Abstract
Using a comprehensive transcriptome analysis, a Z chromosome-linked chicken homolog of hemogen (cHEMGN) was identified and shown to be specifically involved in testis differentiation in early chicken embryos. Hemogen [Hemgn in mice, EDAG (erythroid differentiation-associated gene protein) in humans] was recently characterized as a hematopoietic tissue-specific gene encoding a transcription factor that regulates the proliferation and differentiation of hematopoietic cells in mammals. In chicken, cHEMGN was expressed not only in hematopoietic tissues but also in the early embryonic gonad of male chickens. The male-specific expression was identified in the nucleus of (pre)Sertoli cells after the sex determination period and before the expression of SOX9 (SRY-box 9). The expression of cHEMGN was induced in ZW embryonic gonads that were masculinized by aromatase inhibitor treatment. ZW embryos overexpressing cHEMGN, generated by infection with retrovirus carrying cHEMGN, showed masculinized gonads. These findings suggest that cHEMGN is a transcription factor specifically involved in chicken sex determination.
Keywords: bird, gonadal differentiation
In birds, as in mammals, sex is genetically determined, but males are the homogametic sex (ZZ), and females are heterogametic (ZW). The molecular mechanisms determining sex in birds has been a long-standing mystery. In mammals, the sex-determination gene SRY (sex determining region Y) acts as a transcription factor to activate SOX9 (SRY-box 9) expression directly by binding to the SOX9 enhancer in pre-Sertoli cells in the undifferentiated gonads of XY embryos (1). SOX9 functions in Sertoli cell differentiation in mammals and other vertebrates (2). A strong candidate for male sex determination in chicken is doublesex and mab-3 (Protein MAB-3) related transcription factor 1 (DMRT1), which is on the Z chromosome (3). DMRT1 has been suggested to activate SOX9 indirectly, because there is a time lag between the expression of DMRT1 and SOX9, which are first expressed on day 4.5 and day 6.5 of incubation, respectively (4–6). Therefore, other factors that are likely to be chicken specific must be in the molecular cascade between DMRT1 and SOX9.
Herein we show that chicken homolog of hemogen (cHEMGN), also in the Z chromosome linkage group, is a transcription factor involved in this chicken-specific molecular cascade. In mice, hemogen [Hemgn; also known as EDAG (erythroid differentiation-associated gene protein) in humans] is a recently characterized hematopoietic tissue-specific gene encoding a nuclear protein (7). The expression of Hemgn is restricted to the blood islands of the yolk sac and the fetal liver during embryogenesis, as well as the adult spleen and bone marrow (BM) (7). EDAG shows similar expression patterns. EDAG expression is high in the BM cells in acute myeloid leukemia, suggesting that EDAG may play a modulator role in acute myeloid leukemia (8). Overexpression of Hemgn in hematopoietic cells suppresses lymphopoiesis and enhances myelopoiesis in transgenic mice, suggesting that Hemgn regulates the proliferation and differentiation of hematopoietic cells (9). However, the gene is not expressed in the gonads during embryogenesis in mammals. In chicken, cHEMGN was expressed not only in hematopoietic tissues but also in the early embryonic gonad of male chickens. We present evidence that cHEMGN acts as a transcription factor in the nucleus of (pre)Sertoli cells after the sex-determination period and directly or indirectly triggers the expression of SOX9, suggesting this gene is specifically involved in chicken sex determination.
Results and Discussion
Identification of Chicken Hemogen cDNA and Chromosome Localization.
High coverage expression profiling (HiCEP) was used to conduct a comprehensive transcriptome analysis (10) comparing male and female gonads at day 5.5–6.5 of incubation. A total of 33,962 transcripts were identified. The 18 transcripts that were expressed specifically in males or were more than fivefold higher in males than females were sequenced, and cHEMGN was among these transcripts. The full-length coding sequence of cHEMGN was obtained by RT-PCR and 5′ and 3′ RACE. The full-length coding sequence was 543 bp, and the predicted amino acid sequence was 180 aa. A bipartite nuclear localization signal and a coiled-coil domain that are present in mouse HEMGN were also conserved in cHEMGN (7) (Fig. S1). The amino acid sequence identities between human and chicken, or mouse and chicken, were both 26%.
