Abstract
Polymorphisms in MHC class II molecules, in particular around β-chain position-57 (β57), afford susceptibility/resistance to multiple autoimmune diseases, including type 1 diabetes, through obscure mechanisms. Here, we show that the antidiabetogenic MHC class II molecule I-Ab affords diabetes resistance by promoting the differentiation of MHC-promiscuous autoreactive CD4+ T cells into disease-suppressing natural regulatory T cells, in a β56–67-regulated manner. We compared the tolerogenic and antidiabetogenic properties of CD11c promoter-driven transgenes encoding I-Ab or a form of I-Ab carrying residues 56–67 of I-Aβg7 (I-Ab-g7) in wild-type nonobese diabetic (NOD) mice, as well as NOD mice coexpressing a diabetogenic and I-Ag7–restricted, but MHC-promiscuous T-cell receptor (4.1). Both I-A transgenes protected NOD and 4.1-NOD mice from diabetes. However, whereas I-Ab induced 4.1-CD4+ thymocyte deletion and 4.1-CD4+Foxp3+ regulatory T-cell development, I-Ab-g7 promoted 4.1-CD4+Foxp3+ Treg development without inducing clonal deletion. Furthermore, non–T-cell receptor transgenic NOD.CD11cP-I-Ab and NOD.CD11cP-IAb-g7 mice both exported regulatory T cells with superior antidiabetogenic properties than wild-type NOD mice. We propose that I-Ab, and possibly other protective MHC class II molecules, afford disease resistance by engaging a naturally occurring constellation of MHC-promiscuous autoreactive T-cell clonotypes, promoting their deviation into autoregulatory T cells.
Keywords: autoimmune diabetes, FoxP3+ Treg cells, genetic resistance, major histocompatibility complex class II, T-cell tolerance
The HLA gene region on human chromosome 6 accounts for >50% of the genetic risk for autoimmune diseases (including T1D) (1). Genes encoding HLA-DQ and -DR and their murine counterparts (I-A and I-E, respectively) (2), as well as class I genes (3, 4), play key roles, albeit through poorly understood mechanisms. Human T1D is primarily associated with HLA-DQB1. Alleles encoding DQβ chains with Ser, Ala, or Val at position 57 provide risk, whereas those encoding DQβ chains with Asp at this position afford protection in most, though not all, populations (5–7). The nonobese diabetic (NOD) mouse is homozygous for a unique H-2 haplotype (H-2g7). This haplotype carries a nonproductive I-Eα gene and encodes an I-Aαd/I-Aβg7 heterodimer in which the Pro and Asp found at positions 56 and 57 in most I-Aβ chains are replaced by His and Ser, respectively (5, 6). Early studies of NOD mice expressing non-NOD MHC haplotypes or MHC class II transgenes have proven that class II molecules play a direct role. Evidence for enhanced central T-cell tolerance and T-cell–mediated immunoregulation or immune deviation (8–13) has been provided. However, a unifying conclusion could not be drawn from these individually reported mechanisms, as they could not explain, on their own, MHC class II-mediated resistance to polyclonal T-cell–mediated autoimmune diabetes.
We previously reported that thymocytes carrying a diabetogenic I-Ag7–restricted T-cell receptor (TCR; 4.1) (14) undergo deletion in T1D-resistant H-2g7/b, H-2g7/k, H-2g7/q, and H-2g7/nbl NOD mice by engaging anti-T1D class II molecules on a hematopoietic antigen-presenting cell (APC) type (15, 16). Expression of protective class II transgenes, such as I-Eαk (which restores expression of I-Eβg7), I-Aβd, and I-Aβg7PD (I-Ag7 carrying Pro56 and Asp57) in 4.1-NOD mice also led to various degrees of 4.1-thymocyte tolerance and T1D resistance (17). Interestingly, the I-A molecules capable of deleting 4.1-thymocytes display a conserved stretch of amino acids at β-chain positions 56–67, which differ from those in I-Aβg7 (17). On the basis of these and other observations (18, 19), we hypothesized that protective class II molecules afford T1D resistance by deleting “4.1-like” (MHC-promiscuous and diabetogenic) CD4+ T cells, and that I-Aβ-chain residues 56–67 play a critical role.
The present study was thus initiated to investigate if the MHC class II-induced resistance to T1D in non-TCR transgenic NOD mice can be accounted for by central tolerance of 4.1-like CD4+ T cells, if this phenotype is triggered by thymic epithelial cells or dendritic cells (DCs), and if the polymorphic residues at and around the T1D-associated β-chain position 57 play a key role and, if so, how. This investigation was done by assessing the diabetes resistance of NOD and 4.1-NOD mice expressing wild-type and modified I-Ab transgenes under the control of K14 and CD11c promoters, and following the fate and function of 4.1-thymocytes. Our data demonstrate that antidiabetogenic MHC class II molecules afford T1D resistance both by deleting and skewing the development of MHC-promiscuous autoreactive thymocytes into autoregulatory CD4+ T-cells, and that these two phenotypes are triggered by DCs without any contribution from thymic epithelial cells. Unexpectedly, we find that replacement of I-Aβb residues 56–67 by their I-Aβg7–derived counterparts abrogates the ability of I-Ab to delete 4.1-thymocytes, but not its ability to promote the development of these thymocytes into antidiabetogenic regulatory T cells (Tregs). We propose that polymorphisms proximal to β-chain position 57 contribute to the antidiabetogenic effects of protective MHC class II alleles by enhancing the avidity with which these molecules are recognized by thymocytes expressing MHC-promiscuous autoreactive TCRs.
