Abstract
The activity of protein phosphatase-1 (PP1) inhibitor-1 (I-1) is antithetically modulated by the cAMP-protein kinase A (PKA) and Ca2+-protein kinase C (PKC) signaling axes. β-adrenergic (β-AR) stimulation results in PKA-phosphorylation of I-1 at threonine 35 (Thr35) and depressed PP1 activity, while PKC phosphorylation at serine 67 (Ser67) and/or Thr75 increases PP1 activity. In heart failure, pThr35 is decreased while pSer67 and pThr75 are elevated. However, the role of Ser67/Thr75 phosphorylation in vivo and its effects on Ca2+-cycling are not known. Thus, our aim was to investigate the functional significance of Ser67 and Thr75 phosphorylation in intact hearts. We generated transgenic mice (TG) with cardiac-specific overexpression of constitutively phosphorylated I-1 at Ser67 and Thr75 (S67D/T75D) and evaluated cardiac function. The S67D/T75D cardiomyocytes exhibited significantly depressed Ca2+-kinetics and contractile parameters, compared with wild-type (WT) cells. The decreased Ca2+-cycling was associated with a 27 % increase in PP1 activity, no alterations in PP2 activity and impaired phosphorylation of myosin-binding protein-C (MyBPC). Upon aging, there was cardiac remodeling associated with increases in systolic and diastolic left ventricular internal diameter dimensions (at 16 months), compared with WTs. The results indicate that phosphorylation of I-1 at Ser67 and Thr75 is associated with increased PP1 activity and depressed cardiomyocyte Ca2+-cycling, which manifests in geometrical alterations over the long term. Thus, hyper-phosphorylation of these sites in failing hearts may contribute to deteriorative remodeling.
Keywords: Protein phosphatase 1, Inhibitor-1, Calcium cycling, Cardiac function
Introduction
Heart function relies on highly regulated cyclic variations of intracellular Ca2+ levels, peaking at systole and declining during diastole, when the muscle fills up with blood for the next contraction cycle. During stress conditions, such as exercise and fight or flight responses, the sympathetic nervous system exerts positive inotropic effects mainly by activation of β-adrenergic receptors. As a result, enhanced adenylyl cyclase (AC) leads to increased intracellular cAMP and PKA activity. PKA phosphorylates target enzymes involved in energy metabolism and key Ca2+-cycling proteins that modulate contractility [4, 17]. In the heart, the β-adrenergic-stimulatory effects are reversed mainly by PP1 [30]. The first direct evidence for the role of PP1 in cardiac function in vivo was provided in mouse models with cardiac-specific overexpression of PP1-α that exhibited depressed cardiac function, a blunted β-adrenergic response and remodeling [9]. Thus, PP1 is a negative regulator of cardiac Ca2+-cycling and contractility.
PP1 is often associated with regulatory and inhibitory subunits that confer functional diversity for the PP1 holoenzyme. The inhibitors include inhibitor-1 (I-1) and inhibitor-2 (I-2). I-1 is expressed in all mammalian tissues including the heart [18] and it is activated by β-adrenergic dependent phosphorylation at Thr35, causing decreased PP1 activity [12, 14, 16]. Two additional I-1 phosphorylation sites were suggested to exist in a study of I-1 purified from rabbit skeletal muscle [1]. Serine 67 was one of these sites and its phosphorylation occurred with a slower rate, compared with Thr35. An additional unknown site appeared to be phosphorylated with even slower rate than Ser67 and it was suggested that Thr75 might be the best candidate [1]. The functional significance of Ser67 phosphorylation in the heart was first proposed in a study using a PKCα deficient mouse [8]. In this model, there was decreased Ser67 phosphorylation, decreased PP1 activity and increased contractile function, suggesting an association between phosphorylation of Ser67 and PP1 activity. Subsequently, Thr75 was also identified as an additional PKCα-site on I-1, using purified recombinant systems [34, 35]. In isolated cardiomyocytes, Thr75 phosphorylation was associated with increased PP1 activity and depressed contractile parameters [34, 35]. Thus, while I-1 is activated by PKA to inhibit PP1, this effect is negated by PKC-phosphorylation of Ser67 and/or Thr75. This dual regulation of I-1 activity implies that I-1 may function as a nodal point, integrating the regulatory effects of two major second messengers, cAMP and Ca2+.
Interestingly, human and experimental heart failure is associated with increased PP1 activity as well as decreased protein levels and activity of I-1 [11]. The depressed I-1 activity reflects depressed PKA-phosphorylation as well as increased PKC-phosphorylation of this protein [8, 33, 35]. These observations implicated I-1 as an important player in the pathological changes associated with heart failure [29, 45]. However, although the functional significance of PKA-phosphorylation of I-1 has been elucidated by several research groups [31, 33, 46, 47], similar studies on PKC-phosphorylation of I-1 are not available. Thus, our goal was to investigate the role of Ser67 and Thr75 dual site phosphorylation of I-1 and its modulatory effects on cardiac Ca2+-cycling and function in vivo. The present study suggests that expression of I-1 with constitutively phosphorylated Ser67D and Thr75D results in increased PP1 activity, consistent with depressed cellular contractility and Ca2+ cycling. The impaired Ca2+-handling manifests in cardiac remodeling upon aging, as evidenced by in vivo echocardiographic measurements.
Materials and methods
Experimental animals
Transgenic (TG) mice were generated at the University of Cincinnati by pronuclear microinjection of a ~6 kb cDNA fragment into WT FVBN/n mice. The transgene consisted of α-MHC promoter, the human I-1S67D/T75D and the simian virus 40-polyadenylation site. Three TG lines were generated and all expressed similar levels of I-1S67D/75D (between 16 and 18 fold of endogenous I-1). Line 1 was used and experiments were performed on males separated on two age groups: young mice between 3 and 4 months and old mice between 16 and 18 months.Age-matched WT mice were used as controls.The present study was in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23,revised 1996) and the National Research Council Guide for the Care and Use of Laboratory Animals: 8th Edition published byThe National Academies Press, 2011, Washington, DC. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Cincinnati (Protocol No. 04-04-19-02).
