Abstract
Biologically active forms of vitamin D are important analytical targets in both research and clinical practice. The current technology is such that each of the vitamin D metabolites is usually analyzed by individual assay. However, current LC-MS technologies allow the simultaneous metabolic profiling of entire biochemical pathways. The impediment to the metabolic profiling of vitamin D metabolites is the low level of 1α,25-dihydroxyvitamin D3 in human serum (15–60 pg/mL). Here, we demonstrate that liquid–liquid or solid-phase extraction of vitamin D metabolites in combination with Diels–Alder derivatization with the commercially available reagent 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) followed by ultra-performance liquid chromatography (UPLC)–electrospray/tandem mass spectrometry analysis provides rapid and simultaneous quantification of 1α,25-dihydroxyvitamin D3, 1α,25-dihydroxyvitamin D2, 24R,25-dihydroxyvitamin D3, 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in 0.5 mL human serum at a lower limit of quantification of 25 pg/mL. Precision ranged from 1.6–4.8 % and 5–16 % for 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3, respectively, using solid-phase extraction.
Keywords: 1α,25-Dihydroxyvitamin D3; 25-Hydroxyvitamin D3; 24R,25-Dihydroxyvitamin D3; UPLC; LC-MS; Metabolic profiling; Derivatization
Introduction
Metabolic profiling, defined here as the quantification of metabolites involved in the same metabolic pathway, has become an important tool for determining steady-state concentrations of metabolites and studying the regulation of the corresponding metabolic pathways [1, 2]. Metabolic profiling allows metabolic regulation to be surveyed in a minimally invasive manner using biofluids such as plasma or urine that are subsequently analyzed by GC-MS or LC-MS. The field of vitamin D metabolite analysis has been historically dominated by immunoassays and receptor binding assays [3], although there are examples of the application of LC-UV or LC-MS to the analysis of 25-hydroxyvitamin D2 (25(OH)D2) and 25-hydroxyvitamin D3 (25(OH)D3) [4–6]. LC-MS can potentially detect and measure more than 40 reported vitamin D metabolites [7]. If we draw parallels with other families of steroid hormones, many of the vitamin D metabolites may have other biological roles beyond being mere catabolic products. However, very little is known about the biological roles of most of the downstream vitamin D metabolites, and the development of a comprehensive profiling method would facilitate research on vitamin D metabolism. Currently, most research and diagnostic assays focus on 25(OH)D and 1α,25-dihydroxyvitamin D (1α, 25(OH)2D) produced in a series of oxidations by the cytochromes P450 from their dietary precursor vitamin D (Fig. 1) [8]. Most current analytical methods based on immunoassays are not able to separate forms of vitamin D with different side-chains (mainly D2 and D3). There is a growing body of evidence that the biological activities of these forms may be different [9, 10]. This illustrates a need for analytical methods that are selective for D2 and D3 forms. Analytical methods for vitamin D also are needed for regulatory, quality control and nutritional studies. Biologically, the conversion of vitamin D2 and D3 into corresponding 25 (OH)D forms is rapid, as estimated from the 36–48-hour half-life of vitamin D3 in human circulation [11]. Thus, there is usually little need to analyze the blood levels of vitamin D2 and D3 except supplementation studies. Subsequently, 25 (OH)D3 is converted into biologically active 1α,25(OH)2D3, which binds to the vitamin D nuclear receptor (genomic response) as well as to a putative membrane receptor (rapid response) to initiate a cascade of biological events related to calcium and phosphorus homeostasis, cancer and inflammation [12]. Alternatively, 25(OH)D3 is thought to be deactivated via conversion into 24R,25-dihydroxyvitamin D3 (24R,25(OH)2D3) by 25-hydroxyvitamin D 24-hydroxylase, although independent biological effects of 24R,25(OH)2D3 are also known [13–15]. Furthermore, the same enzyme deactivates 1α,25(OH)2D3 via conversion into 1α,24R,25-trihydroxyvitamin D3 (1,24R,25(OH)3D3). These metabolites can undergo further metabolism by several pathways including further oxidation and conjugation [8].
Fig. 1.
Metabolism of vitamin D. The metabolites measured in this study are highlighted in a darker font on the left. The two major forms of vitamin D and sites of hydroxylation are shown on the right
The development of an LC-MS profiling method for vitamin D metabolites is impeded by their low concentration in human circulation, particularly for 1α,25(OH)2D3, with concentrations ranging from 15 to 60 pg/mL. Recently, an LC-tandem MS method was introduced to measure nonderivatized 1α,25(OH)2D3, but this requires 2 mL of human serum [16]. Vitamin D metabolites have low ionization efficiencies in electrospray (ESI) or atmospheric pressure chemical ionization (APCI) sources because they lack easily charged groups, which would enhance ioniza tion efficiencies. However, the conjugated diene group of vitamin D metabolites makes them a specific target for Diels–Alder derivatization. In fact, several highly reactive 4-substituted 1,2,4-triazoline-3,5-diones (TADs or Cookson-type reagents) have been reported in the literature for the analysis of vitamin D metabolites, their analogs and other dienes, including derivatization reagents for ESI-MS [6, 17–22]. The derivatization reagents introduce polar groups and thus typically result in a 100–1000-fold increase in sensitivity over nonderivatized compounds. However, no method for profiling the major vitamin D metabolites 1α,25(OH)2D2, 1α,25(OH)2D3, 24R,25(OH)2D3, 25(OH)D2 and 25(OH)D3 has been reported that can detect and quantify endogenous levels of 1α,25(OH)2D3. Here, we demonstrate an ultra-performance liquid chromatography (UPLC)–tandem MS method for the quantification of an array of the most biologically important vitamin D metabolites after Diels–Alder derivatization with 4-phenyl-1,2, 4-triazoline-3,5-dione (PTAD). The method was validated for solid-phase extraction (SPE) and liquid–liquid extraction (LLE) of 1α,25(OH)2D3 and 25(OH)D3 from serum and applied to studies of vitamin D metabolism in humans.
Experimental
Chemicals
Hexane, methyl tert-butyl ether, dichloromethane, acetonitrile, ethyl acetate, methanol, formic acid, and K2HPO4 were purchased from Fisher Scientific (Pittsburgh, PA, USA). Deionized water (resistivity of 18.1 MΩ/cm) and distilled water were prepared in-house and used for mobile phase preparation and SPE extraction, respectively. PTAD was purchased from Fluka (St. Louis, MO, USA). Standards of vitamin D metabolites were purchased from Fluka, Sigma–Aldrich (St. Louis, MO, USA) and BIOMOL (Plymouth Meeting, PA, USA) as indicated below. Deuterated surrogates of vitamin D metabolites were purchased from Synthetica (Oslo, Norway) and Medical Isotopes (Pelham, NH, USA). Human serum from healthy male donors for method development purposes was ordered from Fisher Scientific (Pittsburgh, PA, USA).