Results
Lack of Thymocyte Deletion and T1D Resistance in 4.1-NOD Mice Expressing a K14 Promoter-Driven I-Aβb Transgene.
We first followed the fate of 4.1-thymocytes and the incidence of spontaneous diabetes in 4.1-NOD mice expressing high levels of a K14 promoter (K14P)-driven I-Aβb transgene in thymic cortical epithelial cells (where it pairs with endogenous I-Aαd) (20). The 4.1-NOD.K14P-I-Aβb.Rag2+ and 4.1-NOD.K14P-I-Aβb.Rag2−/− mice developed T1D like their non–K14P-I-Aβb transgenic counterparts (Fig. S1 A and B). The thymocyte and splenic flow cytometric profiles of K14P-I-Aβb transgenic and nontransgenic 4.1-NOD mice were also similar (Fig. S1C). Lack of 4.1-thymocyte deletion in these mice was not because of lack of pairing of transgenic K14-driven I-Aβb with endogenous I-Aαd, because 4.1-TCR transgenic mice carrying an I-Aαb−/− chromosome (encoding I-Aβb) and an H-2g7+ chromosome (encoding I-Aαd and I-Aβg7) efficiently deleted 4.1-thymocytes (15). Thus, in agreement with the outcome of previous studies in marrow chimeras (15–17), thymic epithelial cells cannot drive I-Aβb–dependent 4.1-CD4+ thymocyte tolerance and T1D resistance.
Expression of CD11c Promoter-Driven I-Ab and Chimeric I-Aαb/IAβb-g7 (I-Ab-g7) Transgenes.
To evaluate the role of DCs, we produced transgenic NOD mice expressing CD11c promoter (CD11cP)-driven I-Aαb and I-Aβb transgenes (NOD.CD11cP-I-Aαβb mice). To address the role of I-Aβb paired with endogenous I-Aαd, we also backcrossed an existing B6-derived CD11cP-I-Aβb transgene (21) onto the NOD background (NOD.CD11cP-I-Aβb mice). In addition, to investigate whether the tolerogenic or antidiabetogenic properties of I-Ab mapped to β-chain residues 56–67, we replaced the amino acids at positions 56–67 in CD11cP-I-Aβb by their counterparts on I-Aβg7, and microinjected the resulting transgene (CD11cP-I-Aβb-g7) into NOD oocytes along with CD11cP-I-Aαb (to produce NOD.CD11cP-I-Aαβb-g7mice) (Fig. S2A). As shown in Fig. S2B, Upper Left and Center, splenic DCs from NOD.CD11cP- I-Aαβb and NOD.CD11cP-I-Aαβb-g7 mice expressed similar levels of transgenic I-Ab, as determined with an I-Aβb–specific antibody. The levels of transgenic I-Ab were moderate and lower than those seen in splenic DCs from C57BL/6 mice, indicating that they were not overexpressed. This mAb, however, did not stain DCs from NOD.CD11cP-I-Aβb mice (Fig. S2B, Upper Right) or 293T cells transfected with I-Aαd and I-Aβb cDNAs (Fig. S3A), suggesting that I-Aαdβb dimers cannot be recognized by this mAb. Because most available anti–I-Aαb mAbs (22) do not cross-react with I-Aαd (Fig. S3B and Table S1), we investigated this further by introgressing all three I-Aαβ combinations onto NOD.I-Aβb−/− mice, expressing I-Aαb but not I-Aβ. As shown in Fig. S2B (Lower), this mAb stained splenic DCs from all three types of mice equally well, demonstrating that DCs from NOD.CD11cP-I-Aβb mice do indeed express transgenic I-Aβb. Analyses of splenic CD19+ and CD11b+ cells confirmed that transgene expression was restricted to DCs (Fig. S2C). Transgene expression did not cause a reduction in either the levels of endogenous I-Ag7 on DCs (Fig. S2D) or in the ability of antigen-pulsed DCs to support the activation of I-Ag7–restricted BDC2.5 CD4+ T-cells (Fig. S2E), or Kd-restricted 8.3-CD8+ T cells (Fig. S2F). Furthermore, transgene expression did not reduce the percentages of B cells in the peripheral lymphoid organs (Fig. S2G), which caused a reduction in the incidence of diabetes in NOD mice expressing high levels of an I-Ag7 transgene in all professional APC types (23).
Thus, the DCs of NOD.CD11cP-I-Aαβb, NOD.CD11cP-I-Aαβb-g7, and NOD.CD11cP-I-Aβb mice express comparable levels of transgenic MHC class II and this does not interfere with the expression of diabetogenic I-Ag7, the DCs’ antigen-presentation capacity, or B-cell development.
DC-Specific Expression of CD11cP-Driven I-Ab or I-Ab-g7 Transgenes Protect NOD and 4.1-TCR Transgenic NOD Mice from T1D.
