Abstract
Mechanisms controlling the proliferative activity of neural stem and progenitor cells (NSPCs) have a pivotal role to ensure life-long neurogenesis in the mammalian brain1. How metabolic programs are coupled with NSPC activity remains unknown. Here we show that fatty acid synthase (Fasn), the key enzyme of de novo lipogenesis2, is highly active in adult NSPCs and that conditional deletion of Fasn in mouse NSPCs impairs adult neurogenesis. The rate of de novo lipid synthesis and subsequent proliferation of NSPCs is regulated by Spot14, a gene previously implicated in lipid metabolism3–5, that we found to be selectively expressed in low proliferating adult NSPCs. Spot14 reduces the availability of malonyl-CoA6, which is an essential substrate for Fasn to fuel lipogenesis. Thus, we identify here a functional coupling between the regulation of lipid metabolism and adult NSPC proliferation.
Adult neurogenesis, the generation of new neurons throughout adulthood in discrete brain regions, is critically involved in certain forms of cognition and has been associated with neuropsychiatric diseases such as depression and epilepsy1,7. Recently, mechanisms regulating cell metabolism have been shown to control haematopoietic stem cell activity as well as proliferation of certain cancer cells8–11. Whether regulation of cell metabolism governs cell proliferation in mammalian NSPCs remains unknown. Therefore, we here studied the role of de novo lipogenesis and its key enzyme Fasn11,12 in the context of adult NSPC biology.
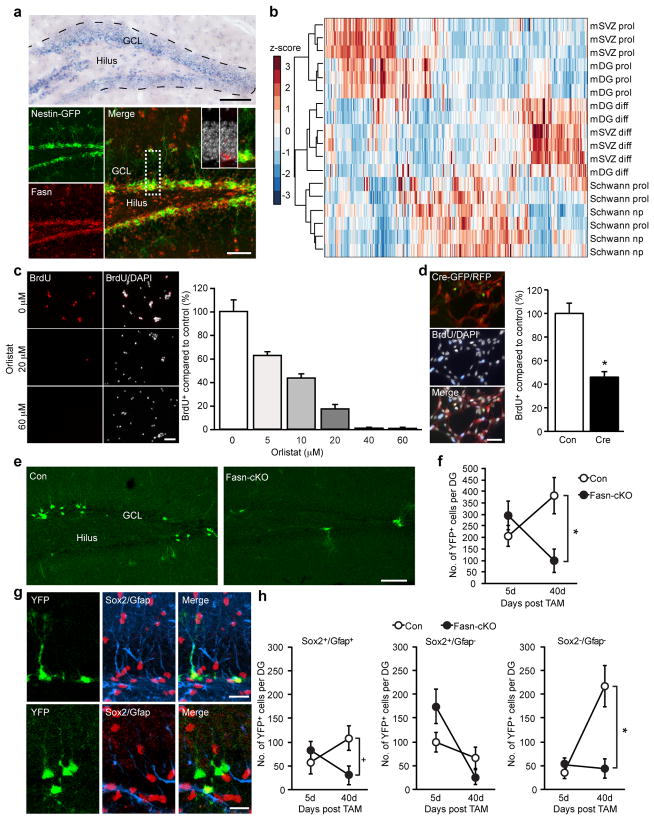
Fasn messenger RNA and protein were expressed in the two main neurogenic areas of the adult brain, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) lining the lateral ventricles (Fig. 1a and Supplementary Fig. 1a–h). Fasn expression was high in proliferating NSPCs but downregulated in vitro after differentiation (Supplementary Fig. 2a). Proliferating NSPCs showed high levels of Fasn enzymatic activity relative to other dividing neural cells, such as non-myelinating Schwann cells, or to NSPC- derived differentiated progeny (Supplementary Fig. 2b, c). High levels of Fasn activity were not due to impaired uptake of fatty acids, as NSPCs were competent to pick up free fatty acids in substantial amounts (Supplementary Fig. 2d). Mass-spectrometry-based metabo- lomics of dividing NSPCs, proliferating non-myelinating Schwann cells and differentiated progeny of NSPCs revealed striking differences in the metabolic profile of these discrete cell populations, confirming that NSPCs are in a distinct metabolic state (Fig. 1b).