Stock and calibration solutions
Neat standards of vitamin D metabolites were diluted and stored at –80 °C; 10 μg 1α,25-dihydroxyvitamin D3 (Sigma–Aldrich) was dissolved in 1000 μL acetonitrile in the original vial and then transferred into an amber glass HPLC vial; 1 mg 1α,25-dihydroxyvitamin D2 (Fluka) was dissolved in 500 μL acetonitrile; 1 mg 25-hydroxyvitamin D3 (Fluka) was dissolved in 500 μL acetonitrile; 1 mg 25-hydroxyvitamin D2 (Fluka) was dissolved in 1000 μL acetonitrile and transferred into an amber glass HPLC vial; 50 μg 24R,25-dihydroxyvitamin D3 (BIOMOL) in 50 μL ethanol was diluted with 950 μL acetonitrile; 1 mg calcipotriol (Sequoia Research Products, Pangbourne, UK) was dissolved in 2000 μL acetonitrile. Calcipotriol was further diluted with acetonitrile to make a 125 ng/mL derivatization quality control spike. The deuterated internal standards (IS) 26,26,26,27,27,27-hexadeuterium-1α,25-dihydroxyvitamin D3 (Medical Isotopes) and 26,26,26, 27,27,27-hexadeuterium-25-hydroxyvitamin D3 (Synthetica) were dissolved in acetonitrile to prepare stock solutions. The stock solutions were combined and diluted to obtain a 12.5 ng/mL d6 1α,25(OH)2D3 and 500 ng/mL d6 25(OH)D3 IS solution. The purity of the derivatized IS was assessed by LC-MS (full scan and MRM) up to 500 ng/mL and no interferences were found, including no traces of natural metabolites. All analytes were individually dissolved in a solution of PTAD (0.5 mg/mL) in acetonitrile at 100 ng/mL and allowed to react at room temperature for 4 h to form the corresponding PTAD Diels–Alder conjugate. No nonderivatized analytes were found in these solutions as analyzed by LC-MS (MRM). To prevent cross-contamination of the 1α,25(OH)2D-PTAD calibration solution, it was prepared separately from the rest of the analytes. Using serial dilutions, 0.1, 0.3, 1.0, 3.0, and 10.0 ng/mL calibration stocks of 1α,25(OH)2D3-PTAD and 1α,25(OH)2D2-PTAD were prepared. Similarly, we prepared calibration solutions of 25(OH)D2-PTAD and 25(OH)D3-PTAD at the levels of 0.1, 0.3, 1.0, 3.0, 10.0, 30.0, 100, 300 and 1000 ng/mL. These calibration solutions also contained 24R,25(OH)2D3-PTAD at tenfold lower levels than 25(OH)D2-PTAD and 25(OH)D3-PTAD; however, 0.01 and 0.03 ng/mL levels of 24R,25(OH)2D3 were not used in practice.
Human samples
Plasma samples for the HIV study were obtained from the Reaching for Excellence in Adolescent Care and Health (REACH) repository [23, 24]. Serum samples from the sun exposure study were acquired from 17 volunteers in fall 2006 and 17 different volunteers in winter 2007 at week 0, week 4, and week 7 (week 8 in winter). Two 10-mL tubes of blood were collected from each participant after a four-hour fast from fat. The tubes were wrapped in foil and allowed to clot at room temperature for one hour. The tubes were centrifuged and then aliquoted in ten 1-mL tubes. These tubes were placed in light-proof boxes and kept frozen at –80 °C. Commercial human serum from male donors (Fisher Scientific) was used for method development and validation except in the extraction reproducibility study, where donor plasma was used.
Sample pretreatment
Sample preparation was adapted from published methods [25]. Briefly, 500 μL aliquotes of human serum in 2 mL plastic tubes (Fisher Scientific) were spiked with 20 μL solution of internal standard (12.5 ng/mL d6 1α,25-dihydroxyvitamin D3 and 500 ng/mL d6 25-hydroxyvitamin D3) in acetonitrile and allowed to equilibrate for 15 min at room temperature. Proteins were precipitated by the addition of 500 μL acetonitrile and by spinning the sample on a Vortex mixer for 1 min at maximum speed followed by 10 min centrifugation at 10,000×g.
Liquid–liquid extraction
The supernatant from protein precipitation was transferred into 2-mL plastic tubes (Fisher Scientific) containing 400 μL 0.4 M K2HPO4 and mixed using a Vortex mixer for 30 s followed by the addition of 500 μL methyl t-butyl ether (MTBE). The tubes were vigorously mixed for 2 min on a Vortex mixer, centrifuged for 5 min at 10,000×g, and the upper organic layer was transferred into 2-mL plastic tubes (Fisher Scientific). For method development, the organic extracts were spiked with 10 μL 25 ng/mL calcipotriol used as a control for derivatization efficiency. Samples were evaporated for 1 h using an RC10.22 vacuum concentrator (Jouan, Winchester, VA, USA), and 100 μL 0.75 mg/mL PTAD in acetonitrile was added to the residue followed by 1 min of mixing. Samples were left at room temperature for 1 h and then stored overnight at +4 °C to allow the derivatization reaction to proceed to completion; then the samples were mixed for 30 s, centrifuged and transferred into 150-μL vial inserts.
Solid-phase extraction
Solid-phase extraction of vitamin D metabolites using Oasis HLB (Waters, Milford, MA, USA) sorbent was adopted from published methods [22, 25]. Oasis HLB cartridges (3 cc 60 mg) were preconditioned with 3 mL ethyl acetate, 3 mL methanol and 3 mL water. Individual valves of cartridges were closed after the water meniscus reached the sorbent surface. Cartridges were loaded with 900 μL supernatant from the protein precipitation protocol and 1 mL 0.4 M K2HPO4. The valves were opened and samples were extracted using gravity only. Cartridges were subsequently washed with 3 mL water and 2 mL of 70% methanol and dried for 2 min by application of negative pressure. The needles of the extraction manifold were wiped to remove residual solvent droplets. Samples were eluted with 1.5 mL of acetonitrile into 2 mL plastic tubes. For method development the organic extracts were spiked with 10 μL 25 ng/mL calcipotriol used as a control for derivatization efficiency. Samples were evaporated for 2.5 h using a vacuum concentrator (RC10.22) and 100 μL 0.75 mg/mL PTAD in acetonitrile was added to the residue followed by 1 min of vigorous mixing. Samples were left at room temperature for 1 h, stored overnight at +4 °C for complete derivatization, vigorously mixed for 30 s, centrifuged and transferred into 150-μL vial inserts.
HPLC and tandem MS conditions
Separation was performed using an ACQUITY UPLC separation module (Waters). Samples were kept in the autosampler in amber glass vials at +10 °C, and 10 μL samples were injected on the column. The UPLC BEH C18 2.1×100 mm 1.7 μm column (Waters) was kept at +40 °C. Aqueous phase A consisted of 10 % v/v acetonitrile in water containing 0.1% formic acid as a modifier. Organic phase B was 100% methanol. Starting gradient conditions were 60% B at 0.4 mL/min flow rate. The following gradient program was used: 0–1 min 60% B; 7 min 72% B. After separation the column was washed with 100% B for 2 min, and equilibrated with 60% B for 2 min at a 0.4 mL/min flow rate. The Quattro Premier tandem mass spectrometer (Waters) was operated in positive electrospray mode with the capillary voltage set to 3.00 kV. Nitrogen gas flow rates were fixed with a cone gas flow of 25 L/h and a desolvation gas flow of 700 L/h. A source temperature of 125 °C and a desolvation temperature of 350 °C were applied. Argon was used as a collision gas at 2.2×10–3 mbar. Other compound-specific settings are listed in Table 1. To obtain acceptable chromatographic peak statistics (12–20 points per peak), MRM (multiple reaction monitoring) functions were divided into three groups with 0.25 s dwell time for each reaction shown in Table 1 (MRM1: 0–4.75 min, calcipotriol, 24R,25(OH)2D3; MRM2: 4.50–6.50 min, 1α,25(OH)2D3, d6 1α,25(OH)2D3, 1α,25(OH)2D2; MRM3: 6.25–8.00 min, 25(OH)D3, d6 25(OH)D3, 25(OH)D2).
Table 1.