Western blot analysis
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) and the appropriate depth of anesthesia was tested by monitoring hind limb reflexes. Hearts were excised, snap frozen in liquid nitrogen and homogenized in 1X cell lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with phenylmethylsulphonyl fluoride (PMSF, 1 mM) and complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The hearts were homogenized using an automated biological sample lyser (Precellys 24, Omni International, Kennesaw, GA) for 15 s at 6,500 rpm and protein concentration was quantified using the Bradford method. Equal amounts of homogenates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblotting analysis was performed using specific primary antibodies (between 1:500 and 1:2,000 dilutions) corresponding to the protein under analysis: I-1 (Covance Inc. Denver, PA); pI-1-Thr35 custom made antibody; calsequestrin (CSQ), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), total PLN and total RyR (Thermo Scientific, Rockford, IL); p-PLN-Ser16, p-PLN-Thr17, p-RyR2-Ser2809 and p-RyR2-Ser2815 (Badrilla Ltd, Leds, West Yorkshire, UK); myosin-binding protein C (MyBPC) and PP1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); p-MyBPC-Ser282 (Alexis Biochemicals, San Diego, CA); troponin I (TnI) and p-Tn I Ser22/23 (Cell Signaling Technology, Boston, MA). The secondary antibody conjugated with horse radish peroxidase was used at a 1:5,000 dilution. On the nitrocellulose membrane, the bands were visualized with SuperSignal West Pico chemiluminescence substrate kit (Pierce, Rockford, IL) or ECLPLUS Western Blotting Detection kit (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). The intensity of the bands was quantified using AlphaEaseFC analysis software (Protein-Simple, Santa Clara, CA). The densitometry values corresponding to TG samples were normalized to WT samples and the data were expressed as fold change relative to control WT. As an internal standard we used calsequestrin or GAPDH.
Assessment of protein phosphatase activity
Whole-heart homogenate samples were prepared in homogenization buffer without NaF and PP1 activity was assessed using RediPlate 96 EnzChek Serine/Threonine Phosphatase Assay Kit (Molecular Probes, Inc., Eugene, OR). Briefly, samples were diluted in 1X reaction buffer and incubated with the substrate for 30 min at 37 °C, protected from light. The PP1 activity was measured in the absence or presence of 10 nM okadaic acid (OA) in the reaction buffer to block protein phosphatase 2A (PP2A) activity. PP1 activity in heart homogenates was also measured in the presence of a PKA inhibitor (H-89, 1 μM, Sigma-Aldrich, St. Louis, MO), which had no effect, when compared with the PP1 activity in the absence of H-89. A negative control was included by adding 1X reaction buffer to the substrate and a positive control was prepared by diluting a PP1 enzyme standard of known activity in the reaction buffer. The product of the enzymatic reaction was measured using a fluorescence microplate reader equipped with the appropriate filters to detect the reaction product that exhibits excitation/emission maxima of 358/452 nm.
Myocyte isolation
Cardiac myocytes were isolated according to a protocol described previously [32]. Briefly, adult mice were anesthetized with sodium pentobarbital (50 mg/kg); hearts were excised and perfused on a Langendorff apparatus with oxygenated solution containing 0.65 units/ml Liberase TH (Roche, Indianapolis, IN), at 37 °C. Following digestion, dissociated cells were placed on laminin-coated coverslips and allowed to attach for 15 min. All experiments were carried out at room temperature (22–25 °C) in standard Tyrode solution containing (mM): 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 10 HEPES, 1.8 CaCl2 and pH 7.4 adjusted with NaOH.
Cell shortening and intracellular calcium measurements ([Ca]i)
Cells were paced with a stimulation frequency of 0.5 Hz using a pair of platinum electrodes that delivered voltage pulses generated by a Grass stimulator (model S88, Grass, West Warwick, RI, USA). Cell contractions were measured using a video edge detector (Crescent Electronics model VED-105, UT, USA) and the signal was digitized and recorded on a computer. For [Ca2+]i measurements, cells were loaded with the membrane-permeable form of the fluorescent Ca2+ indicator fura-2/AM (2 μM; Invitrogen/Molecular probes, Carlsbad, CA, USA) in standard Tyrode solution for 20–30 min at room temperature, followed by a >20 min de-esterification interval in dye-free media. Cells were simultaneously excited at 340 (F340) and 380 nm (F380) and emission signals were collected at 505 nm by a photomultiplier tube. [Ca]i changes were expressed as changes in ratio ΔR = F340/F380.
Ischemia/reperfusion
Mice were anesthetized by intraperitoneal injection with pentobarbital (50 mg/kg body weight) and the adequacy of anesthesia was evaluated by monitoring hind limb reflexes. Excised hearts from TG and WT mice were mounted on a Langendorff apparatus and retrogradely perfused through the aorta with Tyrode solution (37°) containing (mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.5 EDTA 2Na, 25 NaHCO3, 2.5 CaCl2, 11 Glucose, pH 7.4 adjusted with NaOH. The perfused hearts were stabilized for 30 min at constant pressure (65 cm H2O) at 37°. Perfusion was then stopped for 40 min and subsequently a 60-min reperfusion period followed. The following contractile parameters were acquired with a water-filled balloon connected to a pressure transducer and heart performance analyzer (HPA-τ; Micro-Med Ltd, Louisville, KY): end-diastolic pressure (LVEDP), left ventricular developed pressure (LVDP), maximum rates of isovolumic contraction (+dP/dt) and relaxation (–dP/dt).
Echocardiography and Doppler measurements
Echocardiographic images were obtained on unstressed mice following general anesthesia with 1.5–2 % isoflurane. Based on our previous studies [23], this level of anesthesia allowed us to record changes in heart rate and cardiac function in response to injections of inotropes. The chest hair was removed and the mouse placed on a heated platform where the animals were kept at constant body temperature. All images were obtained with a 30-MHz transducer probe (MS 400, Vevo 2100 system, Visualsonics, Toronto, Canada) placed perpendicular and along the long axis of the heart as previously described [36]. Echocardiographic measurements acquired by both 2D and M-mode in the parasternal long axis view included: thickness of septal and posterior LV wall, size of LV cavity (end-diastolic LVEDD and end-systolic LVESD dimensions) and left ventricular diameter. Fractional shortening (FS) was calculated as [(LVEDD – LVESD)/LVEDD] × 100. Ejection fraction (EF) was calculated using Teicholz formula. All images were transferred as 2D gray-scale images to a separate computer station for post-imaging processing VevoStrain software (Vevo 2100, v1.1.1 B1455, Visualsonic, Toronto, Canada).