Figure 1.
NSPCs are in a distinct metabolic state and require Fasn- dependent lipogenesis for proliferation. a, In situ hybridization shows expression of Fasn within the neurogenic dentate niche. Fasn protein is expressed in nestin-GFP1 cells. Insets show high-power views of Fasn- expressing cells with 49,6-diamidino-2-phenylindole (DAPI; grey). GCL, granule-cell layer. b, Metabolomics show a distinct metabolic state of proliferating dentate gyrus and SVZ NSPCs (mDG prol and mSVZ prol) compared to proliferating Schwann cells (Schwann prol), non-proliferating Schwann cells (Schwann nprol), and differentiated progeny of hippocampal and SVZ NSPCs (mDG diff and mSVZ diff). Note the close clustering of distinct cell populations. c, Pharmacological inhibition of Fasn using orlistat leads to a dose-dependent reduction of hippocampal NSPC proliferation. BrdU, 5-bromodeoxyuridine. d, Genetic deletion of Fasn decreases proliferation of adult hippocampal NSPCs. Con, control; Cre, transduction of Cre-expressing virus to delete Fasn. Left panel, cells that were transduced with a red fluorescent protein (RFP)-expressing control virus and Cre-GFP-expressing retrovirus, and pulsed with BrdU. e, Conditional Fasn deletion in NSPCs reduces hippocampal neurogenesis. Shown are YFP-expressing cells in controls and mutant mice (Fasn-cKO). f, Quantification of YFP-labelled cells in the dentate gyrus (DG) 5 days and 40 days after tamoxifen (TAM)-induced recombination. g, Phenotyping of recombined, YFP-labelled cells reveals that conditional Fasn deletion reduces the number of radial glia-like NSPCs expressing Sox2 and Gfap 40 days after tamoxifen and leads to a substantial decrease in differentiated YFP-labelled cells. h, Quantification of phenotypes after conditional Fasn deletion. Error bars represent mean s.e.m. Scale bars represent 100 μm (a top panel, e), 40 μm (a bottom panel), 50 μm (c, d) and 20 μm(g).*P, 0.05
Pharmacological inhibition of Fasn activity using two independent inhibitors of Fasn (orlistat and cerulenin) reduced proliferation of rodent NSPCs in a dose-dependent manner (Fig. 1c and Supplementary Fig. 3a, c, e) without inducing substantial amounts of cell death after short-term treatment (Supplementary Fig. 3b, d). In contrast, proliferation of non-myelinating Schwann cells was not affected by Fasn inhibition (Supplementary Fig. 3f). However, prolonged inhibition of Fasn enhanced apoptotic cell death, suggesting that Fasn activity is also required for NSPC maintenance (Supplementary Fig. 3d). To genetically delete Fasn, we isolated NSPCs from mice carrying floxed alleles of the Fasn gene (Fasnflox/flox)12. Retrovirus-based Cre-recombinase-mediated excision of Fasn phenocopied the effects of pharmacological Fasn inhibition and reduced proliferation of NSPCs (Fig. 1d and Supplementary Fig. 4a–c).
We next conditionally deleted Fasn specifically in adult NSPCs in vivo by crossing Fasnflox/flox mice with mice harbouring tamoxifen- inducible nestin-driven Cre recombinase and yellow fluorescent pro- tein (YFP) reporter alleles in the ROSA locus (Fasn-cKO (conditional knockout))12,13. When analysed 40 days after the last tamoxifen injec- tion, the number of recombined, YFP-expressing cells was drama- tically reduced in both neurogenic areas in Fasn-cKO mice compared to their respective controls (Fig. 1e, f and Supplementary Fig. 4d). Phenotyping of YFP-labelled cells in the dentate gyrus revealed that the defects observed after conditional Fasn deletion manifested themselves early in the neurogenic lineage with a reduction in YFP-labelled cells occurring already at the stage of radial NSPCs and resulting in a dramatic loss of newly generated neurons (Fig. 1g, h and Supplementary Fig. 5a, b). These results show that adult NSPCs require high levels of de novo lipogenesis for proper neurogenesis.