Mass spectrometry conditions
| PTAD derivative | Cone voltage (V) | Precursor ion (m/z) | Collision energy (V) | Product ion (m/z) |
|---|---|---|---|---|
| Calcipotriol | 30 | 570.3 | 20 | 314.0 |
| 24R,25(OH)2D3 | 25 | 574.3 | 20 | 298.0 |
| d6 1 α,25(OH)2D3 | 32 | 580.3 | 18 | 314.0 |
| 1α,25(OH)2D3 | 32 | 574.3 | 18 | 314.0 |
| 1α,25(OH)2D2 | 32 | 586.3 | 18 | 314.0 |
| d6 25(OH)D3 | 20 | 564.3 | 16 | 298.0 |
| 25(OH)D3 | 20 | 558.3 | 16 | 298.0 |
| 25(OH)D2 | 20 | 570.3 | 16 | 298.0 |
| d6 D3 (cholecalciferol) | 35 | 566.3 | 20 | 298.0 |
| D3 (cholecalciferol) | 35 | 560.3 | 20 | 298.0 |
| D2 (ergocalciferol) | 35 | 572.3 | 20 | 298.0 |
Standard addition experiment
For LLE standard addition experiments 400 μL pooled human serum was aliquoted into 2 mL plastic tubes and each group (n=4) was spiked with 10 μL blank, 1.0, 3.0 and 10.0 ng/mL 1α,25(OH)2D3 as well as 125, 250 or 500 ng/mL 25(OH)D3. Samples were extracted and measured independently as described above. For SPE standard addition experiments 500 μL pooled human serum was aliquoted into 2 mL plastic tubes and each group (n=5) was spiked with 10 μL blank, spike 1 (0.75 ng/mL 1α,25(OH)2D3, 10.0 ng/mL 24R,25(OH)2D3, 100 ng/mL 25(OH)D3 and 100 ng/mL 25 (OH)D2), spike 2 (twofold spike 1) and spike 3 (fourfold spike 1). Samples were extracted and measured independently as described above.
Quantification and data analysis
Quantification was performed using a QuantLynx module of MassLynx 4.1 (Waters). Multi-point external calibrations with 1/x weighting were built for all endogenous analytes and one-point calibrations were made for deuterated analogs and calcipotriol. Calibration statistics are shown in Table 2. Calcipotriol was added to ~10% samples as a derivatization quality control. Deuterated 1α,25(OH)2D3 was used as an internal reference for the quantification of 24R,25(OH)2D3, 1α,25(OH)2D2 and 1α,25(OH)2D3, while deuterated 25 (OH)D3 was the internal reference for the quantification of 25(OH)D2 and 25(OH)D3. Prior to integration the chromatograms were smoothed using the Savitzky–Golay algorithm (two iterations using four points). Statistical data analysis and regressions were performed using Microsoft Excel 2003 (Microsoft, Redmond, WA, USA), and SigmaPlot 9.0 (Systat Software, San Jose, CA, USA).
Table 2.
Calibration statistics
| PTAD derivative | tR (min) | Calibration curve equation | R2 | ILD (pg) | LLOQ (pg/mL) |
|---|---|---|---|---|---|
| 24R,25(OH)2D3 | 3.20, 4.40a | y=295.1x + 4.2 | 0.9980 | 0.2 | 25 |
| 1α,25(OH)2D3 | 5.56 | y=300.3x + 3.0 (area) y=3.46x + 121.76 (height)b | 0.9999 (area) 0.9985 (height)b | 0.2 | 25 |
| 1α,25(OH)2D2 | 5.97, 6.13 | y= 195.2x – 24.70 | 0.9964 | 0.3 | 25 |
| 25(OH)D3 | 7.01, 7.47 | y=421.1x + 199.2 | 0.9817 | 0.1 | 25 |
| 25(OH)D2 | 7.39, 7.86 | y=137.1x + 52.4 | 0.9949 | 0.3 | 25 |
ILD, instrumental limit of detection; LLOQ, lower limit of quantification
Retention time of the major isomer peak is given in bold font
Height was used for 1α,25(OH)2D3 calibration and quantification in the samples. Recoveries of internal standards and reproducibility in SPE and LLE extraction methods
25(OH)D RIA
25(OH)D in serum was measured in the Bioanalytical Support Laboratory of the Western Human Nutrition Research Center (WHNRC) using a standard RIA protocol (DiaSorin, Stillwater, MN, USA) according to the manufacturer's instructions with the following modification: the centrifugation following the precipitation step was performed at 3000×g for 60 min at +10 °C instead of the recommended 1800×g for 20 min at +20–25 °C. This modified procedure facilitated the aspiration of the supernatant from above the pellet containing the labeled 25(OH)D. The WHNRC participates in the DEQAS Vitamin D External Quality Assessment Scheme (http://www.deqas.org/) [26] and calibration standards from DEQAS analyzed during this period were all within acceptable limits.
Results and discussion
Derivatization reaction and product stability
TADs are among the most reactive dienophiles known. However, they are unstable in protic solvents. To determine derivatization rates, 1α,25(OH)2D3 and 25(OH)D3 at concentrations of 10 ng/mL (26 nM) and 10 μg/mL (25 μM), respectively, were allowed to react with 0.75 mg/mL (4.3 mM) PTAD at room temperature. Aliquots of the reaction mixtures were taken at fixed time intervals and quenched with equal volumes of methanol. The regression analysis according to a pseudo-first-order kinetics model resulted in t1/2 < 1 min for 25(OH)D3 and t1/2 ~8 min for 1α,25(OH)2D3. Because the deuterium label is distant from the Diels–Alder reaction site, no isotope effects on the reaction kinetics are expected for isotopically labeled 1α,25(OH)2D3 and 25(OH)D3. Thus, >99% yield of derivatization products is achieved after one hour at room temperature, as predicted by the kinetic study. An increase in the PTAD concentration to over 2 mg/mL leads to a significant decrease in yield.
Derivatization of vitamin D metabolites results in an approximately 100-fold increase in the analytical signal in MRM mode, as shown for 1α,25(OH)2D3 in Fig. 2. Moreover, while CID produces multiple fragments of native vitamin D metabolites, it produces a single major fragment for the derivatized products. The fragmentation patterns of native and derivatized 1α,25(OH)2D3 are shown in Fig. 3. This could also potentially be used to screen for additional vitamin D metabolites and other conjugated dienes using the precursor ion scan function. We also examined the stability of the derivatization reaction products. A test solution of derivatized analytes was prepared and stored for one week at –80 °C, –20 °C, +4 °C and room temperature. No significant loss of the analytes was detected in samples stored at room temperature for one week compared to the samples stored at –80 °C.
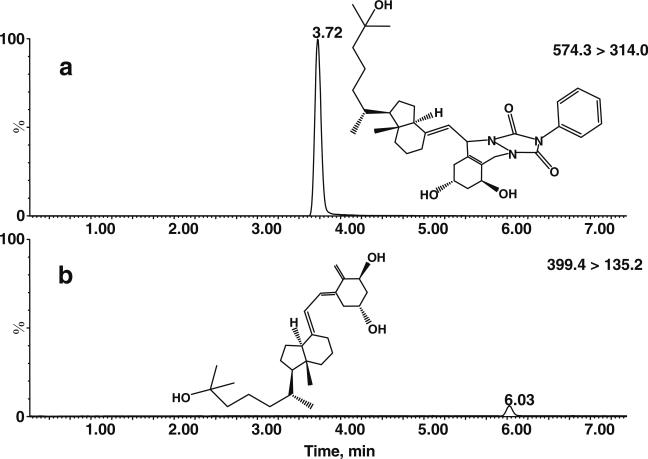
Fig. 2a–b.
Improvement in the sensitivity of MS/MS analysis of 1α,25(OH)2D3 by derivatization with PTAD. Analysis of 1α,25(OH)2D3-PTAD, 1 ng injected on column (574.3>314.0 reaction trace), is shown in panel a, and that of native 1α,25(OH)2D3, 10 ng injected on column (399.4>135.2 reaction trace), is shown in panel b. Both chromatograms were scaled the same way, indicating a 100-fold increase in signal intensity for the derivatized 1α,25(OH)2D3. Standards were injected in 10 μL acetonitrile and separated using gradient elution on a 5-cm UPLC BEH C18 column
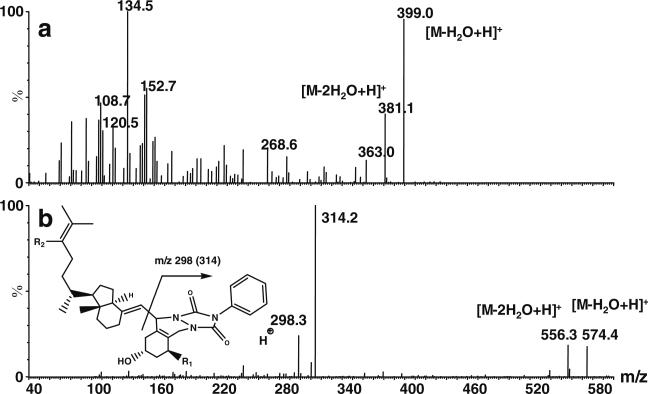
Fig. 3a–b.