We performed FISH using cHEMGN cDNA clone as probe. The fluorescence signals were detected in Zq21 in chicken chromosomes (Fig. S2). This location corresponded with the information of cHEMGN in a chicken genome database (Ensemble, www.ensembl.org/index.html, last accessed October 20, 2012).
Expression Pattern of cHEMGN in Early Embryonic Gonads.
Northern blot analysis demonstrated that cHEMGN mRNA was more highly expressed in male gonads than female gonads at day 7.5 (Fig. 1A). Quantitative RT-PCR (qRT-PCR) analysis revealed that cHEMGN was expressed in the male gonads from day 5.5 onward, and expression increased dramatically to a peak at day 8.5 and was then lost before hatching (Fig. 1B). By contrast, female gonads exhibited only very low expression throughout embryogenesis. The expression in male gonads at day 8.5 was more than 10-fold higher than in female gonads. The cHEMGN protein was detected in male gonads from day 6.5 onward (Fig. S3). Anti-müllerian hormone (AMH) expression in Sertoli cells is one of the earliest markers of sex differentiation in chicken embryo gonads (11). AMH expression was up-regulated between days 5.5 and 6.5, similarly to cHEMGN expression (Fig. 1C). SOX9 expression was present from day 6.5 and was up-regulated by day 8.5 (Fig. 1C). These results are consistent with previous studies that reported that AMH was expressed in the male gonad before significant SOX9 expression (5, 12). The RT-PCR analysis here revealed that cHEMGN was also expressed before SOX9.
Fig. 1.

cHEMGN is highly expressed in early embryonic male chicken gonads. (A) Northern blot analysis of cHEMGN in embryonic tissues at day 7.5. cHEMGN mRNA was highly expressed in the male gonad. 18S rRNA was used as a loading control. (B) qRT-PCR of male and female gonads. In male embryos, cHEMGN expression was detected by day 5.5 (just after sex determination) and achieved a peak at day 8.5. The expression was gradually reduced and lost at hatching. Filled square, male; filled circle, female. Data are mean ± SEM; n ≥ 3. (C) RT-PCR of cHEMGN, AMH, and SOX9 in male gonads at day 5.5, 6.5, 7.5, and 8.5. cHEMGN expression was detected before SOX9 expression. GAPDH is the loading control.
cHEMGN expression was detected in the gonads of male embryos using in situ hybridization of whole embryos (WISH) (Fig. 2A) and in the gonadal medulla using frozen sections of embryos (Fig. 2 C and D). No signals were detected in the gonads of female embryos (Fig. 2 B, E, and F). To identify the cells that expressed cHEMGN, double-label in situ hybridization was conducted using SOX9 and chicken vasa homolog [CVH, also known as DDX4 (DEAD box polypeptide 4)] as markers for Sertoli cells and germ cells (13), respectively. The cHEMGN signal colocalized with the SOX9 signal in the gonadal medulla (Fig. 2 G–J). By contrast, the cHEMGN signal did not colocalize with that of CVH (Fig. 2 K–N). The cHEMGN protein was observed in the nucleus of male gonadal cells by immunohistochemistry (IHC) (Fig. 2 O–R). These results suggested that cHEMGN was a nuclear protein expressed in Sertoli cells.
Fig. 2.
cHEMGN is expressed in the nucleus of Sertoli cells within the medulla of the male gonads. (A and B) WISH of male and female embryos at day 7.5. cHEMGN was expressed throughout the male gonads. The dashed lines indicate the gonads. (Scale bar, 300 μm.) (C–F) cHEMGN in situ hybridization in frozen sections of male and female gonads at day 7.5. cHEMGN expression was localized to the medulla of the male gonad. The negative control used a sense probe for hybridization. (Scale bar, 100 μm.) (G–N) Dual-labeled in situ hybridization of frozen sections of male and female gonads at day 8.5. cHEMGN expression colocalized with SOX9 expression in Sertoli cells. (Scale bars, 100 μm.) (O–Q) IHC of day-8.5 male gonads using a cHEMGN antibody. cHEMGN was expressed in the nucleus of Sertoli cells in the medulla of the male gonad. (Scale bar, 100 μm.) (R) Higher-magnification view of the area indicated by the box in Q. Arrows indicate signals in the nucleus. (Scale bar, 100 μm.)