Histological analysis
Excised hearts from 4- and 18-month-old mice were fixed in 4 % paraformaldehyde. Longitudinal sections of 3 μm were generated at the Cincinnati Children's Pathology Research Core and stained with hematoxylin & eosin (H&E). Images of whole heart sections were captured in bright field with a color camera attached to a stereo microscope, at 100× magnification (Leica Microsystems, Buffalo Grove, IL).
Statistical analysis
The results are presented as mean ± standard error of the mean for the indicated number of samples. Statistical significance was determined using Student's t test for paired or unpaired data. The difference was considered statistically significant at p < 0.05.
Results
Expression of I-1S67D/T75D increases cardiac phosphatase-1 activity
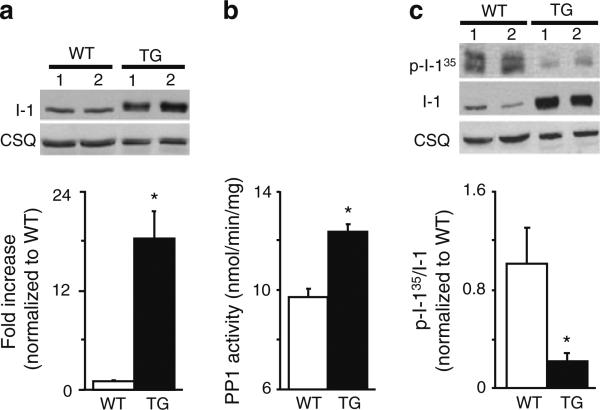
Previous studies using adenovirus-infected rat cardiomyocytes suggested that overexpression of I-1S67D/T75D impaired cellular contractility following forskolin (adenylate cyclase activator) stimulation [34]. We investigated here the in vivo effects of Ser67 and Thr75 phosphorylation, by generating a mouse model with cardiac-specific overexpression of a constitutively phosphorylated I-1 at these sites (S67D/T75D). As illustrated in Fig. 1a, the expression levels of I-1 were ~18-fold higher in TG mice, compared with WTs. Accordingly, PP1 activity was increased by 27 % in TG hearts (Fig. 1b). Furthermore, PP1 activity was measured in membrane and cytosolic fractions, and the TGs exhibited a significant increase only in the cytosolic fraction (~26 %), compared with WTs. This was confirmed by assessment of the PP1 catalytic subunit levels, which were also increased by 30 % in the cytosolic fraction of TGs (data not shown). To determine whether S67D/T75D may influence PKA-phosphorylation of Thr35 in I-1, we used an antibody that specifically recognizes the phosphate group at Thr35 and measured the pThr35 and the total I-1 levels in TG and WT hearts. The results indicate that in TGs, Thr35 phosphorylation levels are reduced compared with WT hearts (Fig. 1c). In addition, the degree of p-Thr35 was examined in cardiac homogenates following isoproterenol stimulation (8 mg/kg and euthanized 5 min later) [37]. The results showed that p-Thr35 levels were significantly increased in both WTs and TGs but they still remained lower in TGs, compared with WTs (data not shown). Thus, expression of I-1S67D/T75D is associated with decreased phosphorylation of the PKA-site.
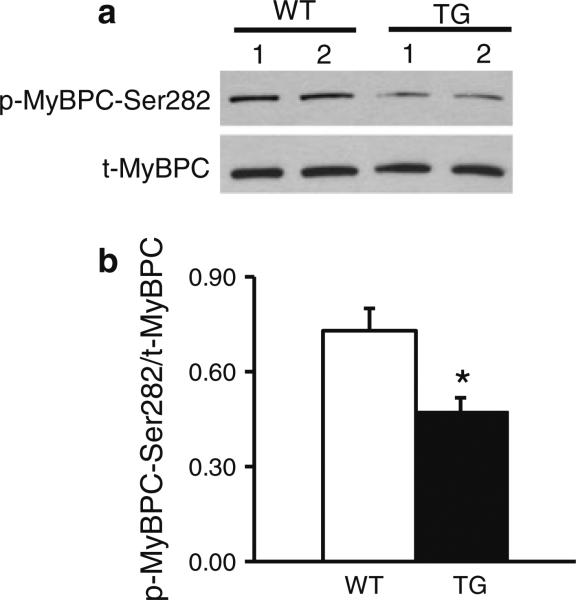
Fig. 1.
Cardiac overexpression of I-1S67D/T75D is associated with increased PP1 activity. a I-1S67D/T75D expression levels are ~18-fold higher in TG heart homogenates compared to WTs. Due to low I-1 abundance of endogenous I-1, 4× more WT protein (120 μg) was loaded on the gel to allow for detection. This adjustment was taken into consideration when I-1 expression levels were quantified (N = 9). b PP1 activity levels in heart homogenates from TG hearts increased by 27 % compared with WTs (N = 10). c Phosphorylation levels of Thr35 in TG heart homogenates are decreased to ~20 % compared with WT control (N = 4); *p < 0.05 versus WT
Depressed cardiomyocyte contractility in TG mice
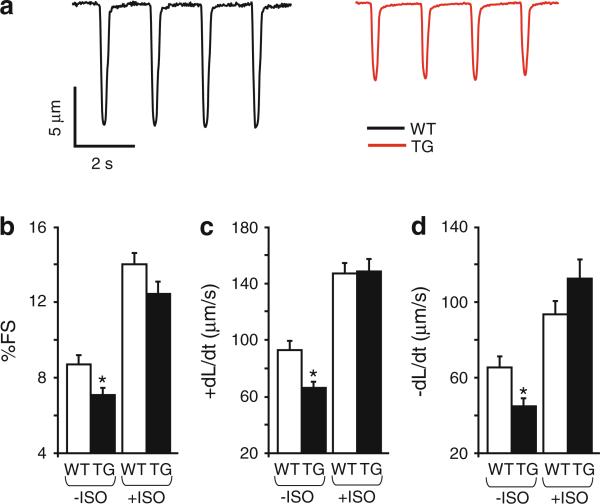
To determine the effect of I-1S67D/T75D expression and increased PP1 activity on cardiac function, we measured basal contractile parameters in isolated cardiomyocytes. Compared with WT, TG myocytes showed depressed contractility (Fig. 2a) as indicated by the degree of fractional shortening (FS: 19 % decrease), the rate of contraction (+dL/dt: 29 % decrease) and the rate of relaxation (–dL/dt: 32 % decrease) (Fig. 2b, c, d). However, isoproterenol (ISO 100 nM) stimulation relieved the inhibitory effects of I-1S67D/T75D under basal conditions and the maximally stimulated parameters were similar between TG and WT cells (Fig. 2b, c, d). Altogether, the observations under basal conditions correlate with the afore-mentioned increased PP1 activity in TG mouse hearts. However, adrenergic stimulation and PKA-activation prevail over increases in PP1 and abolish the differences in contractile parameters.