CID spectra of 1α,25(OH)2D3. Native 1α,25(OH)2D3 a; 1α,25(OH)2D3-PTAD b. Both product ion spectra were acquired for the dominant [M–18+H]+ ion. The general fragmentation reaction for derivatized vitamin D metabolites is shown. We observed a dominant fragment for PTAD derivatives at m/z 298 (m/z 314 for metabolites hydroxylated at position 1)
LC and MS optimization
Cone voltage and collision energies were optimized using injections of diluted standards. No sharp maxima were found and optimized parameters did not differ significantly in the range of ±5 V and ±3 V for cone voltage and collision energy, respectively (Table 1). Both derivatized and nonderivatized vitamin D metabolites containing a hydroxyl group at position 25 easily lose water in the Z-spray source probably due to the formation of a stable tertiary carbocation. We observed similar dominant water loss in the QTRAP4000 Turbo V IonSpray (Applied Biosystems; Foster City, CA, USA) ion source, and presumably this loss is not source-specific. Thus, [M–18+H]+ was used as a precursor ion for all vitamin D metabolites hydroxylated at position 25. Initially, acetonitrile with 0.1% formic acid was used as an organic phase for UPLC separation. However, we found that methanol without modifier gave the best signal intensity, probably due to its higher volatility [27]. Thus, methanol was used as the organic solvent despite a significantly higher backpressure. This demonstrates the additional advantage of using UPLC to improve the sensitivity of LC-MS. The target analytes were separated using gradient elution on a 10-cm BEH C18 UPLC column in 12 min, including column wash and re-equilibration (Fig. 4; Table 2).
Fig. 4.
Chromatographic separation of major vitamin D metabolites using the conditions described in the “Experimental” section
Derivatization of vitamin D metabolites with PTAD produces two epimers, 6S and 6R, corresponding to the position of the dienophile relative to the plane of the A ring. The major isomer peak was used for integration and quantification. We compared results for 25(OH)D3 and 24R,25(OH)2D3 quantification in 50 individual serum samples using integration of either the major or minor peak and found a good correlation between the values (R2=0.9669 and R2=0.8838, respectively). The lower correlation for 24R,25(OH)2D3 can be explained by the low intensity of the minor isomer peak and the lack of a corresponding isotopically labeled standard to correct the measurements. Interestingly, the C18 BEH UPLC stationary phase does not separate isomers of derivatized 1α,25(OH)2D3, probably due to the anti position of hydroxyl groups on its A ring, which make this structure more symmetric. Separation of derivatized 1α,25(OH)2D3 can be achieved using phenyl BEH column chemistry, but this separation is not advantageous for quantitative purposes. While the C18 BEH phase does not separate isomers of 1α,25(OH)2D3-PTAD, it does separate the isomers of 1α,25(OH)2D2-PTAD (see the “Electronic supplementary material”).
In addition to cycloaddition to the locked C-10-19 : C-5-6 cisoid diene, the Diels–Alder reaction can theoretically occur at the C-5-6 : C-7-8 diene if the C-6–C-7 bond rotates into a cisoid conformation. However, this reaction would be unfavorable because of the activation barrier to uncoupling the conjugated triene system and the steric hindrance to forming a planar diene. PTAD is not only a potent dienophile but also a mild oxidizing reagent. Therefore, other possible by-products of derivatization can be form because of the oxidation of secondary alcohols of vitamin D metabolites into corresponding ketones. We surveyed mass chromatograms of derivatized standards and did not find an abundant signal (>1% peak height of derivatized standard) that would correspond to keto- ([M–2+H]+) and diketo- ([M–4+H]+) by-products.
Selectivity of the method was determined by surveying MRM chromatograms of the analytes extracted from human serum extracts. No significant interfering peaks were found for any of the analytes except 1α,25(OH)D3 (Fig. 5). The interference was present in both LLE and SPE human serum extracts. The interfering ion could not be suppressed with increasing quadrupole resolution because of concomitant 1α,25(OH)2D3-PTAD signal loss. The interfering ion is a product of serum matrix derivatization, because it was not found in nonderivatized serum matrix. It was not possible to use a different transition for the detection of 1α,25(OH)2D3-PTAD, because the derivative only produces one dominant fragment ion, as shown above. The low signal intensity of the interfering peak did not allow the product and precursor MS/MS scan experiment to be performed. However, because the interfering compound coelutes with vitamin D metabolites under selective SPE conditions and undergoes Diels–Alder derivatization, we hypothesize that it is an unknown dihydroxyvitamin D3 isomer with two hydroxyl groups on the A ring because of the characteristic m/z 314 fragment. A possible candidate metabolite is 1α,25-dihydroxy-3-epi-vitamin D3, a biologically active product of the catabolic epimerization of 1α,25(OH)2D3 [28]. However, the lack of a commercially available standard does not allow this finding to be confirmed. Interestingly, 25-hydroxy-3-epi-vitamin D3 was found as an interference in another LC-MS assay [29]. Thus, we used a 10-cm column to separate 1α,25(OH)2D3-PTAD from the interfering peak. It is crucial to use isotopically labeled 1α,25(OH)2D3 to assign the correct retention time to this analyte. To increase the precision of quantification, we measured the height of the 1α,25(OH)2D3-PTAD peak while the area was measured for the quantification of other analytes (see “Electronic supplementary material”).
Fig. 5.
Separation of 1α,25(OH)2D3 from coeluting interferences using a 10-cm UPLC BEH C18 column. The identity of the peak was supported by a standard addition experiment (see Table 5) and the use of the deuterated internal standard d6 1α,25(OH)2D3. Hexadeuterated surrogates of vitamin D metabolites were found to elute ~0.03 min earlier than their native analogs (see Fig. 4). Arrows indicate 1α,25(OH)2D3-PTAD. Detection was performed in MRM mode
Sample preparation
Initially, we used liquid–liquid extraction of vitamin D metabolites to demonstrate the feasibility of the method. Initial sample preparation steps such as protein precipitation with acetonitrile and subsequent dilution with 0.4 M K2HPO4 were adopted from known methods of vitamin D extraction to fit the format of 2-mL polypropylene plastic tubes [25]. No losses of 1α,25(OH)2D3 were detected with plastic tubes compared to borosilicate glass tubes (see “Electronic supplementary material”). We tested different modes of serum protein precipitation using acetonitrile, including fast and slow mixing and addition of the serum drop-wise to acetonitrile, and found no significant difference between them (see “Electronic supplementary material”). We also tested three different extraction solvents, MTBE, dichloromethane and ethyl acetate, and found very similar extraction recoveries with the exception of dichloromethane, which produced lower recoveries for 25(OH)D3 (Fig. 6). Thus, we selected MTBE due to its low absorption of water, which deactivates PTAD, and its high volatility. To simplify sample preparation, one extraction step was performed subsequently.
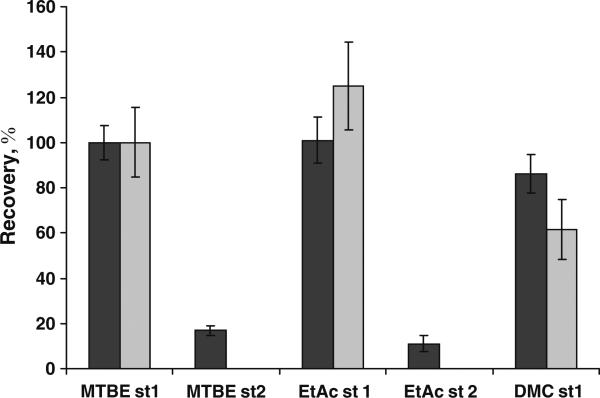
Fig. 6.