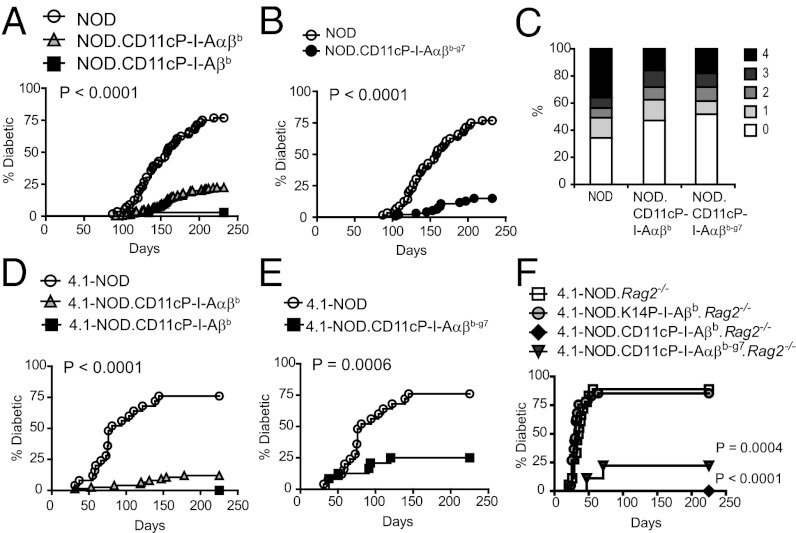
To assess whether the expression of transgenic I-Ab and/or I-Ab-g7 on DCs had antidiabetogenic activity in non-TCR transgenic NOD mice, we monitored transgenic females derived from different founders for development of diabetes and insulitis (two CD11cP-I-Aαβb lines derived from transgenic NOD or 129 mice, respectively; two CD11cP-I-Ab-g7 lines derived from transgenic NOD mice; and one CD11cP-I-Aβb line derived from transgenic B6 mice). NOD.CD11cP-I-Aαβb and NOD.CD11cP-I-Aβb lines developed a lower incidence of diabetes (but not lower insulitis scores) than wild-type NOD mice (Fig. 1 A and C). Surprisingly, NOD.CD11cP-I-Aαβb-g7 mice were also protected (Fig. 1B). Similar patterns of protection were observed in NOD.CD11cP-I-Aαβb, -I-Aβb, and -I-Aαβb-g7 mice expressing the diabetogenic, I-Ag7–restricted 4.1-TCR, even in RAG2-deficient backgrounds (Fig. 1 D–F). Taken together, these data indicate that DC-specific expression of I-Ab is sufficient to confer I-Ab’s anti-T1D effects, that I-Ab´s antidiabetogenic properties are dissociated from polymorphisms at or around β-chain position 57, and that, in 4.1-TCR transgenic mice, I-Ab and I-Ab-g7 afford T1D protection directly via 4.1-CD4+ T-cells.
Fig. 1.
Expression of CD11cP-I-Aβb, CD11cP-I-Aαβb and NOD.CD11cP-I-Aαβb-g7 protects 4.1-TCR transgenic and non-TCR transgenic NOD mice from diabetes. (A) DC expression of I-Aαβb or I-Aβb protected NOD mice from diabetes (n = 56 for NOD, n = 267 for NOD.CD11cP-I-Aαβb, n = 35 for NOD.CD11cP-I-Aβb). (B) DC expression of I-Aαβb-g7 protected NOD mice from diabetes (n = 56 for NOD, n = 93 for NOD.CD11cP-I-Aαβb-g7). (C) Insulitis scores of 32-wk-old NOD vs. NOD.CD11cP-I-Aβb and NOD.CD11cP-I-Aαβb-g7 females. There were no statistically significant differences between the average scores (NOD 1.92 ± 0.32 vs. NOD.CD11cP-I-Aβb 1.59 ± 0.30 vs. NOD.CD11cP-I-Aαβb-g7 1.44 ± 0.51) or percentages of islets that were insulitis-free (35 ± 9% vs. 41 ± 10% vs. 58 ± 15%). (D) DC expression of I-Aαβb or I-Aβb protected 4.1-TCR transgenic mice from diabetes (n = 25 for 4.1-NOD; n = 75 for 4.1-NOD.CD11cP-I-Aαβb; n = 15 for 4.1-NOD.CD11cP-I-Aβb). (E) DC expression of I-Aαβb-g7 protected 4.1-TCR transgenic mice from diabetes (n = 25 for 4.1-NOD; n = 24 for 4.1-NOD.CD11cP-I-Aαβb-g7). (F) Diabetes incidence of 4.1-TCR-transgenic, RAG-2−/− NOD (n = 18), NOD.K14P-I-Aβb (n = 41), NOD.CD11cP-I-Aαβb (n = 10), and NOD.CD11cP-I-Aαβb-g7 (n = 9) females. P values were calculated using Logrank test (A, B, and D–F). Statistical analysis in C was performed on averaged insulitis scores or percentages using Mann–Whitney U test.
Central Tolerance of 4.1-CD4+ Thymocytes by DC-Specific Expression of I-Ab.