Fig. 2.
Contractility in TG cardiomyocytes is depressed. a Representative traces illustrating myocyte contractions at 0.5 Hz stimulation in WT (black) and TG cells (red). Quantification of myocyte fractional shortening (b), contraction rates, (c) and relaxation rates (d) under basal conditions (–ISO) and in the presence of 100 nM isoproterenol (+ISO) (–ISO: WT = 51 cells, 5 hearts; TG = 88 cells, 8 hearts; +ISO: WT = 35 cells, 4 hearts; TG = 35 cells, 4 hearts); values are mean ± SEM based on numbers of cells; *p < 0.05
Depressed [Ca2+]i transients in TG cardiomyocytes and reduced myosin-binding protein C phosphorylation
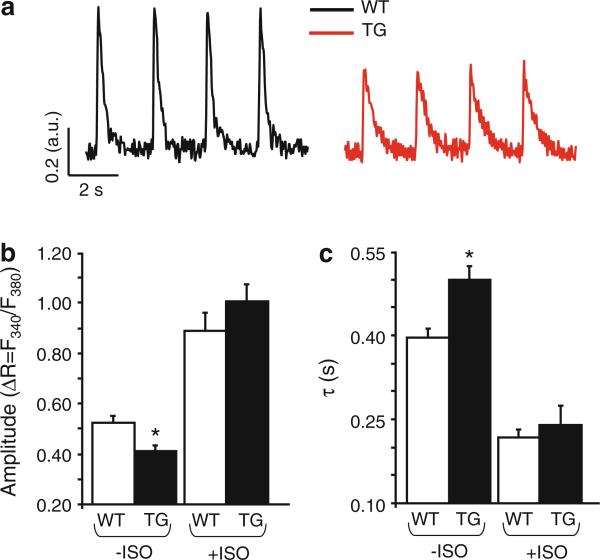
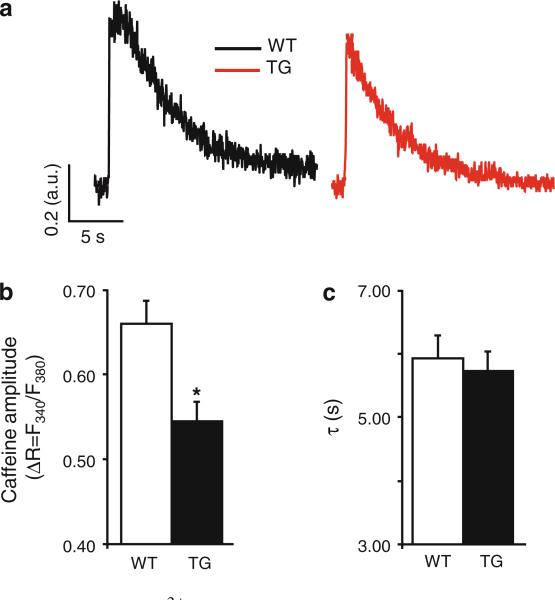
To determine whether the observed alterations in myocyte contractility are accompanied by similar alterations of the intracellular calcium cycling ([Ca]i) (Fig. 3a), we measured basal intracellular [Ca2+]i transients in fura-2AM loaded cells subjected to electrical stimulation in normal Tyrode solution. The [Ca2+]i transient amplitude decreased by 22 % in TG cells (Fig. 3b). Analysis of the kinetic characteristics of the decay phase of the [Ca2+]i transient revealed that the exponential decay time (τ) was significantly prolonged in TG compared with WT (Fig. 3c). Similar to contractility data presented above, adrenergic stimulation significantly enhanced Ca2+-transient amplitude and kinetics compared with basal conditions (Fig. 3b, c) in both TG and WT groups. Moreover, isoproterenol leveled the differences that were seen in basal conditions in TG, compared with WT group, analogous with the aforementioned contractility results. To test if the alterations in basal [Ca2+]i transient characteristics are due to changes in the SR Ca2+ load, we estimated the total SR Ca2+ content by measuring the amplitude of the [Ca2+]i transient evoked by fast application of 10 mM caffeine (Fig. 4a). The analysis of the caffeine-induced [Ca2+]i transient indicated that in TG cells, the caffeine-induced amplitude (Fig. 4b) was decreased by 18 % compared with WT cells, while the decay phase, corresponding to the Ca2+ extrusion mediated mainly through Na/Ca exchanger (NCX) and plasmalemmal Ca2+ ATPase was not significantly changed in TGs, compared with WTs (Fig. 4c). These data suggest that expression of I-1S67D/T75D induces increased PP1 activity which reflects decreases in Ca2+ cycling and depressed contractility.
Fig. 3.
[Ca2+]i cycling in TG cardiomyocytes is impaired. a Representative traces illustrating [Ca2+]i transients measured with fura-2AM in WT (black) and TG (red) cells, while stimulated with 0.5 Hz. b Quantification of [Ca2+]i transient amplitudes expressed as ratiometric fluorescence signals (ΔR = F340/F380) under basal conditions and in the presence of 100 nM isoproterenol. c Mono-exponential constant of the Ca2+ transient decay in the absence (–ISO: WT = 81 cells, 6 hearts; TG = 72 cells, 6 hearts) and in the presence of 100 nM isoproterenol (+ISO: WT = 29 cells, 4 hearts; TG = 27 cells, 3 hearts; 4 months old); values are mean ± SEM based on measured numbers of cells; *p < 0.05
Fig. 4.