Selection of solvents for liquid–liquid extraction. Human serum was spiked with deuterated 1α,25(OH)2D3 and prepared as described in the “Experimental” section. Extraction was performed in two steps (st) with methyl tert-butyl ether (MTBE), dichloromethane (DCM) and ethyl acetate (EtAc). No second extraction was performed for DCM, which formed an emulsion that was difficult to separate. Data are normalized to MTBE recoveries in the first step. No d6 25(OH)D3 was available at the time of the experiment, and so native 25(OH)D3 was used to measure the relative recovery. Four samples were analyzed independently in each sample group (black bars, d6 1α,25(OH)2D3; gray bars, d6 25(OH)D3). Error bars represent standard deviations
The SPE method was adopted from the literature [22, 25]. The effects of SPE sample dilution, cartridge washing and elution solvents are shown in Fig. 7. The resulting data correspond to standard guidance for vitamin D sample preparation, including sample dilution with 1 v. 0.4 M K2HPO4 at pH 10.4 and cartridge washing with 70% methanol. Although a 30% methanol wash resulted in the highest recoveries of d6 1α,25(OH)2D3, we selected 70% methanol wash as a standard procedure because samples obtained with <50% methanol wash contained a substantial amount of residual moisture that slows down sample concentration and may interfere with the yield of the derivatization reaction (Fig. 7b). We found that an additional wash with hexane was not critical to sample preparation and thus omitted it to avoid forming a biphasic solution in SPE wastes. We selected acetonitrile as elution solvent because ethyl acetate and especially methanol negatively affected the yield of the derivatization reaction, as monitored with the derivatization control compound calcipotriol in the extraction matrix (Fig. 7c). To elute residual analytes remaining on the cartridges after the first 1 mL of eluent, we increased the final elution volume to 1.5 mL.
Fig. 7a–c.
Selection of solvents for sample loading, SPE wash, and elution. Supernatant from the protein precipitation was transferred into SPE cartridges (Oasis HLB) and diluted with given volume of water (W), 0.4 M K2HPO4 (K), or 0.4 M Na2HPO4 (Na). a SPE cartridges were washed with 50 % methanol and eluted with 1.5 mL ethyl acetate. b SPE cartridges loaded with diluted supernatant were washed with 2 mL 70% methanol, 2 mL 50% methanol, 2 mL 50% methanol and 2 mL hexane, 2 mL 30% methanol, and water and eluted with 1.5 mL ethyl acetate. c SPE cartridges were loaded with diluted supernatant, washed with 70% methanol and eluted from Oasis HLB cartridges with 3×1 mL of acetonitrile (MeCN), ethyl acetate (EtAc) or methanol (MeOH). Each 1 mL sample was spiked with calcipotriol to study the effect of matrix on derivatization efficiency. Four samples were analyzed independently in each sample group (black bars, d6 1α,25(OH)2D3; gray bars, d6 25(OH)D3; white bars, calcipotriol). All serum samples were spiked with deuterated surrogates prior to sample preparation. Error bars represent standard deviations
The recoveries of the deuterated surrogates d6 1α,25(OH)2D3 and d6 25(OH)D3 were determined in 25 individual human plasma samples extracted by optimized liquid–liquid extraction and 50 individual human serum samples extracted by optimized SPE (Table 3). Because these recoveries also include the yield of the derivatization reaction, we spiked the organic extracts with the 1α,25(OH)2D3 analog calcipotriol (calcipotriene) to determine the effect of the sample matrix on the derivatization yield. Compared with liquid–liquid extraction, SPE results in a lower derivatization yield but comparable extraction recoveries. The lower derivatization yield in SPE is probably due to traces of protic solvents (water and methanol) that deactivate PTAD while the low solubility of water in MTBE makes the LLE extracts the preferred matrix for the derivatization reagent. LLE and SPE give statistically the same results for vitamin D metabolites except for 24R,25(OH)2D3. These data were generated by extracting six aliquots of the same human plasma sample by SPE (n=3) and LLE (n=3) methods (Table 3). The lower values obtained for 24R,25(OH)2D3 in the SPE procedure compared to LLE can be explained by the relatively high polarity of 24R,25(OH)2D3, which could result in losses during the SPE wash, and the lack of a deuterated internal standard (d6 1α,25(OH)2D3 was used as a reference for 24R,25(OH)2D3 quantification).
Table 3.
Recoveries of internal standards and reproducibilities for SPE and LLE extractionmethods
| Recoveries | Solid-phase extraction | Liquid–liquid extraction |
|---|---|---|
| Calcipotriol derivatization yield (%) | 83.3±9.6 (n=50) | 105±11 (n=25) |
| d6 1α,25(OH)2D3recovery (%) | 70.5±6.7 (n=50) | 85.8±8.8 (n=25) |
| d6 25(OH)D3recovery (%) | 78.1±4.7 (n= 50) | 81.0±8.0 (n=25) |
| Inter-sample reproducibility | ||
| 25(OH)D2 (ng/mL) | 0.5±0.1 (n=3) | 0.6±0.1 (n=3) |
| 25(OH)D3 (ng/mL) | 26.8±0.9 (n=3) | 29.9±2.5 (n=3) |
| 1α,25(OH)2D3 (pg/mL) | 36±3 (n=3) | 41±9 (n=3) |
| 24R,25(OH)2D3 (ng/mL) | 1.2±0.2 (n=3) | 1.8±0.1 (n=3) |
In addition, SPE results in more precise measurements than LLE. Also, the throughput of SPE sample preparation is practically twice as fast as LLE. Thus, we suggest that SPE is the preferred technique for samples in clinical settings where precise measurements of 1α,25(OH)2D2, 1α,25(OH)2D3, 25(OH)D2 and 25(OH)D3 are critical. However, the LLE procedure is suitable for further explorations in vitamin D metabolic profiling, such as the addition of 1α,24R,25(OH)3D3 to the method, as well as the analysis of biohazardous human samples (e.g., HIV or hepatitis positive) that would otherwise require a special SPE extraction manifold.
Reverse-phase SPE is known for its very high retention of vitamin D2 and D3, which makes its application problematic. Only ~30% of vitamin D2 and D3 are eluted from SPE cartridges under the conditions selected for our method (1.5 mL acetonitrile), as monitored by UPLC-UV. Using a stronger solvent such as ethyl acetate would decrease the purity of the sample and the yield of the derivatization reaction. Surprisingly, we found that LLE also results in poor recoveries of vitamin D2 and D3. The addition of vitamin D2 and D3 standards to extracted serum matrix prior to derivatization revealed that the reason for the poor recoveries is a low derivatization reaction yield. However, poor recoveries were not obtained for other more polar forms of vitamin D. Taking into consideration the severe matrix effect present during the derivatization of vitamin D2 and D3 and the need for longer chromatographic runs, we omitted these analytes from the method.
Method validation
Calibration curves were linear over the entire range of selected concentrations (Table 2). Because all analytes in the vitamin D pathways have similar structures and produce similar fragments in CID, we obtained relatively similar instrumental limits of detection (ILD) for all analytes. Instrumental limit of detection was determined as the lowest concentration in a series of dilutions that produces a chromatographic peak with a root mean square (RMS) signal-to-noise ≥ 3 under the standard chromatographic conditions of the method. Lower limits of quantification in plasma and serum were set to 25 pg/mL, estimated using the lowest calibration point of 100 pg/mL corrected for a sample dilution factor of 5 and 80% recovery (100/0.8/5). From the subsequent standard addition experiments we found that 1α,25(OH)2D3 measurements above the LLOQ have precisions of <20% for both LLE and SPE (Tables 4 and 5).
Table 4.