We next compared the fate of the 4.1-thymocytes in 4.1-NOD, 4.1-NOD.CD11cP-I-Ab, and 4.1-NOD.CD11cP-I-Ab-g7 mice to ascertain if DC-specific expression of I-Ab and I-Ab-g7 triggers 4.1-thymocyte deletion.
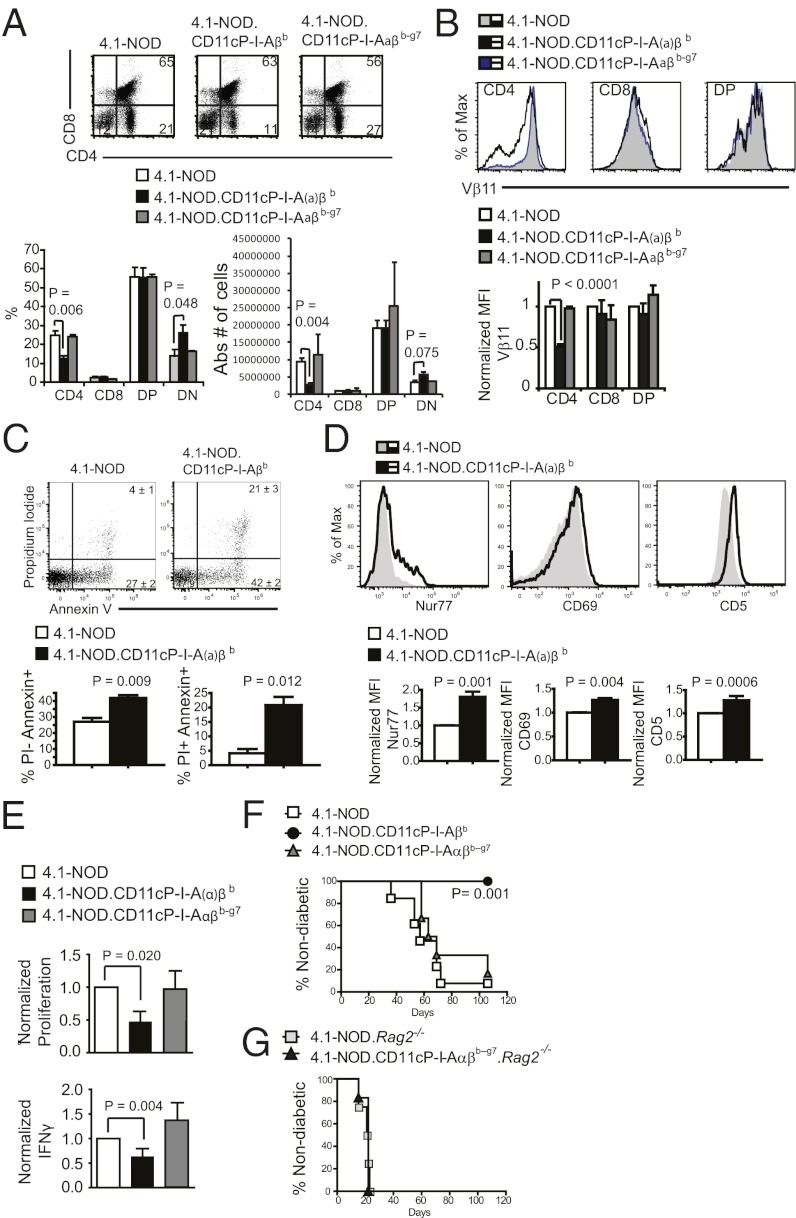
As was the case in H-2g7/b-congenic 4.1-NOD mice (15), DC-specific expression of I-Aαβb or I-Aβb in 4.1-NOD mice [herein collectively referred to as 4.1-NOD.CD11cP-I-A(α)βb mice] led to enhanced negative selection of 4.1-CD4+CD8– thymocytes, as demonstrated by an increase in the size of the CD4–CD8– thymocyte subset at the expense of the CD4+CD8– population in the thymic and, to a lesser extent, the splenic pools (Fig. 2A and Fig. S4A, respectively). This result was accompanied by other markers of negative selection (24–27): significant increases in the percentages of Annexin V+/propidium iodide– and Annexin V+/propidium iodide+ cells in the thymic CD4+CD8– subset (Fig. 2C), and up-regulation of Nur77, CD5 and CD69 (Fig. 2D) and down-regulation of the transgenic and total TCR levels (Fig. 2B, and Fig. S4 B–D) in CD4+CD8– thymocytes. Functionally, the splenic CD4+CD25– T-cells of 4.1-NOD.CD11cP- I-A(α)βb mice proliferated significantly less and secreted lower levels of IFN-γ when challenged with NOD islet cells (but not anti-CD3 mAb) than those isolated from 4.1-NOD mice (Fig. 2E). In addition, these cells could not transfer disease to NOD.scid hosts (Fig. 2F), indicating that DC-specific expression of I-Ab affords diabetes protection to 4.1-TCR transgenic mice, at least in part, by tolerizing 4.1-CD4+ T cells during their development.
Fig. 2.