Basal SR Ca2+-load assessed by fast caffeine exposure is reduced in TG cells. a Representative traces illustrating [Ca2+]i transients measured in response to 10 mM caffeine in WT (black) and TG (red) cells. The fluorescence signals were measured with fura-2AM in cells paced at 0.5 Hz. b Quantification of [Ca2+]i transient amplitudes expressed as ratiometric fluorescence signals (ΔR = F340/F380) under basal conditions. c Mono-exponential constant of the Ca2+ transient decay (WT = 68 cells, 6 hearts; TG = 56 cells, 6 hearts); values are mean ± SEM based on numbers of cells; *p < 0.05
The altered cellular Ca2+ cycling and contractility prompted us to investigate the main phosphoproteins that control cardiac contractility. Thus, we investigated the total and phosphorylation levels of PLN at Ser16 (PKA site) and Thr17 (CaMKII site), RyR phosphorylation at Ser2809 (PKA site) and Ser2815 (CaMKII site), troponin I (p-TnI-Ser22/23) and myosin-binding protein C (p-MyBPC-Ser282). Quantitative analysis indicated that the only protein that was significantly dephosphorylated in TGs compared with WTs was myosin-binding protein C (p-MyBPC-Ser282) (Fig. 5). These results suggest that the observed increased PP1 activity alters phosphorylation of MyBPC, resulting in depressed myocyte contractility.
Fig. 5.
Cardiac overexpression of I-1S67D/T75D is associated with decreased MyBPC phosphorylation at Ser282. a Representative western blots and b quantification of phosphorylation levels of MyBPC at Ser282 showing that in TG heart homogenates, Ser282 phosphorylation levels are decreased by ~35 % compared with WT controls (N = 4); *p < 0.05 versus WT
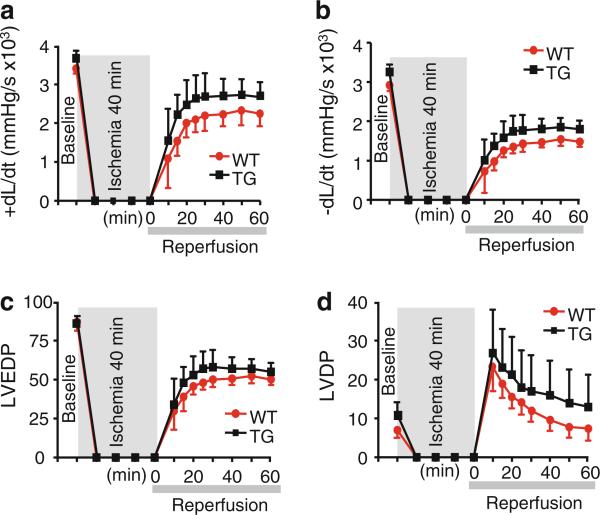
Recovery of function is not altered in TG hearts upon ischemia/reperfusion injury
Previous studies have implicated I-1 as a modulator of contractile recovery upon ischemia/reperfusion injury (I/R) and suggested that expression of a truncated, constitutively active I-1 lacking the PKC sites (Thr35D, aa 1–65) enhanced cardiac performance and attenuated apoptosis post I/R [31, 33, 43]. Therefore, we examined the effect of I-1S67D/T75D on functional recovery of Langendorff-perfused hearts subjected to 40-min ischemia followed by 60-min reperfusion (I/R). Upon reperfusion, TG hearts showed similar rates of contraction and relaxation as WT hearts (Fig. 6a, b). In addition, diastolic (LVEDP) and systolic (LVDP) functional parameters were comparable during reperfusion in both TG and WT hearts (Fig. 6c, d).
Fig. 6.
Contractile recovery of TG hearts is similar to WTs upon ischemia/reperfusion injury. Heart performance evaluated by measuring a maximal rates of contraction (+dP/dt), b maximal rates of relaxation (–dP/dt) c left ventricular end diastolic pressure (LVEDP) and d left ventricular developed pressure (LVDP). The results indicate comparable recovery of isolated TG perfused hearts following ischemia–reperfusion versus WT hearts (N = 3, 4 months old, *p < 0.05)
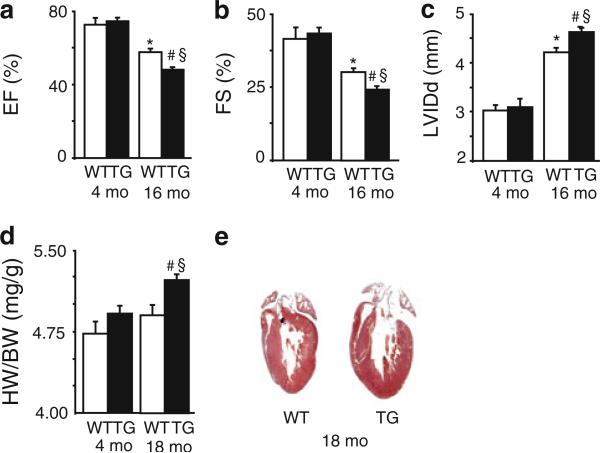
Contractile dysfunction and remodeling in aging TG mice
Echocardiography measurements in intact animals indicated that there were no contractile or geometrical alterations in I-1S67D/T75D compared with WT mice at 4 months of age (Fig. 7a, b, c; Table 1). Correspondingly, cardiac morphology showed no significant differences between TGs and WTs (data not shown). However, upon aging to 16-months of age, there were significant decreases in EF (Fig. 7a) and FS (Fig. 7b) in TGs compared with WTs (Table 1). Furthermore, there was a significant increase in left ventricular internal diameter (LVIDd; Fig. 7c) and dilation of LV chambers as illustrated by the increased systolic and diastolic LV volume in TGs, compared with WTs (Table 1). Accordingly, assessment of HW/BW ratio in aging mice confirmed the increased hypertrophy in TGs (Fig. 7d) and histological evaluation of longitudinal heart sections confirmed the thicker LV wall in TGs compared with WTs (Fig. 7e). We also monitored the survival rates of a cohort of TG (n = 19) and WT mice (n = 29) and we observed that by 21 months of age, 63 % of TG had died compared with 34 % of WTs, indicating increased mortality in TGs, compared with WTs. Altogether, these data indicate that the depressive effects of I-1S67D/T75D expression on cardiomyocytes Ca2+-cycling in young hearts lead to depressed in vivo cardiac function and remodeling over the long-term.