Standard addition experiment using liquid–liquid extraction
| 25(OH)D3 standard addition | 25(OH)D3 measured (ng/mL) |
|---|---|
| Native serum (n=4) | 22.5 (±0.9%)a |
| Native serum + 1.25 ngb 25(OH)D3 (n=4) | 26.1 (102.±2%) |
| Native serum + 2.5 ng 25(OH)D3 (n=4) | 28.4 (98.9±4.8%) |
| Native serum + 5 ng 25(OH)D3 (n=4) | 34.3 (98.0±3.3%) |
| 1α,25(OH)2D3 standard addition | 1α,25(OH)2D3 measured (pg/mL) |
| Native serum (n=3) | 60 (±9%) |
| Native serum + 10 pgb 1α,25(OH)2D3 (n=4) | 86 (103±16%) |
| Native serum + 30 pg 1α,25(OH)2D3 (n=4) | 160 (120±20%) |
| Native serum + 100 pg 1α,25(OH)2D3 (n=3) | 343 (111±5%) |
Concentration (accuracy (analyte recovery corrected to recovery of corresponding deuterated internal standard) ± precision (RSD))
Quantity added to 0.4 mL serum prior to protein precipitation and extraction
Table 5.
Standard addition experiment using SPE
| Native serum | Spike 1a | Spike 2 | Spike 3 | |
|---|---|---|---|---|
| 24R,25(OH)2D3 (ng/mL) | 2.83 (±7.1%)b | 3.03 (99.9±11.1%) | 3.08 (95.2±6.5%) | 3.19 (88.0±5.4%) |
| 1α,25(OH)2D3 (pg/mL) | 18 (±23%)c | 31 (94±16%) | 52 (108±16%) | 90 (116±7%) |
| 25(OH)D3 (ng/mL) | 16.2 (±2.3%) | 17.4 (95.6±1.9%) | 18.7 (92.4±2.2%) | 21.5 (88.8±4.0%) |
| 25(OH)D2 (ng/mL) | 0.3 (±7.7%) | 2.3 (101.4±6.1%) | 4.3 (100.6±3.9%) | 8.3 (99.8±8.6%) |
Spike 1: 0.75 ng/mL 1α,25(OH)2D3, 10 ng/mL 24R,25(OH)2D3, 100 ng/mL 25(OH)D3and 25(OH)D2
Spike 2: 1.50 ng/mL 1α,25(OH)2D3, 20 ng/mL 24R,25(OH)2D3, 200 ng/mL 25(OH)D3and 25(OH)D2
Spike 3: 3.00 ng/mL 1α,25(oh)2D3, 40 ng/mL 24R,25(oh)2D3, 400 ng/mL 25(OH)D3and 25(OH)D2
10 μL spike was added to 0.5 mL serum resulting in ~50-fold spike dilution
Concentration (accuracy (analyte recovery corrected to recovery of corresponding deuterated internal standard) ± precision (RSD))
Below LOQ and above 20% precision criterion
The lowest calibration point (100 pg/mL) for all analytes produced chromatographic peaks with RMS signal to noise > 10. Because vitamin D metabolites are endogenous compounds, we studied method accuracy and precision using the addition of known quantities of vitamin D metabolites to human serum and extracted it by LLE and SPE. Separate standard addition experiments were performed for samples extracted by LLE (Table 4) and SPE (Table 5). For LLE, separate experiments for 1α,25(OH)2D3 and 25(OH)D3 standard spikes were performed, while for SPE, serum was spiked with a mixture of 24R,25(OH)2D3, 1α,25(OH)2D3, 25(OH)D2 and 25(OH)D3. The differences in metabolite concentrations in native serum between LLE and SPE experiments are due to the different batches of pooled human serum used in the experiments. We set quality control criteria for all extractions as precision (RSD) <20% and accuracy (analyte recovery corrected to the recovery of a corresponding deuterated internal standard) in the range of 75–125%. All samples passed these criteria except 1α,25(OH)2D3 measurement in native serum after SPE, which was below the LLOQ and above the 20% precision criterion. Average accuracies in the spiked samples were 99.6% for 25(OH)D3 and 111% for 1α,25(OH)2D3 in the LLE experiments and 92.3% for 25(OH)D3, 100.6% for 25(OH)D2, 106% for 1α,25(OH)2D3 and 94.4% for 24R,25(OH)2D3 in the SPE experiments. SPE results in more precise measurements of 25(OH)D3, while the accuracy of LLE for 25(OH)D3 is higher. Both extraction methods tend to overestimate 1α,25(OH)2D3 levels while precision is slightly better with SPE, which corresponds to the data obtained by direct comparison of both techniques (Table 3). Linear regression curves built for standard addition of 1α,25(OH)2D3 are more linear if the height of the peak (R2=0.9996) is used instead of peak area (R2=0.9850). For comparison, the R2 values of the linear regression curves for 24R,25(OH)2D3, 25(OH)D2 and 25(OH)D3 were 0.9985, 1.000, and 0.9993, respectively (see the “Electronic supplementary material”). The poor slope («1) obtained in the standard addition experiment for 24R,25(OH)2D3 can be explained with its higher polarity compared to 1α,25(OH)2D3, thus resulting in extraction losses that could not be accurately corrected due to the lack of the corresponding isotopically labeled standard.
In addition, 50 individual human serum samples were analyzed with a 25(OH)D RIA (Diasorin). We found a good correlation between LC-MS and RIA data (Fig. 8). However, the results from the LC-MS method were systematically higher than the RIA 25(OH)D results. Both methods of 25(OH)D analysis were validated by the analysis of DEQAS samples [26] and found to give results within the desirable inter-laboratory mean (±σ). Interestingly, other LC-MS methods of 25(OH)D analysis in DEQAS studies tend to produce systematically higher measurements than RIA methods (data not shown). This LC-MS bias might be explained by the more accurate estimation of 25(OH)D2 and 25(OH)D3 recoveries using isotopically labeled analogs. The development of internal standards for calibrating immunoassays remains problematic.
Fig. 8.
Comparison of RIA (DiaSorin) and LC-MS data for 50 individual measurements of 25(OH)D in serum samples collected in the fall study. LC-MS data are plotted as the sum of the 25(OH)D2 and 25(OH)D3 concentrations
Biological applications
Photosynthesis of vitamin D is a route parallel to dietary supplementation in humans (Fig. 1). UV light (290–315 nm) induces cleavage of the C-9–C-10 bond of 7-dehydrocholesterol with subsequent proton migration and conformational changes producing vitamin D3. For example, one minimal erythemal dose of UV exposure from sunlight resulted in the production of 10,000–20,000 IU (0.25–0.5 mg) of vitamin D3 [30]. Typically, vitamin D photosynthesis in skin is thought to satisfy most human dietary requirements. However, the photosynthesis of vitamin D3 in skin becomes less efficient in people with darker skin living in higher latitudes, where sun exposure is insufficient to generate enough vitamin D3, or alternatively in people with mostly indoor lifestyles and those who habitually use sunscreens. An LC-MS method specific for D3 forms of vitamin D is particularly useful for studies of the photosynthesis of vitamin D3 in skin because it avoids interference with D2 metabolites from the diet. Because the major aim of our method development was to study the effect of skin pigmentation on vitamin D, we used a LLE version of the method to study the vitamin D deficiency in an HIV-positive urban Afro-American population previously reported to be 25(OH)D-deficient. The SPE version of the method was used for high-throughput phenotyping of vitamin D metabolism in subjects with different levels of sun exposure and different levels of skin pigmentation. The occurrence of D2 group metabolites was sporadic in studied groups and was therefore omitted from the discussion.