Expression Pattern of cHEMGN in Hematopoietic Tissues.
The expression of cHEMGN in chicken hematopoietic tissues was determined. Northern blot analysis identified cHEMGN expression in the spleen and BM, as well as in the blood, of both sexes, similar to mammals (7) (Fig. 3A). The cHEMGN protein was localized in the nuclei of blood cells by IHC (Fig. 3 B–D). The expression levels were compared between sexes by qRT-PCR, and males expressed two- to threefold higher levels than females in the spleen, BM, and blood (Fig. 3E). The chicken Z chromosome has no gene dosage compensation system such as X chromosome inactivation in mammals, with the net result that a large number of genes on the avian Z chromosome are expressed at a higher level in males (ZZ) than in females (ZW). Previous studies measured the male to female (M:F) ratio of Z-linked genes in chicken and reported that Z genes had M:F expression ratios ranging from 0.4 to 2.7 (14–16). Thus, the differences in expression of cHEMGN between sexes in the hematopoietic tissues may reflect the Z-linked gene dosage of cHEMGN. Furthermore, the 10-fold higher expression in male embryonic gonads relative to female (Fig. 1B) suggests the existence of a specific enhancer for expression in male gonads.
Fig. 3.
cHEMGN is expressed in hematopoietic tissues, similar to mammals. (A) Northern blot analysis of cHEMGN in spleen, BM, and blood of male and female embryos at day 8.5. Loading control, 18S rRNA. High cHEMGN expression was detected in blood; weak expression was detected in spleen and BM at longer exposure times. (B–D) IHC of day-8.5 embryo blood cells. The cHEMGN signal was localized to the nucleus. (Scale bar, 10 μm.) (E) qRT-PCR of spleen, BM, and blood from day-8.5 embryos. White bars, females; black bars, males. Expression was two- to threefold higher in males than females. Data are mean ± SEM; n ≥ 3.
Expression in Masculinized ZW Gonads by Aromatase Inhibitor Treatment.
cHEMGN was expressed in the nucleus of (pre)Sertoli cells, suggesting that this gene functioned on testis differentiation. However, there was a possibility that this expression was insignificant, because mammalian hemogen did not function on testis differentiation in humans and mice. Therefore, we analyzed the expression of cHEMGN in masculinized ZW embryo to prove that the expression actually associated with testis differentiation.
Day-10.5 masculinized ZW embryos were produced by fadrozole treatment. In females, the right gonad regresses early in embryonic development, and asymmetric gonadal development is observed (Fig. 4A). By contrast, bilateral development is observed in early embryonic development in males (Fig. 4B). The gonads in masculinized ZW embryos showed the bilateral development characteristic of the male morphology (Fig. 4C). The left gonad of female embryos possesses a thickened outer cortex and fragmented medulla (Fig. 4D). The gonads of male embryos are characterized by a dense medulla with seminiferous cords and a reduced cortex (Fig. 4E). A section from the left gonad of a masculinized ZW embryo exhibited the male-like morphology with dense medulla and a reduced cortex, although a slight fragmented medulla was observed (Fig. 4F). The expression of cytochrome P450, family 19, subfamily A, polypeptide 1 (CYP19A1, also known as aromatase) was detected in ZW female embryos but not in ZZ male or masculinized ZW embryos (Fig. 4 G–I) by in situ hybridization. SOX9 and cHEMGN were expressed in the gonads of ZZ male and masculinized ZW embryos (Fig. 4 J–O). The cHEMGN expression level was compared among ZW female, ZZ male, and masculinized ZW embryo gonads by qRT-PCR. An increase in cHEMGN expression was detected in masculinized ZW embryos by day 8.5 (Fig. 4P), suggesting that cHEMGN associated with testis differentiation.
Fig. 4.