Fig. 7.
Contractile dysfunction and remodeling in aging TG mice. Comparison of heart performance evaluated in vivo by echocardiographic measurement of a ejection fraction (%EF), b fractional shortening (%FS) and c end-diastolic left ventricular internal dimension (LVIDd) in 16-month-old mice versus 4-month-old mice (N = 6–7 mice). d Hypertrophy assessed by heart weight/body weight ratio (HW/BW; WT = 6, TG = 4 hearts. e Illustrative examples of longitudinal heart sections obtained from 18-month-old WT and TG hearts stained with H&E; *p < 0.05 versus young WT mice; #p < 0.05 versus young TG mice; §p < 0.05 versus WT old mice
Table 1.
Echocardiography parameters from male FVB/n mice at different ages
| WT |
TG |
|||
|---|---|---|---|---|
| 4 months | 16 months | 4 months | 16 months | |
| LV Vol;d (μl) | 36.56 ± 3.11 | 78.89 ± 3.56* | 39.24 ± 5.65 | 97.94 ± 5.05#,§ |
| LV Vol;s (μl) | 10.20 ± 2.11 | 33.77 ± 1.94* | 10.11 ± 1.87 | 51.19 ± 2.50#,§ |
| EF (%) | 72.47 ± 3.66 | 57.33 ± 1.70* | 74.15 ± 2.21 | 47.83 ± 1.16#,§ |
| FS (%) | 41.47 ± 2.88 | 29.96 ± 1.20* | 43.02 ± 2.10 | 24.05 ± 0.72#,§ |
| LVID;d (mm) | 3.03 ± 0.18 | 4.20 ± 0.11* | 3.10 ± 0.18 | 4.60 ± 0.10#,§ |
| LVID;s (mm) | 1.78 ± 0.14 | 2.94 ± 0.07* | 1.75 ± 0.10 | 3.50 ± 0.07#,§ |
LV Vol;d left ventricular volume (diastole), LV Vol;s left ventricular volume (systole), EF ejection fraction, FS fractional shortening, LVID;d left ventricular internal diameter (diastole), LVID;s left ventricular internal diameter (systole)
p < 0.05; WT at 16 months versus WT at 4 months
p < 0.05; TG at 16 months versus TG at 4 months
p < 0.05; TG at 16 months versus WT at 16 months
Discussion
The inhibitor-1 of PP1 has emerged as an important regulator of Ca2+-cycling and contractility in the heart [29]. While the functional role of its PKA-mediated phosphorylation at Thr35 has been extensively investigated in vivo [10, 31, 33, 47], the significance of PKC-phosphorylation on Ser67 and Thr75 in cardiac contractility remains unclear. The present study shows that constitutive phosphorylation of these sites (I-1S67D/T75D) is associated with increased PP1 activity, impaired Ca2+-cycling and depressed contractility, which leads to cardiac remodeling upon aging. Additionally, the I-1S67D/T75D hearts exhibit a significant decrease of Thr35 phosphorylation compared with WTs, supporting the notion that phosphorylation of Ser67/Thr75 converts I-1 into a weaker substrate for PKA [34]. Several studies reported that the failing human heart is characterized by increased PP1 activity [8, 27], decreased I-1 expression levels [12], attenuated phosphorylation at Thr35 [9, 11] and increased phosphorylation at Ser 67 and Thr75 [8, 35]. Additionally, it was demonstrated that heart failure is associated with an increase in PKCα protein levels and activity [6, 44]. Therefore, heart failure favors both dephosphorylation of Thr35 and phosphorylation of Ser67/Thr75, attenuating the inhibitory function of I-1. This leads to increased PP1 activity, further compromising the deteriorated contractility of failing hearts.
Pathological increases in PP1 activity have been investigated in PP1α overexpressing mouse models [9] and notably, the depressed cardiac function could be restored by PKCα deletion [8], suggesting that PKC signaling through I-1 may alter PP1 activity. Further in vivo studies have shown that PKCα phosphorylates I-1 at both Ser67 and Thr75 [8, 35]. Additionally, purified recombinant I-1 protein studies have indicated that the two sites appear to be phosphorylated independently of each other and to the same extent [35]. The role of PP1 in the control of cardiac function was further addressed in studies of TG mice overexpressing a truncated form of the PP1 inhibitor-2 (I-2: ~40-fold). I-2 overexpression resulted in decreased PP1 activity, increased Ca2+ cycling in isolated myocytes and enhanced in vivo contractile function [21]. In addition, cell permeant organic inhibitors of PP1 activity protected the heart during reperfusion [15] and induced positive inotropic effects on contractile responses of mouse aorta and pulmonary artery [22] in isolated heart preparations [28] and failing human hearts [25], substantiating the role of PP1 activity in smooth and cardiac muscle contractility.
The present study indicates that constitutively phosphorylated I-1S67D/T75D increases PP1 activity and impairs myocyte Ca2+-kinetics and mechanical performance in vivo. The underlying mechanisms may involve reduced association of I-1S67D/T75D with PP1, rendering a more active enzyme, and/or lower efficiency of I-1S67D/T75D to serve as substrate for PKA and thus, to reduce PP1 activity. Indeed, we detected enhanced phosphorylation of I-1 at Thr35 in WT hearts under basal conditions, in accordance with previous observations [13], but lower phosphorylation at Thr35 in TG hearts. Moreover, in vitro studies in rat myocytes expressing I-1S67D/T75D indicated that the mutant shows significantly less inhibitory effects on PP1 activity, compared with GFP-infected myocytes [34] under PKA stimulation. The increased PP1 activity reflected dephosphorylation of MyBPC at its PKA/CaMKII-site (Ser282) in thick myofilaments, compared with WTs. Constitutive dephosphorylation of MyBPC at PKA sites (Ser273, -282, -302) was previously shown to associate with contractile dysfunction both in vitro [42] and in vivo as well as with hypertrophy in transgenic mice [40]. On the other hand, MyBPC phosphorylation at PKA sites appears to be cardioprotective [39] except in heart failure, when PKC-mediated phosphorylation of MyBPC (Ser273, -302) depresses systolic function [24]. Nevertheless, the phosphorylation status of Ser282 appears to be critical in modulating myocardial function [38].