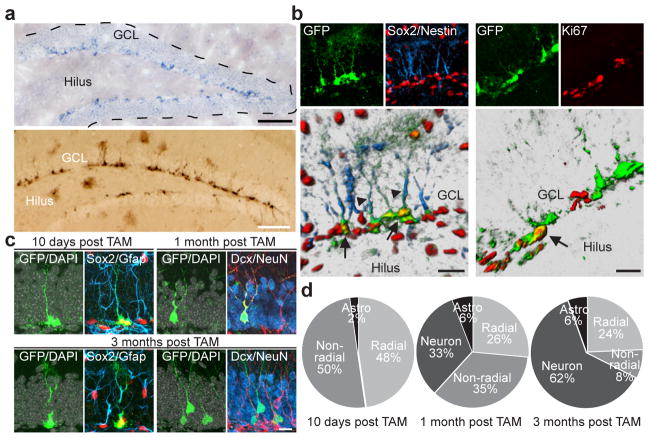
We next sought to identify genes that could regulate de novo lipogenesis in adult NSPCs and consequently alter proliferation of NSPCs. One gene that has previously been implicated as a context-dependent modulator of de novo lipogenesis is Spot14 (also known as thyroid hormone responsive protein (Thrsp))3–5. In situ hybridization revealed that Spot14 mRNA expression was highly confined to the two neurogenic areas of the adult brain (Fig. 2a and Supplementary Fig. 6a–c, e). To study Spot14-expressing cells in more detail we used a bacterial artificial chromosome (BAC) transgenic mouse driving green fluorescent protein (GFP) under the control of the regulatory elements of the Spot14 gene (Spot14-GFP). In agreement with the mRNA expression, Spot14-GFP-expressing cells (hereafter called Spot14+ cells) were largely restricted to the SGZ with only scattered expression outside the dentate gyrus, unlike previously described NSPC markers such as nestin, which also labels a substantial number of cells outside the adult neurogenic niches (Fig. 2a and Supplementary Fig. 6d, f). Spot14+ cells in the dentate gyrus showed a radial and non-radial cell morphology14, and co-labelled with NSPC markers such as Sox2 (92.7 6 15%), glial fibrillary acidic protein (Gfap; 85.3 6 4.2% in radial cells) and nestin (73.4 6 5.7% in radial cells) (Fig. 2b and Supplementary Fig. 6g, h)14. Of all Spot141 cells, only 9.36 6 1.1% were dividing. Non-radial Spot14+ cells showed higher proliferation rates (7.86 6 1.0%) compared to radial Spot14+ cells (1.7 6 0.5%), as measured by Ki67 co- labelling (Fig. 2b and Supplementary Fig. 6i), indicating that the majority of Spot14+ cells are not dividing under resting conditions. Confirming the relative quiescence of Spot14+ cells, we found that out of all Ki67+ cells within the dentate gyrus, only 5.1 6 0.7% were positive for Spot14-GFP, whereas in the nestin-GFP mouse, 46.6 6 3.7% of all Ki67+ cells were GFP positive (Supplementary Fig. 6j).
Figure 2.
Spot14 is expressed in adult hippocampal NSPCs. a, In situ hybridization shows restricted expression of Spot14 in the SGZ of the dentate gyrus (top panel). Enhanced GFP expression under the regulatory elements of the Spot14 gene labels cells in the SGZ (bottom panel). b, Spot14-GFP co-labels with NSPC markers Sox2 and nestin. Arrows, GFP and Sox2 co-labelled cells; arrowheads, GFP and nestin co-labelled radial glia-like processes (bottom-left panel). A fraction of Spot14-GFP cells divide. Arrow, a Ki67-positive, Spot14-GFP co-labelled cell (bottom-right panel). c, YFP expression in radial and non-radial NSPCs labelled by Sox2 and Gfap 10 days after tamoxifen (TAM) injections in Spot14-CreERT2 mice. One month after tamoxifen injections, YFP-expressing cells co-label with Dcx, and 3 months after tamoxifen injections these cells co-label with the mature neuronal marker NeuN (bottom-right panel). Note also that radial NSPCs were labelled with YFP 3 months after tamoxifen injections (bottom-left panel). DAPI is shown in grey. d, Quantification of cellular phenotypes generated 10 days, 1 month and 3 months after tamoxifen injections in Spot14-CreERT2 mice. Astro, astrocytes. Scale bars represent 100 μm (a) and 20 μm (b, c).