Effect of HIV infection on vitamin D profile
As a pilot study we analyzed eight plasma samples from HIV-negative females and compared their vitamin D profile with the vitamin D profile in seven plasma samples of HIV-positive females. The samples were acquired from the REACH depository [23, 24]. We found good agreement with previously acquired immunoassay data on their 25(OH)D status, showing that both groups are vitamin D-deficient (25(OH)D < 32 ng/mL), with no statistical difference between the groups (Fig. 9) [31]. No difference in the 24R,25(OH)2D3 level was found either, which corresponds to low 25(OH)D3 status. However, 1α,25(OH)2D3 tends to be higher in the HIV-positive group (p<0.08; t-test). Therefore, the study of a larger population is required to increase the power of the statistical analysis and test this initial observation. The literature data on 1α,25(OH)2D3 status in patients with HIV infections are controversial. While some studies found suppressed levels of 1α,25(OH)2D3, presumably caused by antiretroviral treatment [32, 33], there are many cases of reported increases in 1α,25(OH)2D3 [34–37]. The increase in 1α,25(OH)2D3 in HIV-positive subjects can be caused by extrarenal production of 1α,25(OH)2D3 in macrophages activated by chronic inflammation or in tumors in the final stages of the disease. This case shows the importance of not simply measuring 25(OH)D to assess vitamin D nutritional status but to access the whole cascade by also measuring its biologically active 1α,25(OH)2D3 form. In individuals with depressed 25(OH)D level but elevated 1α,25(OH)2D3 production, dietary supplementation with vitamin D or sun exposure may have a deleterious effect because of possible 1α,25(OH)2D3 deregulated extra-renal production and the resulting toxicity, as observed for example in some lymphomas [38], sarcoidosis [39] and tuberculosis [40].
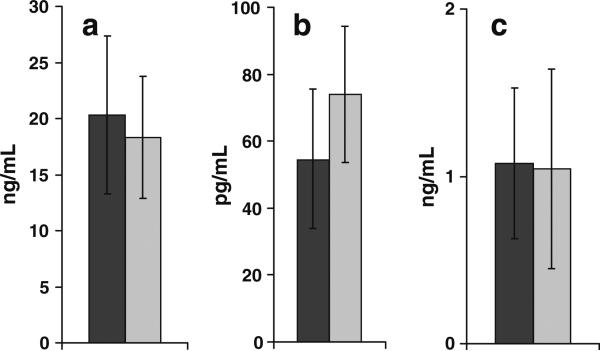
Fig. 9a–c.
Metabolic profile of (a) 25(OH)D3, (b) 1α,25(OH)2D3 and (c) 24R,25(OH) 2D3 in HIV-positive (black bars; n=7) and -negative (gray bars; n=8) female subjects. Error bars represent standard deviations
Seasonal variations in vitamin D metabolites
We analyzed 102 individual serum samples acquired from 34 subjects with different levels of sun exposure and different levels of skin pigmentation. The preliminary data showed that the majority of the studied population had a very low level of 25(OH)D2 (< 3.0 ng/mL), which corresponds to the fact that the diet is supplemented predominantly with the D3 form. However, in three of the 34 subjects the level of 25(OH)D2 was high (16.6, 9.2, and 6.3 ng/mL measured as the average of three time points). Those three subjects reported taking multivitamin supplements or drinking soy milk supplemented with vitamin D2. The ability to separate 25(OH)D2 and 25(OH)D3 forms of vitamin D is a specific feature of our method compared to typically used immunoassays. There is evidence that 25(OH)D2 does not have the same properties as 25(OH)D3 [9, 10]. In addition, in contrast to vitamin D3, which can be produced by photosynthesis in vivo, vitamin D2 is adsorbed solely from the diet (Fig. 1). Thus, separate measurement of the D2 and D3 forms of vitamin D is especially valuable for studying the photosynthesis of this vitamin.
As expected from the seasonal variation in sun exposure, there was a significant difference in 25(OH)D3 levels between the fall and winter study groups (Fig. 10). Interestingly, while the level of 24R,25(OH)2D3 correlates well with the level of 25(OH)D3 in two groups of 51 (17 subjects × 3 time points) measurements (R2=0.8065 in fall and R2=0.7452 in winter), the level of 1α,25(OH)2D3 correlates with the level of 25(OH)D3 poorly (R2=0.4633 in fall and R2=0.1823 in winter). The difference in the correlations of the two major products of 25(OH)D3 oxidation with their precursor can be rationalized by much stricter biological control of 25-hydroxyvitamin D 1α-hydroxylase compared to 25-hydroxyvitamin D 24-hydroxylase (Fig. 1). However, if 25(OH)D3 status is sufficient (>32 ng/mL), as in the fall study group, the production of 1α,25(OH)2D3 becomes less strictly controlled. Thus, in the case of elevated 25-hydroxyvitamin D 1α-hydroxylase activity that is associated with some disease states, excess 25(OH)D3 may lead to 1α,25(OH)2D3-associated toxicity.
Fig. 10a–c.
Seasonal variations in (a) 25(OH)D3 [15.5 to 67.8 ng/mL; 8.0 to 50.7 ng/mL], (b) 1α,25(OH)2D3 [25 to 128 pg/mL; 25 to 108 pg/mL], and (c) 24R,25(OH) 2D3 [0.9 to 9.6 ng/mL; 0.3 to 4.3 ng/mL] in two different groups of healthy subjects studied in fall (black bars; n=17) and winter (gray bars; n=17). Values in the brackets shows the ranges of concentrations in fall and winter, respectively. Error bars represent standard deviations
While there are arguments that measuring 24R,25(OH)2D3 status has little biological value, it has been shown that this metabolite is active in bone tissue [14, 15]. Also, the level of 24R,25(OH)2D3 can be a measure of 1α,25(OH)2D3 clearance because 1α,25(OH)2D3 is also oxidized by 25-hydroxyvitamin D 24-hydroxylase, forming the inactive 1α,24R,25(OH)3D3. In addition, measurement of 24R,25(OH)2D3 can be a quality control for 1α,25(OH)2D3 analysis. It is well known that 25-hydroxyvitamin D 24-hydroxylase expression is positively regulated by 1α,25(OH)2D3 via a VDRE in its promoter [41]. Thus, an elevated 1α,25(OH)2D3 level (>50–60 pg/mL) corresponds to high production of 24R,25(OH)2D3 if 25(OH)D3 status is sufficient.
Conclusions
Metabolic profiling is a promising tool for assessing entire metabolic pathways and studying their regulation. While in the field of vitamin D analysis different forms of protein binding assays remain the dominant procedures, they do not have the flexibility to measure multiple analytes in the same run or to separate the D2 and D3 forms of vitamin D. However, measurements of both 1α,25(OH)2D3 and 25(OH)D3 have become an important diagnostic factor for the assessment of dysregulated 1α,25(OH)2D3 extrarenal production in cancerous and inflammatory states. Thus, a method to measure 1α,25(OH)2D3 and 25(OH)D3 simultaneously could become a valuable tool in clinical practice. While this method represents a considerable improvement on the assessment of 25(OH)D3 by LC-MS, the very low circulating levels of 1α,25(OH)2D3, its thermal instability and its low polarity impede the direct measurement of this biologically important hormone with LC-MS or GC-MS. However, the sensitivity of 1α,25(OH)2D3 detection can be significantly improved using Diels–Alder derivatization with PTAD. Thus, the application of Diels–Alder derivatization allows the entire vitamin D cascade to be surveyed in a single LC-MS run, including routine direct measurements of 1α,25(OH)2D3, which has not been achieved before using LC-MS or GC-MS methods. In addition, the improved sensitivity of 25(OH)D detection allows the development of a method for the assessment of dietary 25(OH)D in very small plasma or serum samples (<50 μL). While current immunoassay methods for 1α,25(OH)2D3 quantification are superior in terms of their limits of detection, the reported LC-MS method can be used to detect excessive 1α,25(OH)2D2 and 1α,25(OH)2D3 production associated with some cancerous states and inflammation. It can also simultaneously assess the vitamin D dietary status via measurements of both 25(OH)D2 and 25(OH)D3 levels and estimate the rate of 1α,25(OH)2D3 clearance by 25-hydroxyvitamin D 24-hydroxylase via measurements of the 24R,25(OH)2D3 level. The method may be improved with the development of novel derivatization reagents with stronger ionic properties for more efficient electrospray ionization. By further expanding the list of analytes covered by this metabolic profiling method, we may gain unexpected insights into the biology of the vitamin D family of molecules.