Thymic deletion and down-regulation of the 4.1-TCR in 4.1-NOD mice expressing CD11cP-I-Aβb or CD11cP-I-Aαβb but not in 4.1-NOD mice expressing CD11cP-I-Aαβb-g7. (A) Representative flow cytometric profiles of 4.1-NOD (n = 16), 4.1-NOD.CD11cP-I-A(α)βb (n = 26), and 4.1-NOD.CD11cP-I-Aαβb-g7 (n = 7) thymocytes stained for CD4 and CD8 (Left) and percentages of CD4 (CD4), CD8 (CD8), CD4/CD8-double positive (DP), and CD4/CD8-double-negative (DN) cells (Right). Data are represented as mean ± SEM. (B) Representative flow cytometric profiles of 4.1-NOD (n = 11, 10), 4.1-NOD.CD11cP-I-A(α)βb (n = 24, 14), and 4.1-NOD.CD11cP-I-Aαβb-g7 (n = 6, 3) thymocytes stained for CD4, CD8, and the transgenic TCR-β (Vβ11), with the averaged mean fluorescence intensities (MFI) normalized to 4.1-NOD (Lower). Data are represented as mean ± SEM. (C) Annexin V and propidium iodide (PI) staining of 4.1-NOD (n = 4) vs. 4.1-NOD.CD11cP-I-A(α)βb (n = 5) thymocytes. Shown are representative flow cytometric profiles (Upper) and averaged percentages of PI+ AnnexinV+ and PI− AnnexinV+ thymocytes gated on the Vβ11hi CD4+CD8− population. (D) Nur77 (Left), CD69 (Center), and CD5 (Right) expression levels of 4.1-NOD (n = 4–5 mice) vs. 4.1-NOD.CD11cP-I-A(α)βb (n = 5–13 mice) thymocytes. Shown are representative flow cytometric profiles (Upper) and normalized MFI (Lower) of the respective markers on the Vβ11hi CD4+CD8– population. (E) Proliferation and IFN-γ secretion of CD4+CD25– T-cells from 4.1-NOD.CD11cP-I-A(α)βb and 4.1-NOD.CD11cP-I-Aαβb-g7 mice in response to stimulation with islet cells (n = 4). Data are normalized to values obtained from 4.1-NOD-derived CD4+CD25– T-cells, presented as mean ± SEM. (F) Diabetes incidence of NOD.scid females transferred with CD4+CD25– T-cells from indicated strains (6-wk-old; n = 8 and 6). (G) Diabetes incidence of NOD.scid females transferred with CD4+CD25– T cells from indicated RAG-2–deficient strains (6-wk-old; n = 4 and 6). P values were calculated using Mann–Whitney U test (A–E) and logrank test (F and G).
DC-Specific Expression of I-Ab-g7 Affords Diabetes Resistance to 4.1-CD4+ T-Cell–Induced Diabetes Without Inducing Deletional Tolerance or Anergy.
Surprisingly, 4.1-NOD.CD11cP-I-Ab-g7 mice did not exhibit any of these phenotypes (Fig. 2 A–E and Fig. S4), despite the fact that they displayed similar resistance to diabetes than 4.1-NOD.CD11P-I-Ab mice (Fig. 1 E and F). In fact, the splenic CD4+CD25– T-cells of both Rag2+ and Rag2– 4.1-NOD.CD11cP-I-Ab-g7 mice could transfer diabetes efficiently into NOD.scid hosts (Fig. 2 F and G). Because 4.1-NOD.CD11cP-I-Ab-g7.Rag2−/− mice are resistant to diabetes (Fig. 1F), we conclude that DC-specific expression of I-Ab-g7 affords diabetes resistance by a mechanism dissociated from central tolerance of 4.1-like CD4+ cells. That is, I-Aβ residues 56–67 are important for mediating central tolerance of 4.1-like CD4+ cells, but are dispensable for diabetes resistance.
I-Ab and I-Ab-g7 Promote 4.1-CD4+ Treg Development and Function.