To show directly that cells expressing Spot14 possess neurogenic stem-cell potential in the adult brain we generated a BAC transgenic mouse expressing tamoxifen-inducible Cre under the regulatory elements of the Spot14 gene (Spot14-CreERT2). Spot14-CreERT2 mice were crossed with a ROSA reporter line expressing YFP upon Cre-based recombination, enabling in vivo lineage tracing of Spot14-expressing cells (Supplementary Fig. 7a). Soon after tamoxifen injections (after 10 days) we found YFP labelling of radial (Sox2+Gfap+) and non-radial (Sox2+Gfap−) NSPCs in the dentate gyrus as well as very few astrocytes (Fig. 2c, d). At later time points after tamoxifen injections (1 month and 3 months later) we found YFP labelling of new granule cells expressing doublecortin (Dcx) and the pan-neuronal marker NeuN (neuronal nuclei; also known as RNA binding protein Fox1 homologue 3) besides labelling of radial and non-radial NSPCs, both in the dentate gyrus and SVZ (Fig. 2c, d and Supplementary Fig. 7b–d). These findings indicate that Spot14-expressing cells constitute neurogenic NSPCs within the adult brain.
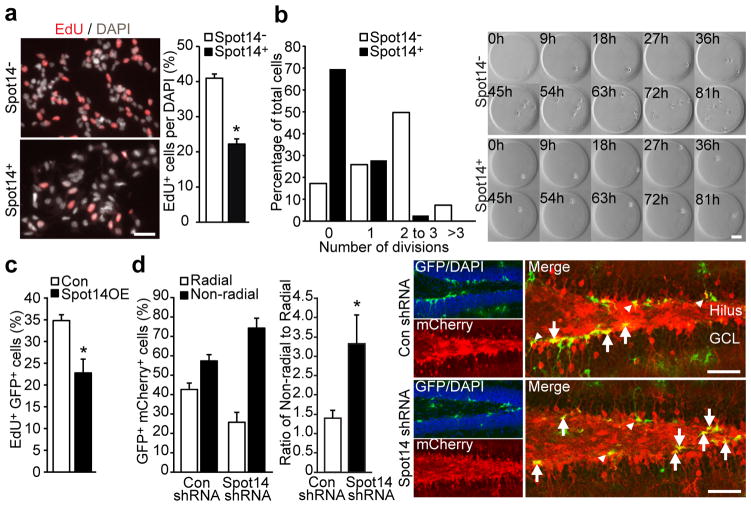
To further characterize Spot14+ cells on functional and molecular levels, we prospectively isolated NSPCs from Spot14-GFP transgenic mice and used FACS to separate Spot14+ and Spot14− NSPCs (Supplementary Fig. 8a, b). Both Spot14+ and Spot14− NSPCs expressed Sox2 and nestin (data not shown), self-renewed over an extended period of time in vitro and gave rise to neuronal and glial cells, indicating multipotency (Supplementary Fig. 8c–e). However, we observed reduced proliferation of Spot14+ cells compared to Spot14- cells using monolayer cultures and clonal time-lapse imaging (Fig. 3a, b and Supplementary Videos 1–4)15, suggesting that the low proliferative activity of Spot14+ cells within the dentate niche is maintained during culture conditions, a finding that was also supported by gene expression profiling (Supplementary Fig. 9a, b and Supplementary Tables 1–6).
Figure 3.