Expression of cHEMGN is induced in the gonads of masculinized ZW embryos. Gonads on top of the mesonephros of female (A), male (B), and masculinized ZW (C) embryos at day 10.5. The gonads of masculinized ZW embryos showed bilateral development similar to male gonads. Dashed lines indicate gonads. (Scale bar, 1 mm.) (D–F) H&E staining of gonad sections from female, male, and masculinized ZW embryos. The left gonad of masculinized ZW has a testis-like phenotype with a dense medulla and thin cortex, although a slight fragmented medulla was observed. The dashed line indicates the border between the cortex and medulla in the female gonad. (Scale bar, 100 μm.) (G–O) CYP19A1, SOX9, and cHEMGN in situ hybridization in male, female, and masculinized ZW gonad frozen sections at day 10.5. Aromatase was identified in female gonads, but no expression was observed in male or masculinized ZW gonads. By contrast, the expression of SOX9 and cHEMGN was not detected in female gonads but was present in male and masculinized ZW gonads. (P) qRT-PCR of cHEMGN in gonads from female (black bars), masculinized ZW (gray bars), and male (white bars) embryos at days 6.5, 8.5, and 10.5. The expression of cHEMGN was induced in masculinized ZW gonads by day 8.5.
Masculinization of the ZW Gonads Overexpressing cHEMGN.
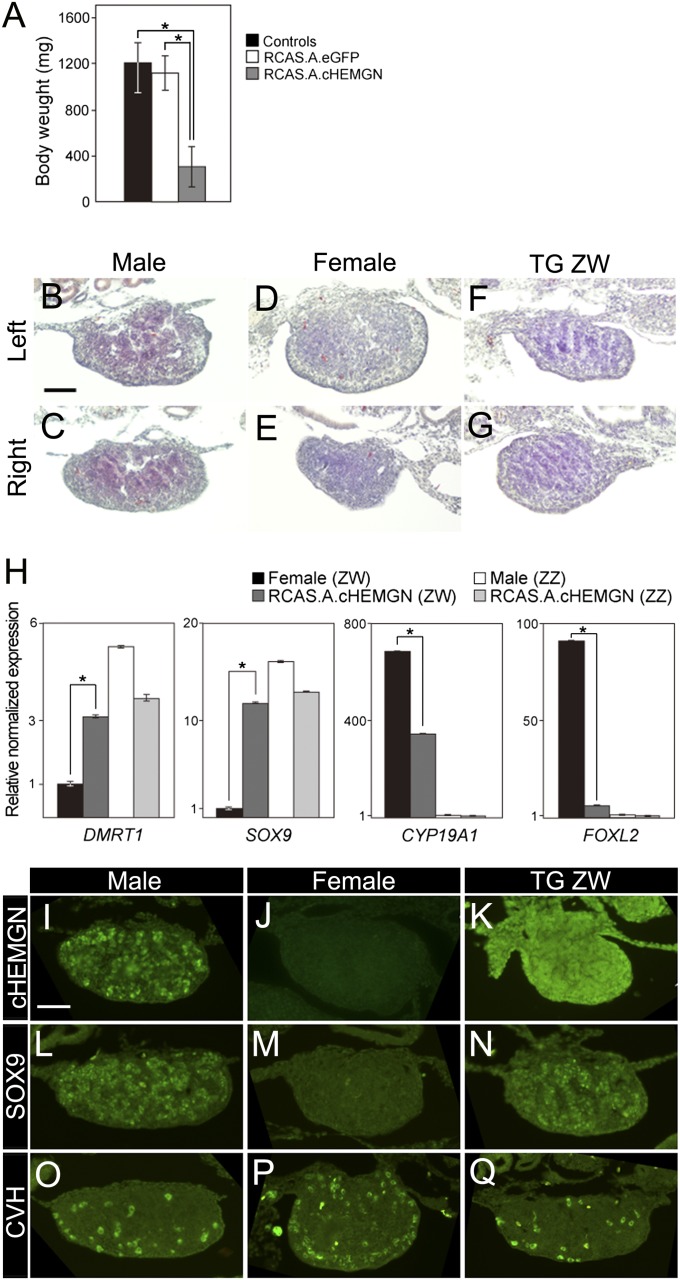
Transgenic embryos overexpressing cHEMGN were produced by infecting chicken embryos with an avian retroviral vector, RCAS.A (17), carrying the cHEMGN gene (RCAS.A.cHEMGN). The number of embryos used in experiments is shown in Table S1. Embryos infected with RCAS.A. carrying the enhanced green fluorescent protein (RCAS.A.eGFP) and uninfected embryos were used as negative controls. The embryos overexpressing cHEMGN showed significantly deficient growth compared with the two negative controls (Fig. 5A and Fig. S4 A and B). The average body weights of control, RCAS.A.eGFP, and RCAS.A.cHEMGN embryos were 1,211, 1,128, and 308 mg, respectively. Furthermore, embryos overexpressing cHEMGN exhibited early lethality. Survival to day 8.5 was 36.4% (63 of 173; Table S1), and there were no embryos that survived past day 9.5. These results suggest that forced expression of cHEMGN throughout the embryo results in abnormal transcription in cells and causes growth deficiency and early lethality. Such abnormalities have not been reported in transgenic Hemgn mice in which the overexpression was restricted to hematopoietic tissues as a result of using the human CD11a promoter (9). DMRT1 overexpression experiments in chicken using a retroviral system caused embryo lethality by day 4, and this early lethality was proposed to be due to the global effects of the transcription factor (3).