Additional evidence showed that the I-1 homolog, DARPP-32 (dopamine- and cyclic AMP-regulated phosphoprotein with MW of 32 kDa), is phosphorylated at Thr75 by cdk5, and transforms DARPP-32 into a PKA inhibitor, reducing its ability to phosphorylate DARPP-32 and other substrates [26]. Thus, I-1S67D/T75D may activate PP1 and inhibit PKA at the same time, contributing to the observed depressed function. Further in vitro studies have indicated that although the contractility of myocytes expressing I-1S67D, I-1T75D or I-1S67D/T75D was enhanced under forskolin stimulation, the overall function remained depressed compared with controls [34]. However, isoproterenol stimulation in the current study enhanced the contractile response and Ca2+ cycling in TGs and the overall function was similar to WTs, suggesting that sympathetic stimulation prevails over the increased PP1 activity. This may also explain why in our model, the alterations in isolated I-1S67D/T75D cardiomyocytes did not translate into depressed contractility in whole hearts or in vivo in the younger mice. Isolated cardiomyocytes represent an unloaded system, free of extracellular matrix and geometric constrains, while the intact heart is affected by additional extrinsic factors such as hemodynamic load and hormonal input that might override the cellular impairment, resulting in normal in vivo cardiac function [41]. Nonetheless, we observed a decline in basal contractility as well as heart remodeling in aging mice, suggesting that long-term I-1S67D/T75D overexpression is detrimental. Aging is considered a physiological stress and it has been shown to be associated with ventricular hypertrophy, fibrosis and a propensity toward apoptosis [5, 7, 19, 20]. Accordingly, aging WT mice exhibited gradual depression of cardiac function, measured by echocardiography, which was comparable to previous studies [2, 3]. However, TG mice showed accelerated cardiac dysfunction and hypertrophy compared with WTs.
Altogether, our present study shows that I-1S67D/T75D expression is associated with alterations in contractility and Ca2+ homeostasis at the cellular level, which leads to remodeling under the physiological stress of aging and further suppression of contractile function. These findings underline the physiological role of the Ser67 and Thr75 phosphorylation sites in I-1 regulation and provide further insight into the modulatory role of I-1 in cardiac function.
Acknowledgments
We thank Dr. Wen Zhao for advice on isolation of myocytes, Ms. Andrea Collins for help with the Western Blot technique and Ms. Valerie Lasko for technical expertise in performing mouse catheterization. This work was supported by National Institutes of Health Grants: HL064018 and HL26057.
Contributor Information
Stela Florea, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Ahmad Anjak, Internal Medicine, Division of Cardiovascular Diseases, College of Medicine, University of Cincinnati, Cincinnati, OH 45267, USA.
Wen-Feng Cai, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Jiang Qian, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Elizabeth Vafiadaki, Molecular Biology Division, Center for Basic Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece.
Sarah Figueria, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Kobra Haghighi, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Jack Rubinstein, Internal Medicine, Division of Cardiovascular Diseases, College of Medicine, University of Cincinnati, Cincinnati, OH 45267, USA.
John Lorenz, Department of Molecular and Cellular Physiology, College of Medicine, University of Cincinnati, Cincinnati, OH 45267, USA.
Evangelia G. Kranias, Department of Pharmacology and Cell Biophysics, College of Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267, USA Litsa.Kranias@uc.edu Molecular Biology Division, Center for Basic Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece.
References
- 1.Aitken A, Bilham T, Cohen P. Complete primary structure of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1982;126:235–246. doi: 10.1111/j.1432-1033.1982.tb06771.x. doi:10.1111/j.1432-1033.1982.tb06771.x. [DOI] [PubMed] [Google Scholar]
- 2.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. doi:10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 3.Anjak A, Koch SE, Jiang MV, Jones WK, Rubinstein J. Age and gender related changes in myocardial function in wild type FVB/N mice evaluated by advanced echocardiography. JASE. 2011;24:B7. [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. doi:10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. doi:10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 6.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. doi: 10.1161/01.CIR.99.3.384. [DOI] [PubMed] [Google Scholar]
- 7.Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, Angeli F, Yeghiazarians Y, Lee R. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. doi:10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. doi:10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 9.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. doi:10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Zhou X, Florea S, Qian J, Cai W, Zhang Z, Fan GC, Lorenz J, Hajjar RJ, Kranias EG. Expression of active protein phosphatase 1 inhibitor-1 attenuates chronic beta-agonist-induced cardiac apoptosis. Basic Res Cardiol. 2010;105:573–581. doi: 10.1007/s00395-010-0106-3. doi: 10.1007/s00395-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. doi:10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann WH, Jackel E, Harding SE, Boknik P, Neumann J, Eschenhagen T. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. doi:10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 13.El-Armouche A, Wittkopper K, Fuller W, Howie J, Shattock MJ, Pavlovic D. Phospholemman-dependent regulation of the cardiac Na/K-ATPase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB J. 2011;25:4467–4475. doi: 10.1096/fj.11-184903. doi:10.1096/fj.11-184903. [DOI] [PubMed] [Google Scholar]
- 14.Endo S, Zhou X, Connor J, Wang B, Shenolikar S. Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry. 1996;35:5220–5228. doi: 10.1021/bi952940f. doi:10.1021/bi952940f. [DOI] [PubMed] [Google Scholar]