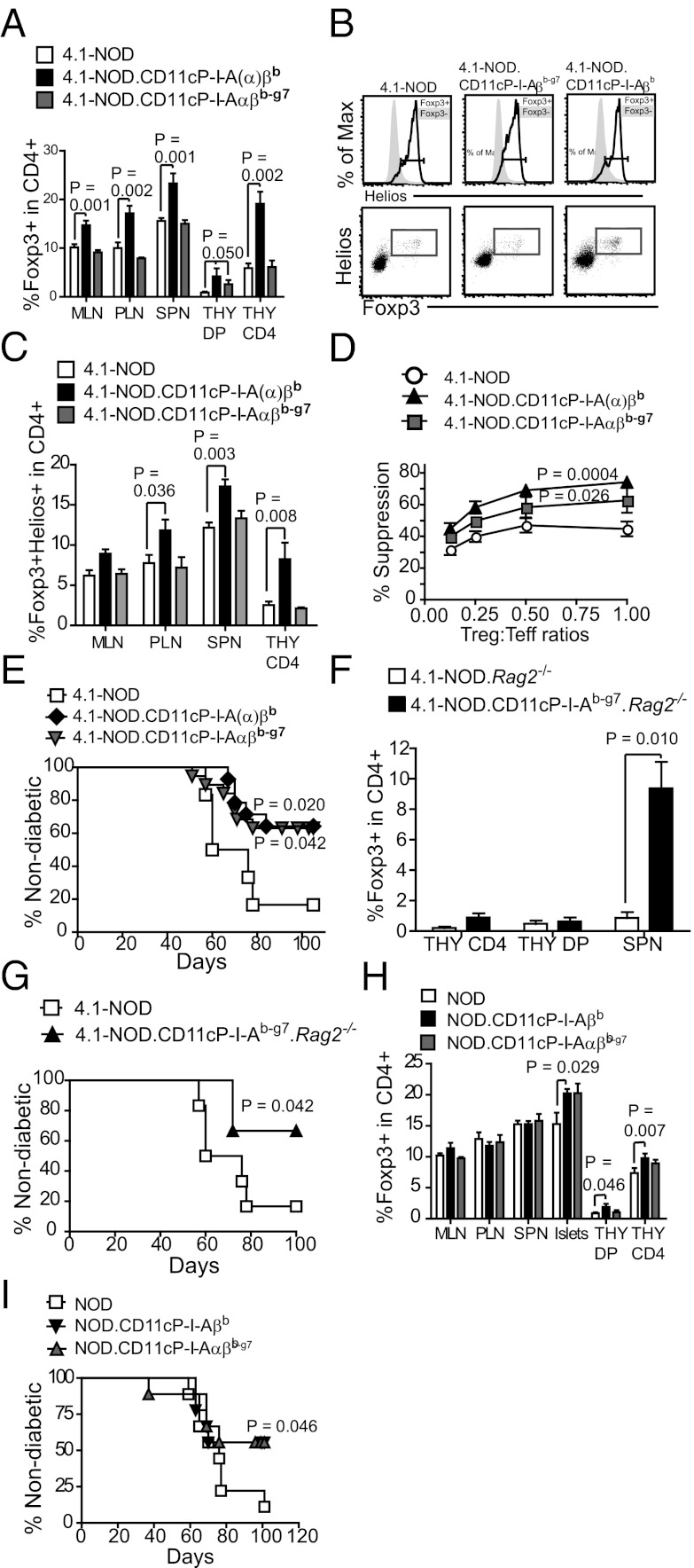
Because natural CD4+CD25+Foxp3+ Treg cells arising from the thymus represent the majority of Tregs found in the periphery and are crucial for the maintenance of self-tolerance (28), we considered the possibility that DC-specific expression of I-Ab and/or I-Ab-g7 might afford diabetes resistance by promoting their development or function. Flow cytometric analyses indicated that 4.1-NOD.CD11cP-I-Aβb and I-Aαβb mice did in fact harbor significantly higher percentages of CD4+Foxp3+ Tregs in the thymic CD4+CD8+ and CD4+CD8– pools and in the peripheral CD4+ T-cell subset of all lymphoid organs examined (Fig. 3A). Helios staining confirmed that these differences were largely attributable to thymic derived Foxp3+ Tregs (Fig. 3 B and C).
Fig. 3.
Enhanced Treg function in NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice, (A) Percentages of Foxp3+ cells within the CD4+ subset in the thymus (THY), spleen (SPN), PLN, and MLN of indicated strains (n = 4–13 each). Data are presented as mean ± SEM. (B) Representative flow cytometric profiles of NOD vs. NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 thymocytes, showing Helios staining on Foxp3+ vs. Foxp3− cells (Upper), or Foxp3 plotted against Helios (Lower) to show gating strategy for C, in the CD4 single-positive population. (C) Percentages of Helios+Foxp3+ cells within the CD4+ subset in the THY, SPN, PLN, and MLN of indicated strains (n = 3–7 each). Data are presented as mean ± SEM. (D) CD4+CD25+ Tregs from 4.1-NOD.CD11cP-I-A(α)βb and 4.1-NOD.CD11cP-I-Aαβb-g7 mice are more suppressive than 4.1-NOD Tregs in vitro. Purified CD4+CD25+ Tregs were activated with anti-CD3/IL-2 for 2 d and cocultured with 8.3-NOD CD8+ T-cells and NRP-A7–pulsed irradiated splenocytes. IFN-γ secretion was determined at 48 h by ELISA. Data are averages ± SEM of three separate experiments; n = 1–2 mice per strain type per experiment. (E) Diabetes incidence of NOD.scid females transferred at 5–7 wk of age with CD4+CD25+ Tregs from 4.1-NOD (n = 5), 4.1-NOD.CD11cP-I-A(α)βb (n = 13), or 4.1-NOD.CD11cP-I-Aαβb-g7 (n = 11) followed by an infusion of CD4+ and CD8+ T cells from prediabetic NOD females. (F) Percentages of Foxp3+CD4+ cells in the spleens of 4.1-NOD.Rag2−/− vs. 4.1-NOD.CD11cP-I-Aαβb-g7.Rag2−/− mice (solid column). Data are presented as mean ± SEM. (G) Adoptive transfer of freshly isolated CD4+CD25+ Tregs from 4.1-NOD (n = 6) or 4.1-NOD.CD11cP-I-Aαβb-g7.Rag2−/− mice (n = 4) into 6-wk-old NOD.scid females, followed by an infusion of splenic T-cells from prediabetic NOD females (7–9 wk old). (H) Percentages of Foxp3+ cells within the CD4+ or CD4+CD8+ populations in the THY, MLN, PLN, SPN, and islet infiltrates of NOD, NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice, as determined by intracellular Foxp3 staining and flow cytometry (n = 8–15 mice each). Data are presented as mean ± SEM. (I) CD4+CD25+ Tregs (5 × 105) from NOD (n = 18), NOD.CD11cP-I-A(α)βb (n = 4) or NOD.CD11cP-I-Aαβb-g7 (n = 5) mice were injected into 6-wk-old NOD.scid female mice, followed by an infusion of splenic T-cells (2 × 107) from prediabetic NOD females (7- to 9-wk-old). Diabetes was monitored for at least 100 d. P values were calculated using Mann–Whitney U test (A, C, F, H), two-way ANOVA (D), logrank test (E, G), and χ2 analysis (I).