Fig. 5.
Masculinization of ZW embryos after overexpression of cHEMGN. (A) Comparison of body weight among controls (black bar), embryos overexpressing eGFP (RCAS.A.eGFP, white bar), and embryos overexpressing cHEMGN (RCAS.A.cHEMGN, gray bar). The body weights of embryos overexpressing cHEMGN were significantly reduced. Data presented are mean ± SD; *P < 0.001; n = 40. H&E staining of left and right gonad sections from male (B and C), female (D and E), and ZW overexpressing cHEMGN (F and G). The ZW gonad overexpressing cHEMGN showed a male-like morphology characterized by a dense medulla with seminiferous cords. (Scale bar, 100 μm.) (H) qRT-PCR of DMRT1, SOX9, CYP19A1, and FOXL2 in gonads of female (black bars), cHEMGN ZW overexpressing (RCAS.A.cHEMGN ZW, dark gray bars), male (white bars), and cHEMGN ZZ overexpressing (RCAS.A.cHEMGN ZZ, light gray bars) embryos at day 8.5. The expression of DMRT1 and SOX9 was increased and expression of CTP19A1 and FOXL2 was reduced in ZW embryos overexpressing cHEMGN compared with control female embryos. Data presented are mean ± SEM; *P < 0.001; n ≥ 3. (I–Q) IHC of cHEMGN, SOX9, and CVH in gonad sections of male, female, and cHEMGN ZW overexpressing (RCAS.A.cHEMGN ZW) embryos at day 8.5. cHEMGN was detected in male gonads and at very high levels in the gonad of cHEMGN overexpressing ZW embryos. SOX9 protein was also detected in gonads of both male and cHEMGN overexpressing ZW embryos. The gonad of cHEMGN overexpressing embryo showed male-like (interior) distribution of germ cells. (Scale bar, 100 μm.) Male and female embryos uninfected with any viruses were used as negative controls (B–E, H–J, L, M, O, and P).
The gonads in ZW embryos overexpressing cHEMGN showed the bilateral development characteristic of the male morphology (Fig. S4C). We performed histological analysis by H&E staining in sections of gonads from male (Fig. 5 B and C), female (Fig. 5 D and E), and ZW overexpressing cHEMGN at day 8.5 (Fig. 5 F and G). After this, male and female embryos uninfected with any viruses were used as negative controls in all experiments. Although a little difference was observed in gonads at day 8.5 between control male and female compared with gonads at day 10.5 (Fig. 4 D and E), The ZW gonad overexpressing cHEMGN showed a male-like morphology characterized by a dense medulla with seminiferous cords (Fig. 5 F and G).