Supplementary Material
Acknowledgement
We thank John Newman who provided the UPLC for the initial method development experiments. We thank Theresa Pedersen and Katrin Georgi for discussion of the extraction procedure, Mike Eskander for help with preparation of standards and MS optimization, Alina Wettstein for help with preparation of REACH samples and Leslie Woodhouse and Manuel Tengonciang for 25(OH)D RIA analysis. P.A.A. was supported by NIEHS Advanced Training in Environmental Toxicology Grant T32 ES007059. L.M.H. was supported by NIH Grant P60 MD00222-01. C.B.S. was supported by USDA-ARS Project 5306-51530-006-00D. K.D. was supported in part by BayGene. This research was supported in part by California Dairy Research Foundation Grant 07 HAB-01-NH, Bristol-Meyers/Squibb Freedom to Discover Award, NIEHS Grant R37 ES02710, NIEHS Superfund Basic Research Program P42 ES004699, NIEHS Center grant P30 ES05707, and NIEHS Center for Children's Environmental Health & Disease Prevention Grant P01 ES11269.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-008-2095-8) contains supplementary material, which is available to authorized users.
Contributor Information
Pavel A. Aronov, Department of Entomology, University of California, Davis, CA 95616, USA
Laura M. Hall, Department of Nutrition and USDA Western Human Nutrition Research Center, University of California, Davis, CA 95616, USA
Katja Dettmer, Institute of Functional Genomics, University of Regensburg, Regensburg, Germany.
Charles B. Stephensen, Department of Nutrition and USDA Western Human Nutrition Research Center, University of California, Davis, CA 95616, USA
Bruce D. Hammock, Department of Entomology and U.C. Davis Cancer Research Center, University of California, Davis, CA 95616, USA bdhammock@ucdavis.edu
References
- 1.Fiehn O. Plant Mol Biol. 2002;48(1–2):155–171. [PubMed] [Google Scholar]
- 2.Dettmer K, Aronov PA, Hammock BD. Mass Spectrom Rev. 2007;26(1):51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerwekh JE. Ann Clin Biochem. 2004;41(4):272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 4.Maunsell Z, Wright DJ, Rainbow SJ. Clin Chem. 2005;51(9):1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 5.Vogeser M, Kyriatsoulis A, Huber E, Kobold U. Clin Chem. 2004;50(8):1415–1417. doi: 10.1373/clinchem.2004.031831. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T, Awada D, Shimada K. Biol Pharm Bull. 2001;24(7):738–743. doi: 10.1248/bpb.24.738. [DOI] [PubMed] [Google Scholar]
- 7.Bouillon R, Okamura WH, Norman AW. Endocr Rev. 1995;16(2):200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 8.Prosser DE, Jones G. Trends Biochem Sci. 2004;29(12):664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Armas LA, Hollis BW, Heaney RP. J Clin Endocrinol Metab. 2004;89(11):5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 10.Houghton LA, Vieth R. Am J Clin Nutr. 2006;84(4):694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 11.Avioli LV, Lee SW, McDonald JE, Lund J, DeLuca HF. J Clin Invest. 1967;46(6):983–992. doi: 10.1172/JCI105605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman AW. Endocrinol. 2006;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 13.Boyan BD, Sylvia VL, Dean DD, Del Toro F, Schwartz Z. Crit Rev Oral Biol Med. 2002;13(2):143–154. doi: 10.1177/154411130201300205. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz Z, Ehland H, Sylvia VL, Larsson D, Hardin RR, Bingham V, Lopez D, Dean DD, Boyan BD. Endocrinol. 2002;143(7):2775–2786. doi: 10.1210/endo.143.7.8889. [DOI] [PubMed] [Google Scholar]
- 15.Boyan BD, Sylvia VL, Dean DD, Schwartz Z. Steroids. 2001;66(3–5):363–374. doi: 10.1016/s0039-128x(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 16.Kissmeyer AM, Sonne K. J Chromatogr A. 2001;935(1–2):93–103. doi: 10.1016/s0021-9673(01)00985-2. [DOI] [PubMed] [Google Scholar]
- 17.Higashi T, Awada D, Shimada K. Biomed Chromatogr. 2001;15(2):133–140. doi: 10.1002/bmc.43. [DOI] [PubMed] [Google Scholar]
- 18.Higashi T, Yamauchi A, Shimada K. Anal Sci. 2003;19(6):941–943. doi: 10.2116/analsci.19.941. [DOI] [PubMed] [Google Scholar]
- 19.Higashi T, Homma S, Iwata H, Shimada K. J Pharm Biomed Anal. 2002;29(5):947–955. doi: 10.1016/s0731-7085(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SR, Tulchinsky ML, Wu Y. Bioorg Med Chem Lett. 1993;3(9):1805–1808. [Google Scholar]
- 21.Aronov PA, Dettmer K, Christiansen JA, Cornel AJ, Hammock BD. J Agric Food Chem. 2005;53(9):3306–3312. doi: 10.1021/jf0485842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murao N, Ishigai M, Sekiguchi N, Takahashi T, Aso Y. Anal Biochem. 2005;346(1):158–166. doi: 10.1016/j.ab.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Anon J Adolesc Health. 2001;29(3, Suppl 1):5–6. [Google Scholar]
- 24.Rogers AS, Futterman DK, Moscicki AB, Wilson CM, Ellenberg J, Vermund SH. J Adolesc Health. 1998;22(4):300–311. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW. Detection of vitamin D and its major metabolites. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Elsevier; Amsterdam: 2005. [Google Scholar]
- 26.Carter GD, Carter CR, Gunter E, Jones J, Jones G, Makin HL, Sufi S. J Steroid Biochem Mol Biol. 2004;89–90(1–5):467–471. doi: 10.1016/j.jsbmb.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Giorgianni F, Cappiello A, Beranova-Giorgianni S, Palma P, Trufelli H, Desiderio DM. Anal Chem. 2004;76(23):7028–7038. doi: 10.1021/ac0493368. [DOI] [PubMed] [Google Scholar]
- 28.Astecker N, Reddy GS, Herzig G, Vorisek G, Schuster I. Mol Cell Endocrinol. 2000;170(1–2):91–101. doi: 10.1016/s0303-7207(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 29.Singh RJ, Taylor RL, Reddy GS, Grebe SK. J Clin Endocrinol Metab. 2006;91(8):3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 31.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Am J Clin Nutr. 2006;83(5):1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 32.Madeddu G, Spanu A, Solinas P, Calia GM, Lovigu C, Chessa F, Mannazzu M, Falchi A, Mura MS, Madeddu G. Q J Nucl Med Mol Imaging. 2004;48(1):39–48. [PubMed] [Google Scholar]
- 33.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. Aids. 2003;17(4):513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 34.Delahunt JW, Romeril KE. J Acquir Immune Defic Syndr. 1994;7(8):871–872. [PubMed] [Google Scholar]
- 35.Ahmed B, Jaspan JB. Am J Med Sci. 1993;306(5):313–316. doi: 10.1097/00000441-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Adams JS, Fernandez M, Gacad MA, Gill PS, Endres DB, Rasheed S, Singer FR. Blood. 1989;73(1):235–239. [PubMed] [Google Scholar]
- 37.Aly ES, Baig M, Khanna D, Baumann MA. Int J Clin Pract. 1999;53(3):227–228. [PubMed] [Google Scholar]
- 38.Freeman NJ, Holik D. J Clin Oncol. 2003;21(1):170–172. doi: 10.1200/JCO.2003.21.1.170. [DOI] [PubMed] [Google Scholar]
- 39.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. N Engl J Med. 1981;305(8):440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 40.Barnes PF, Modlin RL, Bikle DD, Adams JS. J Clin Invest. 1989;83(5):1527–1532. doi: 10.1172/JCI114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Endocrinol. 1997;138(6):2233–2240. doi: 10.1210/endo.138.6.5170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.