Spot14 regulates proliferative activity of adult NSPCs.a, Hippocampal Spot14+ cells are less proliferative than Spot14- NSPCs.b, Time-lapse imaging of single hippocampal NSPCs cultured in hydrogel- based microwells reveals reduced proliferation of Spot14+ cells compared to Spot14- NSPCs on a single-cell level. Shown are snapshots every 9 h for Spot14- cells (top panels) and Spot14+ (bottom panels), and the quantification of the number of cell divisions over 81 h. c, Retroviral overexpression of Spot14 (Spot14OE) reduces proliferation of hippocampal NSPCs. d, shRNA-mediated knockdown of Spot14 (Spot14 shRNA) leads to a shift from radial towards non- radial Spot141 NSPCs compared to a non-targeting, shRNA-expressing lentivirus (Con shRNA). Arrows, non-radial NSPCs; arrowheads, radial NSPCs. Error bars represent mean s.e.m. Scale bars represent 40 μm (a) and 100 μm (b, d). *P, 0.05.
Given the low proliferation rate of Spot14+ cells in vivo and in vitro we tested whether manipulation of Spot14 expression levels impacts NSPC proliferation. Retrovirus-mediated overexpression of Spot14 reduced proliferation of cultured NSPCs (Fig. 3c and Supplementary Fig. 10a). To test for a function of Spot14 in NSPC activity in vivo, we injected lentiviruses expressing short hairpin RNAs (shRNAs) and the fluorescent label mCherry into Spot14-GFP reporter mice. Knock- down of Spot14 mediated by shRNAs led to a shift in Spot14+ cells towards non-radial, more-proliferative NSPCs, compared to control cells expressing non-targeting shRNAs (Fig. 3d and Supplementary Fig. 10b).
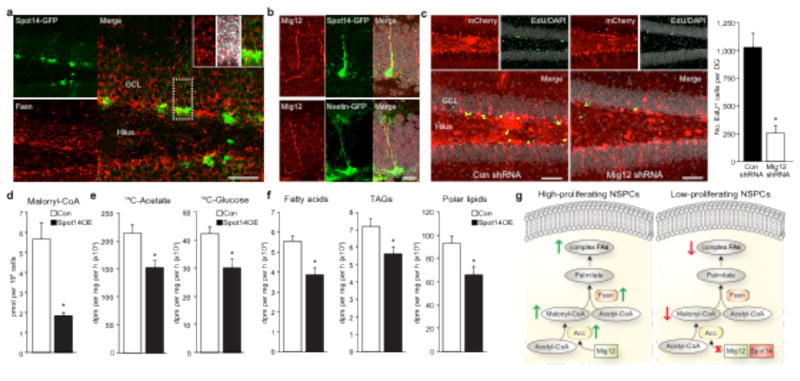
We next sought to identify a mechanism by which Spot14 controls NSPC proliferation. Spot14 has been shown to interact with Mig12 (Mid1-interacting protein 1), an activator of acetyl-coenzyme A (CoA)-carboxylase (Acc), the enzyme that catalyses the production of malonyl-CoA, which is a substrate for Fasn to generate new palmitate16; however, heterodimerization with Spot14 impairs the ability of Mig12 to activate Acc, leading to decreased Acc activity6. We found expression of Fasn in Spot141 cells and high levels of Mig12 in adult NSPCs (Fig. 4a, b and Supplementary Fig. 11a–d). In addition, Spot14 and Mig12 were both localized to the cytoplasm and co-immunoprecipitated (Supplementary Fig. 11e, f), suggesting that an interaction between Spot14 and Mig12 takes place in adult NSPCs. Retroviral overexpression of Mig12 rescued the Spot14-induced decrease in NSPC proliferation, indicating that a Spot14–Mig12 interaction is involved in the regulation of NSPC proliferation (Supplementary Fig. 11g). Furthermore, virus-mediated knockdown of Mig12 reduced proliferation of NSPCs within the adult hippocampus in vivo and in vitro, as measured by EdU (5-ethynyl-29-deoxyuridine) pulse labelling (Fig. 4c and Supplementary Fig. 11h, i). Using targeted liquid chromatography tandem mass spectrometry we found that Spot14 overexpression led to a significant drop in malonyl-CoA levels without affecting expression levels of Fasn protein (Fig. 4d and Supplementary Fig. 10c), suggesting that Spot14 decreases malonyl- CoA levels and as a consequence lowers substrate availability for Fasn to fuel de novo lipogenesis. To directly assess levels of de novo lipo- genesis upon Spot14 overexpression we measured total de novo lipid production by quantifying incorporation of radioactively labelled 14C-acetate and 14C-labelled glucose into lipids. We found that Spot14 overexpression significantly decreased levels of lipid synthesis (Fig. 4e). These data support previous reports that Spot14 can act as a context-dependent modulator of de novo lipogenesis, either as an inhibitor3 (as shown here for adult NSPCs) or activator of lipid syn- thesis4,5,17. In support of an essential role for Fasn and Spot14, we found that genetic deletion of Fasn or overexpression of Spot14 (both leading to reduced de novo lipogenesis) induced a shift in the metabolic profile of NSPCs, as determined by principal component analyses of metabolomics experiments (Supplementary Fig. 12). We next analysed the fate of newly synthesized lipids using thin-layer chromatography (TLC) and found that the majority of them (91.9%; Fig. 4g) ended up in the membrane lipid-containing fractions, suggesting that NSPCs require a large part of new lipids for structural membranes.