The expression of male and female markers in the gonads of the embryos overexpressing cHEMGN was examined. Key markers of testicular differentiation are DMRT1 and SOX9, whereas markers of ovarian development are forkhead box L2 (FOXL2) and CYP19A1. DMRT1 has been proposed as a putative testis-determining gene in birds, and high-level expression of the gene is necessary for testis differentiation during embryogenesis after sex determination (18). A conserved role for FOXL2 has been indicated in chicken embryos because this gene is activated female-specifically just before gonad differentiation (19, 20). Furthermore, the temporal and spatial colocalization profiles suggest that FOXL2 activates the estrogen-synthesizing enzyme aromatase (20, 21). At the mRNA level, DMRT1 and SOX9 expression increased, and FOXL2 and CYP19A1 expression decreased, in ZW gonads overexpressing cHEMGN relative to controls (Fig. 5H). In control male embryos at day 8.5, the cHEMGN and SOX9 proteins were expressed normally in the nuclei of Sertoli cells (Fig. 5 I and L), but control female gonads lacked the expression of these proteins (Fig. 5 J and M). The ZW embryos overexpressing cHEMGN possessed high levels of cHEMGN protein throughout the gonad (Fig. 5K), whereas SOX9 protein was found only in the medullary region of the gonad (Fig. 5N). CVH staining showed distribution of germ cells within the interior of control male gonads (Fig. 5O), but control female gonads exhibited cortical distribution of germ cells (Fig. 5P). The ZW embryos overexpressing cHEMGN showed male-like (interior) distribution of germ cells in the gonads (Fig. 5Q). These results indicate that the ZW gonads were masculinized as a result of high cHEMGN expression. Four of six ZW embryos overexpressing cHEMGN showed clear expression of SOX9 and male-like distribution of germ cells demonstrated by CVH expression (Table S1). In the remaining two embryos, significant signals could not be detected by IHC, because the gonads were very fragile and good sections of gonads could not be obtained in the two embryos.
Expression of DMRT1 starts from day 4.5 and is before cHEMGN expression, suggesting that cHEMGN operates downstream of DMRT1. The expression of DMRT1 is kept in Sertoli cells after sex determination and the gene becomes to express in germ cells with progress of developmental stage (18). These facts indicate that DMRT1 has multiple functions in embryonic gonads and at least two functions in Sertoli cells: triggering of testis differentiation and testis differentiation after sex determination. Therefore, we supposed that each expression was independently regulated, resulting from the masculinization by overexpression of cHEMGN-induced DMRT1 expression in gonads of ZW embryo.
This report has shown that cHEMGN is a gene involved specifically in early events in sex determination in chicken and that cHEMGN functions in the molecular cascade between DMRT1 and SOX9 as a transcription factor in (pre)Sertoli cells. Our data indicate that cHEMGN acts as a transcription factor in the pre-Sertoli cells to induce directly or indirectly SOX9 expression after sex determination. In the 5′-flanking region of mouse and human Hemgn/EDAG, binding sites for GATA1 (GATA binding protein 1) and HOXB4 (homeobox B4) (only reported in Hemgn) are present, and Hemgn/EDAG has been shown to be a direct transcriptional target of these genes in hematopoetic cells (22–25). Future studies should determine the presence of a gonad-specific enhancer and transcription factors that mediate the gonadal expression of cHEMGN, as well as identify direct targets of cHEMGN. Further investigation of the role of cHEMGN will reveal the chicken-specific mechanisms of sex determination.
Materials and Methods
Chicken Strains.
Fertilized chicken eggs (Gallus gallus domesticus) were purchased from Takeuchi Hatchery(Nara, Japan). This study used the Hy-Line Maria chicken strain. Fertilized eggs were incubated at 37.8 °C. All the animal experiments in this study were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals, Hokkaido University.
HiCEP Screening.
We performed HiCEP screening according to a previous report (10). Details of the procedure are provided in SI Materials and Methods.
Cloning of Chicken cHEMGN cDNA.
A detailed procedure is provided in SI Materials and Methods, and the sequences of primers used are shown in Table S2.
Chromosome Preparation and FISH Mapping.
The preparation of R-banded chromosomes and FISH were performed as previously described (26, 27). Details of the procedures are provided in SI Materials and Methods.
RT-PCR and qRT-PCR.
Details of the procedure are provided in SI Materials and Methods, and the sequences of primers used are shown in Table S2.
Northern Blot Analysis.
Details of the procedure are provided in SI Materials and Methods.