- 15.Fan WJ, van Vuuren D, Genade S, Lochner A. Kinases and phosphatases in ischaemic preconditioning: a re-evaluation. Basic Res Cardiol. 2010;105:495–511. doi: 10.1007/s00395-010-0086-3. doi:10.1007/s00395-010-0086-3. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RC, Neumann J, Watanabe AM, Lesch M, Sabbah HN. Evidence for presence and hormonal regulation of protein phosphatase inhibitor-1 in ventricular cardiomyocyte. Am J Physiol. 1996;270:H1159–H1164. doi: 10.1152/ajpheart.1996.270.4.H1159. [DOI] [PubMed] [Google Scholar]
- 17.Haworth RS, Cuello F, Avkiran M. Regulation by phosphodiesterase isoforms of protein kinase A-mediated attenuation of myocardial protein kinase D activation. Basic Res Cardiol. 2011;106:51–63. doi: 10.1007/s00395-010-0116-1. doi:10.1007/s00395-010-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 19.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. doi:10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 20.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. doi:10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhefer U, Baba HA, Boknik P, Breeden KM, Mavila N, Bruchert N, Justus I, Matus M, Schmitz W, Depaoli-Roach AA, Neumann J. Enhanced cardiac function in mice over-expressing protein phosphatase Inhibitor-2. Cardiovasc Res. 2005;68:98–108. doi: 10.1016/j.cardiores.2005.05.019. doi:10.1016/j.cardiores.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Knapp J, Aleth S, Balzer F, Gergs U, Schmitz W, Neumann J. Comparison of contractile responses in isolated mouse aorta and pulmonary artery: influence of strain and sex. J Cardiovasc Pharmacol. 2006;48:820–826. doi: 10.1097/01.fjc.0000232062.80084.4f. doi:10.1097/01.fjc.0000232062.80084.4f. [DOI] [PubMed] [Google Scholar]
- 23.Koch SE, Gao X, Haar L, Jiang M, Lasko VM, Robbins N, Cai W, Brokamp C, Varma P, Tranter M, Liu Y, Ren X, Lorenz JN, Wang HS, Jones WK, Rubinstein J. Probenecid: a novel use as a non-injurious positive inotrope through cardiac TRPV2 stimulation. J Mol Cell Cardiol. 2012;53:134–144. doi: 10.1016/j.yjmcc.2012.04.011. doi:10.1016/j.yjmcc.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios C, Stienen GJ, van der Velden J. Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol. 2010;105:289–300. doi: 10.1007/s00395-009-0053-z. doi:10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linck B, Boknik P, Knapp J, Muller FU, Neumann J, Schmitz W, Vahlensieck U. Effects of cantharidin on force of contraction and phosphatase activity in nonfailing and failing human hearts. Br J Pharmacol. 1996;119:545–550. doi: 10.1111/j.1476-5381.1996.tb15706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Neumann J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res Cardiol. 2002;97(Suppl 1):I91–I95. doi: 10.1007/s003950200036. [DOI] [PubMed] [Google Scholar]
- 28.Neumann J, Boknik P, Herzig S, Schmitz W, Scholz H, Gupta RC, Watanabe AM. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am J Physiol. 1993;265:H257–H266. doi: 10.1152/ajpheart.1993.265.1.H257. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. doi:10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolaou P, Kranias EG. Role of PP1 in the regulation of Ca cycling in cardiac physiology and pathophysiology. Front Biosci. 2009;14:3571–3585. doi: 10.2741/3472. doi:10.2741/3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar RJ, Jones K, Kranias EG. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. doi:10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 33.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H762–H769. doi: 10.1152/ajpheart.00104.2007. doi:10.1152/ajpheart.00104.2007. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez P, Mitton B, Waggoner JR, Kranias EG. Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem. 2006;281:38599–38608. doi: 10.1074/jbc.M604139200. doi:10.1074/jbc.M604139200. [DOI] [PubMed] [Google Scholar]
- 36.Rubinstein J, Aloka F, Abela GS. Statin therapy decreases myocardial function as evaluated via strain imaging. Clin Cardiol. 2009;32:684–689. doi: 10.1002/clc.20644. doi:10.1002/clc.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, Copeland V, Oh M, Bush E, Shelton JM, Bibb JA, Hill JA, Rothermel BA. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ Res. 2011;108:437–445. doi: 10.1161/CIRCRESAHA.110.235309. doi: 10.1161/CIRCRESAHA.110.235309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadayappan S, Gulick J, Osinska H, Barefield D, Cuello F, Avkiran M, Lasko VM, Lorenz JN, Maillet M, Martin JL, Brown JH, Bers DM, Molkentin JD, James J, Robbins J. A critical function for Ser-282 in cardiac Myosin binding protein-C phosphorylation and cardiac function. Circ Res. 2011;109:141–150. doi: 10.1161/CIRCRESAHA.111.242560. doi: 10.1161/CIRCRESAHA.111.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. doi:10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 2006;103:16918–16923. doi: 10.1073/pnas.0607069103. doi:10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Q, Schmidt AG, Hahn HS, Carr AN, Frank B, Pater L, Gerst M, Young K, Hoit BD, McConnell BK, Haghighi K, Seidman CE, Seidman JG, Dorn GW, 2nd, Kranias EG. Rescue of cardiomyocyte dysfunction by phospholamban ablation does not prevent ventricular failure in genetic hypertrophy. J Clin Invest. 2003;111:859–867. doi: 10.1172/JCI16738. doi:10.1172/JCI16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res. 2008;103:974–982. doi: 10.1161/CIRCRESAHA.108.177683. doi:10.1161/CIRCRE SAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Totzeck A, Boengler K, van de Sand A, Konietzka I, Gres P, Garcia-Dorado D, Heusch G, Schulz R. No impact of protein phosphatases on connexin 43 phosphorylation in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H2106–H2112. doi: 10.1152/ajpheart.00456.2008. doi:10.1152/ajpheart.00456.2008. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Liu X, Sentex E, Takeda N, Dhalla NS. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H2277–H2287. doi: 10.1152/ajpheart.00142.2002. doi:10.1152/ajpheart.00142.200. [DOI] [PubMed] [Google Scholar]
- 45.Wittkopper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological beta-adrenoceptor signalling. Cardiovasc Res. 2011;91:392–401. doi: 10.1093/cvr/cvr058. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 46.Wittkopper K, Eschenhagen T, El-Armouche A. Phosphatase-1-inhibitor-1: amplifier or attenuator of catecholaminergic stress? Basic Res Cardiol. 2010;105:569–571. doi: 10.1007/s00395-010-0107-2. doi:10.1007/s00395-010-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittkopper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsold B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. doi:10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]