Figure 4.
Spot14 modulates malonyl-CoA levels and de novo lipogenesis. a, Fasn protein is expressed in Spot14+ cells. Insets show high-power views of a Spot14-GFP and Fasn co-labelled cell. b, Mig12 is expressed in adult NSPCs. c, shRNA-mediated knockdown of Mig12 in vivo (Mig12 shRNA, right panel) reduces proliferation of hippocampal NSPCs. d, Retroviral overexpression of Spot14 reduces malonyl-CoA levels in NSPCs. dpm, disintegrations per minute; pmol, picomol. e, Radioactive tracing using 14C-acetate and 14C- glucose shows a decrease in de novo lipogenesis after Spot14 overexpression. f, Lipid separation by thin-layer chromatography after 14C-acetate labelling shows a reduction in de novo synthesized free fatty acids, TAGs (triacylglycerols) and polar lipids in Spot14-overexpressing hippocampal NSPCs. g, Scheme showing the proposed mechanism: high-proliferating NSPCs require Fasn-dependent lipogenesis. Spot14 downregulates NSPC proliferation by reducing the Fasn-dependent generation of new complex fatty acids (FAs). Error bars represent mean 6 s.e.m. Scale bars represent 40 μm (a), 20 μm (b) and 50 μm (c). *P, 0.05.
We identify here a novel mechanism governing adult neurogenesis by showing that adult NSPCs require Fasn-dependent lipogenesis for proliferation and that Spot14, a gene highly enriched in low proli- ferating NSPCs, downregulates NSPC proliferation by reducing lipid synthesis (Fig. 4g). Thus, Spot14 seems to act as a molecular brake on Fasn-dependent lipogenesis, potentially also integrating other signals, such as thyroid hormone-regulated pathways, to ensure relative quiescence of adult NSPCs. However, future studies will have to further dissect and characterize the exact cellular mechanisms and NSPC-subtype during which high levels of lipogenesis are required for proper proliferation.
The targeting of metabolic pathways may hold potential for novel therapeutic approaches to treat diseases associated with failing adult neurogenesis, such as major depression or age-related cognitive decline.