WISH and in Situ Hybridization.
Chicken embryos were fixed in 4% (wt/vol) pareformaldehyde and processed for WISH according to a previous report (28). The urogenital tissue of chicken embryos was slowly frozen in tissue-tek (Sakura Finetek USA) and kept at −80 °C until use and processed for in situ hybridization, as previously reported (29). Details of the procedures are shown in SI Materials and Methods.
Preparation of Recombinant Protein and Rabbit Antibodies.
Details of the procedure are provided in SI Materials and Methods.
Western Blot Analysis.
Details of the procedure are provided in SI Materials and Methods.
Immunohistochemistry Analysis.
The primary antibodies, rabbit anti-SOX9 and rat anti-CVH, were a kind gift from R. Lovell-Badge (London, UK) and M.-A. Hattori (Fukuoka, Japan), respectively. Details of the procedure are provided in SI Materials and Methods.
Aromatase Inhibitor Treatments.
To obtain sex reversal chickens (female to male), we performed aromatase inhibitor treatments according to a previous report (18), with slight modifications. Details of the procedure are provided in SI Materials and Methods.
Preparation of RCAS.A.cHEMGN and RCAS.A.eGFP Virus.
The cHEMGN transgenic chicken embryos were produced by infection with Replication-Competent Avian Leucosis Sarcoma virus LTR with Splice acceptor, and subgroup An env (envelope protein) gene (RCAS.A). RCAS.A proviral DNA was the kind gift of Stephen H. Hughes (National Institutes of Health, Bethesda, MD) (17). Both primers included an artificial ClaI site for cloning into the RCAS.A. The products were subcloned using the pGEM T-easy Vector System (Promega). The identity of the insert was confirmed by sequencing, and the pGEM T-easy-cHEMGN plasmid DNA was subsequently digested with ClaI and cloned into ClaI-digested RCAS.A.
For negative controls, RCAS.A.eGFP was used, as previously reported (30). This construct was provided by ARK-Genomics, The Roslin Institute.
Injection of Embryos with RCAS.A.cHEMGN.
Embryo injections were performed as described in a previous study (31), with a slight modification. Details of the procedure are provided in SI Materials and Methods.
Measurement of Body Weight.
Details of the procedure are provided in SI Materials and Methods.
Accession Codes.
Homo sapiens EDAG, NM_018437.3; Mus musculus Hemgn, NM_053149.2; Gallus gallus HEMGN, XM_430508.3.
Supplementary Material
Acknowledgments
We thank H. Yunokawa and Y. Mikami for help with HiCEP screening; S. H. Hughes and A. Ferris for providing RCAS vectors; R. Lovell-Badge, S. Guioli and M.-A. Hattori for providing the SOX9 and CVH antibody, respectively; and H. Yoshioka, Y. Ishimaru, Y. Atsumi, A. P. Kimura, Y. Matsuda, and C. Nishida-Umehara for helpful suggestions regarding experimental techniques and this research. This work was supported by Grant-in-Aid for Scientific Research 23132501 and the F3 Project Support office for female researchers at Hokkaido University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218714110/-/DCSupplemental.