This finding suggested that enhanced Treg development in mice expressing I-Ab on DCs might be a consequence of a high-avidity interaction between the autoreactive 4.1-TCR and peptide/I-Ab complexes on thymic DCs that concurrently leads to negative selection. Curiously, 4.1-NOD.CD11cP-I-Aαβb-g7 mice did not harbor increased percentages of Foxp3+ Treg cells (Fig. 3 A–C). To ascertain if DC-specific expression of I-Aαβb-g7 promoted Treg function without increasing the thymic Treg output, we compared the regulatory potential of the peripheral CD4+CD25+Foxp3+ Treg subset of 4.1-NOD, 4.1-NOD.CD11cP-I-A(α)βb and 4.1-NOD.CD11cP-I-Aαβb-g7 mice in in vitro suppression assays. Tregs from 4.1-NOD.CD11cP-I-A(α)βb or 4.1-NOD.CD11cP-I-Aαβb-g7 mice consistently demonstrated higher suppressive activity than Tregs from 4.1-NOD mice, over a range of Treg:Tresponder ratios (Fig. 3D). Furthermore, CD4+CD25+ T-cells from both 4.1-NOD.CD11cP-I-A(α)βb and 4.1-NOD.CD11cP-I-Aαβb-g7 mice had superior antidiabetogenic activity than those derived from 4.1-NOD donors in an adoptive transfer model of diabetes in NOD.scid hosts (Fig. 3E).
Given that both RAG2+ and RAG2– 4.1-NOD.CD11cP-I-Ab-g7 mice are equally resistant to T1D despite absence of 4.1-CD4+ T-cell tolerance (Figs. 1 and 2), we reasoned that engagement of the 4.1-TCR by I-Ab and I-Ab-g7 on thymic DCs might promote the differentiation of 4.1-CD4+CD8+ thymocytes into Tregs (I-Ab) or enhance the suppressive activity of 4.1-Tregs (I-Ab-g7). Additional support for this hypothesis came from two other observations: that CD4+CD25– T cells isolated from 4.1-NOD.CD11c-I-Ab-g7 mice are as capable of transferring diabetes into NOD.scid mice as those derived from 4.1-NOD donors (Fig. 2F), and that 4.1-NOD.CD11cP-I-Ab-g7.Rag2−/− mice are diabetes resistant despite the fact that their CD4+CD25– T cells possess strong diabetogenic activity (Figs. 1F and 2G). Indeed, 4.1-NOD.CD11c-I-Ab-g7.Rag2−/− mice contained significantly higher percentages of Foxp3+ Treg cells than 4.1-NOD.Rag2−/− mice (Fig. 3F), and these T-cells could protect NOD.scid mice from the transfer of diabetes by splenic T-cells from wild-type NOD mice (Fig. 3G). Thus, I-Ab-g7 can directly promote the development of 4.1-CD4+Foxp3+ Tregs.
I-Ab and I-Ab-g7 Promote the Development and Function of Antidiabetogenic Tregs in non-TCR Transgenic NOD Mice.
If protective MHC class II molecules afford diabetes resistance by promoting the development of autoreactive Tregs from T-cell precursors expressing pathogenic, MHC-promiscuous autoreactive TCRs, DC-specific expression of I-Ab and I-Ab-g7 should also promote the Treg development and function in non-TCR transgenic NOD mice, albeit from a much smaller pool of T-cell precursors. NOD.CD11cP-I-A(α)βb mice displayed significant increases in the frequency of CD4+Foxp3+ Tregs within the CD4+CD8+ and CD4+CD8– thymocyte subsets (Fig. 3H), albeit not in the peripheral T-cell subsets other than the islet-infiltrating one (enriched for autoreactive clonotypes), presumably because of the significantly reduced frequency of 4.1-like TCRs in non-TCR transgenic mice. Furthermore, Tregs purified from the pooled pancreatic-draining lymph nodes (PLNs) (but not spleen) of both NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice exhibited different TCR use than those isolated from the PLNs of NOD mice, as determined using a V family-specific nested-PCR approach (29) (Fig. S5 A and B). These separate lines of evidence suggested that the Foxp3+CD4+ Treg subset developing in non-TCR transgenic NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice is also enriched for autoreactive Treg cell specificities, leading to enhanced recruitment and retention in their PLNs. To substantiate this further, we compared the accumulation of splenic carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled Tregs from NOD.CD11cP-I-Aβb and NOD donors (labeled with low and high CFSE concentrations, respectively, and pooled at a 1:1 ratio) into the mesenteric lymph nodes (MLNs) and PLNs of wild-type NOD hosts. As shown in Fig. S5C, NOD.CD11cP-I-Aβb-derived Tregs accumulated in the PLNs more efficiently than NOD-derived Tregs, supporting the idea that I-Ab expression promotes the development of islet-antigen autoreactive Tregs but is dispensable for enhanced recruitment of these T cells into the PLNs.