METHODS SUMMARY
Mouse strains used were Spot14-GFP (Mutant Mouse Regional Resource Centres; MMRRC), nestin-GFP18, Fasn floxed12, nestin-CreERT2-R26YFP13 and Spot14-CreERT2. The Spot14-CreERT2 BAC transgenic line was generated according to a published protocol19. All animal experiments were approved by the veterinary office of the Canton of Zurich, Switzerland. Cre-mediated recombination was induced by five consecutive intraperitoneal injections of tamoxifen (180 mg kg21) at the age of 6 to 8 weeks. Retro- and lentiviral constructs, their production and stereotactic injections were carried out as described previously20. Brain tissue was sectioned and stained using methods published previously20. Murine NSPCs were cultured in DMEM with Ham’s F12, supplemented with N2 supplement plus EGF, FGF and heparin. Fasn enzyme activity was determined using an assay described previously12. Spot141 and Spot142 cells were live sorted and collected for RNA extraction, and gene expression analysis was then carried out using Affymetrix GeneChip arrays. To measure de novo lipid production in NSPCs overexpressing Spot14 and controls, cells were incubated with [1–14C]acetate or
Supplementary Material
Acknowledgments
We thank S. Aigner, D. C. Lie, F. H. Gage and members of the Jessberger group for conceptual input; S. Kobel, C. Fischer, K. Walter, P. Sidiropoulos, T. Buch, B. Becher, P. Pelczar, P. Lötscher, A. J. Eisch and D. C. Lagace for experimental help or reagents; and the Light Microscopy and Screening Center (LMSC) of the ETH Zurich and the BioImaging and Optics Platform (BIOP) of the EPFL for help with imaging. This study was supported by the NCCR Neural Plasticity and Repair, Swiss National Science Foundation, TH grant (ETH-01 08-1), Zurich Neuroscience Center (ZNZ), Novartis Foundation, Theodore Ott Foundation, and the EMBO Young Investigator program (to S.J.). M.K. was supported by the Janggen-Pöhn foundation.
Footnotes
Author Contributions M.K. contributed to the concept, carried out experiments, analysed data and co-wrote the paper. S.M.G.B. carried out experiments and analysed data. L.Z., C.v.S. and R.A.C.M. carried out experiments. N.Z. carried out the metabolomics experiments. M.J.A.B. analysed the array data. M.R. and M.P.L. contributed to the time-lapse imaging of NSPCs. W.J.K. contributed to the lipid metabolism experiments. Ö. K., U.S. and C.F.S. provided reagents. All authors revised the manuscript. S.J. developed the concept and wrote the paper.
References
- 1.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Maier T, Jenni S, Ban N. Architecture of mammalian fatty acid synthase at 4.5 A° resolution. Science. 2006;311:1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q, et al. Spot 14 gene deletion increases hepatic de novo lipogenesis. Endocrinology. 2001;142:4363–4370. doi: 10.1210/endo.142.10.8431. [DOI] [PubMed] [Google Scholar]
- 4.Moncur JT, Park JP, Memoli VA, Mohandas TK, Kinlaw WB. The “Spot 14” gene resides on the telomeric end of the 11q13 amplicon and is expressed in lipogenic breast cancers: implications for control of tumor metabolism. Proc Natl Acad Sci USA. 1998;95:6989–6994. doi: 10.1073/pnas.95.12.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell MC, Anderson GW, Mariash CN. Human spot 14 glucose and thyroid hormone response: characterization and thyroid hormone response element identification. Endocrinology. 2003;144:5242–5248. doi: 10.1210/en.2002-0008. [DOI] [PubMed] [Google Scholar]
- 6.Colbert CL, et al. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc Natl Acad Sci USA. 2010;107:18820–18825. doi: 10.1073/pnas.1012736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 8.Gan B, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurumurthy S, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarthy MV, et al. Brain fatty acidsynthase activates PPARa to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox21 neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells. 2008;26:2586–2594. doi: 10.1634/stemcells.2008-0498. [DOI] [PubMed] [Google Scholar]
- 16.Kim CW, et al. Induced polymerization of mammalian acetyl-CoA carboxylase by MIG12 provides a tertiary level of regulation of fatty acid synthesis. Proc Natl Acad Sci USA. 2010;107:9626–9631. doi: 10.1073/pnas.1001292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aipoalani DL, O’Callaghan BL, Mashek DG, Mariash CN, Towle HC. Overlapping roles of the glucose-responsive genes, S14 and S14R, in hepatic lipogenesis. Endocrinology. 2010;151:2071–2077. doi: 10.1210/en.2009-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter–GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 19.Johansson T, et al. Building a zoo of mice for genetic analyses: a comprehensive protocol for the rapid generation of BAC transgenic mice. Genesis. 2010;48:264–280. doi: 10.1002/dvg.20612. [DOI] [PubMed] [Google Scholar]
- 20.Karalay O, et al. Prospero-related homeobox1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.