References
- 1.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453(7197):930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 2.Koopman P. Sry and Sox9: Mammalian testis-determining genes. Cell Mol Life Sci. 1999;55(6-7):839–856. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith CA, et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461(7261):267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 4.Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215(2):208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- 5.Smith CA, Smith MJ, Sinclair AH. Gene expression during gonadogenesis in the chicken embryo. Gene. 1999;234(2):395–402. doi: 10.1016/s0378-1119(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 6.Chue J, Smith CA. Sex determination and sexual differentiation in the avian model. FEBS J. 2011;278(7):1027–1034. doi: 10.1111/j.1742-4658.2011.08032.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang LV, Nicholson RH, Kaplan J, Galy A, Li L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech Dev. 2001;104(1-2):105–111. doi: 10.1016/s0925-4773(01)00376-8. [DOI] [PubMed] [Google Scholar]
- 8.An LL, et al. High expression of EDAG and its significance in AML. Leukemia. 2005;19(8):1499–1502. doi: 10.1038/sj.leu.2403808. [DOI] [PubMed] [Google Scholar]
- 9.Li CY, et al. Overexpression of a hematopoietic transcriptional regulator EDAG induces myelopoiesis and suppresses lymphopoiesis in transgenic mice. Leukemia. 2007;21(11):2277–2286. doi: 10.1038/sj.leu.2404901. [DOI] [PubMed] [Google Scholar]
- 10.Fukumura R, et al. A sensitive transcriptome analysis method that can detect unknown transcripts. Nucleic Acids Res. 2003;31(16):e94. doi: 10.1093/nar/gng094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josso N, Boussin L, Knebelmann B, Nihoul-Fékété C, Picard JY. Anti-Müllerian hormone and intersex states. Trends Endocrinol Metab. 1991;2(6):227–233. doi: 10.1016/1043-2760(91)90029-m. [DOI] [PubMed] [Google Scholar]
- 12.Oreal E, et al. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev Dyn. 1998;212(4):522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127(12):2741–2750. doi: 10.1242/dev.127.12.2741. [DOI] [PubMed] [Google Scholar]
- 14.McQueen HA, McBride D, Miele G, Bird AP, Clinton M. Dosage compensation in birds. Curr Biol. 2001;11(4):253–257. doi: 10.1016/s0960-9822(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 15.Kuroiwa A, et al. Biallelic expression of Z-linked genes in male chickens. Cytogenet Genome Res. 2002;99(1-4):310–314. doi: 10.1159/000071609. [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y, et al. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6(1):2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CA, Katz M, Sinclair AH. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol Reprod. 2003;68(2):560–570. doi: 10.1095/biolreprod.102.007294. [DOI] [PubMed] [Google Scholar]
- 19.Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144(7):3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- 20.Govoroun MS, et al. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev Dyn. 2004;231(4):859–870. doi: 10.1002/dvdy.20189. [DOI] [PubMed] [Google Scholar]
- 21.Hudson QJ, Smith CA, Sinclair AH. Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary. Dev Dyn. 2005;233(3):1052–1055. doi: 10.1002/dvdy.20388. [DOI] [PubMed] [Google Scholar]
- 22.Yang LV, et al. The GATA site-dependent hemogen promoter is transcriptionally regulated by GATA1 in hematopoietic and leukemia cells. Leukemia. 2006;20(3):417–425. doi: 10.1038/sj.leu.2404105. [DOI] [PubMed] [Google Scholar]
- 23.Li CY, et al. Suppression of EDAG gene expression by phorbol 12-myristate 13-acetate is mediated through down-regulation of GATA-1. Biochim Biophys Acta. 2008;1779(10):606–615. doi: 10.1016/j.bbagrm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Ding YL, et al. Over-expression of EDAG in the myeloid cell line 32D: Induction of GATA-1 expression and erythroid/megakaryocytic phenotype. J Cell Biochem. 2010;110(4):866–874. doi: 10.1002/jcb.22597. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, et al. Hemgn is a direct transcriptional target of HOXB4 and induces expansion of murine myeloid progenitor cells. Blood. 2010;116(5):711–719. doi: 10.1182/blood-2009-07-235341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda Y, et al. Location of the mouse complement factor H gene (cfh) by FISH analysis and replication R-banding. Cytogenet Cell Genet. 1992;61(4):282–285. doi: 10.1159/000133423. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda Y, Chapman VM. Application of fluorescence in situ hybridization in genome analysis of the mouse. Electrophoresis. 1995;16(2):261–272. doi: 10.1002/elps.1150160142. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka H, et al. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94(3):299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara S, Takahashi T, Kimura AP. Epigenetic patterns at the mouse prolyl oligopeptidase gene locus suggest the CpG island in the gene body to be a novel regulator for gene expression. Gene. 2010;465(1-2):17–29. doi: 10.1016/j.gene.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Das RM, et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev Biol. 2006;294(2):554–563. doi: 10.1016/j.ydbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Smith CA, Roeszler KN, Sinclair AH. Robust and ubiquitous GFP expression in a single generation of chicken embryos using the avian retroviral vector, RCASBP. Differentiation. 2009;77(5):473–482. doi: 10.1016/j.diff.2009.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.