vps34 complex protein level. For example, we found that Beclin1 phosphorylation was increased upon ectopic Atg14L expression (Fig.5B), indicating that VIC3 subpopulation is increased. Therefore, we were not comfortable to present these overexpression-based immunofluorescence results. Indeed, when we tried to establish immunofluorescence of tagged Vps34 complex (Vps34, Beclin1, or Atg14L) in MEFs, we realized that it was impractical to obtain expression of all subunits at the levels same as the endogenous proteins. There is indirect evidence showing different localization of Atg14L and UVRAG and moreover, partial overlap of Vps34 with either Atg14L or UVRAG (Itakura et al., 2008).
In this study, we demonstrate that AMPK is necessary and sufficient for Vps34 regulation upon glucose starvation. Previously, we and others have shown AMPK in ULK1 regulation (Egan et al., 2011; Kim et al., 2011). Although lines of evidence indicate ULK1 acting upstream of Vps34 (Di Bartolomeo et al., 2010; Itakura and Mizushima, 2010), it is unclear how ULK1 regulates Vps34. Some reports show that ULK1 is dispensable for autophagy, possibly explained by redundancy with ULK2 (Cheong et al., 2011; Egan et al., 2011). The lack of constitutively active ULK1 or Vps34 hampers us to reach a definitive conclusion. Interestingly, we observed that long-term glucose starvation could activate ULK1-independent autophagy (Fig.S7H), whereas ULK1 is required for the short-term starvation (Fig.S7I). In contrast, Vps34 complex is necessary for both short and long term starvation, revealing complexity of autophagy regulation.
In conclusion, this study reveals a molecular mechanism for the differential regulation of different Vps34 complexes under energy starvation (Fig.7F), in which AMPK inhibits non-autophagy Vps34 complex by phosphorylating Vps34, and activates pro-autophagy Vps34 complex by phosphorylating Beclin1. The presence of autophagy-factor, Atg14L, inhibits Vps34 phosphorylation and promotes Beclin1 phosphorylation, thereby converting the AMPK-mediated inhibition of Vps34 to activation.
Experimental Procedures
in vitro Vps34 lipid kinase assay
Vps34 immune-complexes were incubated with the KA buffer [20 mM HEPES, pH7.4, 1 mM EGTA, 0.4 mM EDTA, 5 mM MgCl2, and 0.05 mM DTT] containing 0.1 mg/ml phosphatidylinositol (PI, Sigma), 50 μM cold ATP, 5 μCi 32P-ATP, 5 mM MnCl2, and 50 μM DTT at 37 for 30 min. Reactions were terminated by adding 1/5 vol. of 1N HCl and the lipid was extracted by 2 vol. of CHCl3:MeOH (1:1). 32P-PI(3)P was separated on thin-layer-chromatography plate (Whatman) under CHCl:MeOH:NH4OH:Water (129:100:4.29:24) mixture.
See the supplemental experimental procedures for additional information.
Supplementary Material
Highlight.
Different Vps34 complexes are distinctly regulated upon energy stress
AMPK activates pro-autophagy Vps34 complex by phosphorylating Beclin1
AMPK inhibits non-autophagic Vps34 complex by phosphorylating Vps34
ATG14L determines whether the Vps34 complex is activated or inhibited by AMPK
Acknowledgements
We thank members of the Guan lab for discussions and reagents. This work was supported by grants from NIH and DOD (KLG), and by the Bio&Medical Technology Development Program of National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST, No.2012M3A9C6049936 to KJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- Furuya T, Kim M, Lipinski M, Li J, Kim D, Lu T, Shen Y, Rameh L, Yankner B, Tsai LH, et al. Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell. 2010;38:500–511. doi: 10.1016/j.molcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EE, Overmeyer JH, Gunning WT, Maltese WA. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J Cell Sci. 2006;119:1219–1232. doi: 10.1242/jcs.02833. [DOI] [PubMed] [Google Scholar]
- Kametaka S, Okano T, Ohsumi M, Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J Biol Chem. 1998;273:22284–22291. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KY, Viollet B, Yoo OJ. CCCP induces autophagy in an AMPK-independent manner. Biochem Biophys Res Commun. 2011;416:343–348. doi: 10.1016/j.bbrc.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, Noda T, Yoshimori T. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J Cell Biol. 2010;190:511–521. doi: 10.1083/jcb.200911141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Noble CG, Dong JM, Manser E, Song H. Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1. J Biol Chem. 2008;283:26274–26282. doi: 10.1074/jbc.M804723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Ohsumi Y. PtdIns 3-Kinase Orchestrates Autophagosome Formation in Yeast. J Lipids. 2011;2011:498768. doi: 10.1155/2011/498768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Sekito T, Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes--Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ropolo A, Grasso D, Pardo R, Sacchetti ML, Archange C, Lo Re A, Seux M, Nowak J, Gonzalez CD, Iovanna JL, et al. The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem. 2007;282:37124–37133. doi: 10.1074/jbc.M706956200. [DOI] [PubMed] [Google Scholar]
- Sagona AP, Nezis IP, Pedersen NM, Liestol K, Poulton J, Rusten TE, Skotheim RI, Raiborg C, Stenmark H. PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat Cell Biol. 2010;12:362–371. doi: 10.1038/ncb2036. [DOI] [PubMed] [Google Scholar]
- Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Deretic V. The role of PI3P phosphatases in the regulation of autophagy. FEBS Lett. 2010;584:1313–1318. doi: 10.1016/j.febslet.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.