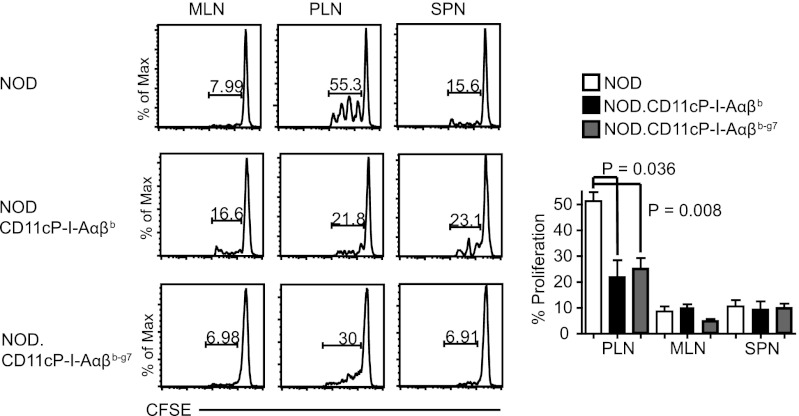
Furthermore, in vivo experiments showed that Tregs from NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice were significantly more suppressive in the NOD.scid diabetes transfer model than Tregs isolated from NOD mice (Fig. 3I). In agreement with this, the PLNs of both NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice were significantly less able to support the proliferation of CFSE-labeled 8.3-CD8+ T cells than the PLNs of NOD mice (Fig. 4). Thus, DC-specific expression of I-Ab and I-Ab-g7 promote the development of antidiabetogenic Tregs.
Fig. 4.
Enhanced Treg function in NOD.CD11cP-I-A(α)βb and NOD.CD11cP-I-Aαβb-g7 mice correspond with decreased antigen-induced proliferation of diabetogenic T-cells in the PLNs. In vivo proliferation of 8.3-CD8+ T-cells in the PLN and MLN of NOD (n = 5) vs. NOD.CD11cP-I-Aβb (n = 3) and NOD.CD11cP-I-Aαβb-g7 (n = 5) mice. 8.3-NOD splenic CD8+ T-cells (5 × 106) were labeled with CFSE (0.5 μM) and injected into respective hosts. SPNs, PLNs, and MLNs were analyzed for the dilution of CFSE via flow cytometry 7 d later. Shown are representative flow cytometric profiles (Left) and averaged percentages of CFSE dilution ± SEM (Right). P values were calculated using Mann–Whitney U test.
Discussion
Here, we have addressed two separate issues relevant to I-Ab–mediated diabetes protection. We asked whether thymic cortical epithelial cells or DCs are responsible for mediating the T1D-protective effects of I-Ab. We followed the fate of β-cell–autoreactive 4.1-CD4+ T-cells in transgenic mice expressing I-Ab either on thymic cortical epithelial cells or on DCs. T1D incidence data and phenotypic/functional characterization of the I-Ab transgenic 4.1-NOD strains conclusively demontrated that DCs, but not thymic cortical epithelial cells, are required for I-Ab-mediated T1D protection. To assess the contribution of the I-Aβb polymorphic residues around position 57 to T-cell tolerance and diabetes protection, we produced transgenic NOD mice expressing, in DCs, I-Ab (I-Aαβb) or a chimeric I-Ab molecule carrying residues 56–67 of I-Aβg7 (referred to as I-Aαβb-g7 or I-Ab-g7). We found that one of the major 4.1-CD4+ T-cell–tolerogenic effects of I-Ab, that of central deletion, was completely absent in mice expressing I-Ab-g7, indicating that this effect requires residues 56–67 of I-Aβb. On the other hand, mice expressing I-Ab-g7 remained resistant to T1D, indicating that these residues are dispensable for T1D resistance, and that I-Ab's anti-T1D effect cannot be explained only by its ability to induce 4.1-like T-cell deletion. Additional work showed that the 4.1-tolerogenic activity of the I-Ab transgene was associated with significant increases in the percentage of the thymic and peripheral 4.1-CD4+Foxp3+ subsets, suggesting that I-Ab induces down-regulation of the 4.1-TCR and Treg differentiation simultaneously. This result was not seen in mice expressing I-Ab-g7, indicating that such increase in Treg pool size was likely induced by a β56–67-dependent, high-avidity TCR-pMHC interaction associated with deletion. Additional studies in Rag2−/− 4.1-NOD.CD11cP-I-Aαβb-g7 mice confirmed that, in the absence of endogenous TCRs, DC-specific expression of I-Aαβb-g7 remains capable of promoting the differentiation of 4.1-thymocytes into Foxp3+ Tregs. Furthermore, CD4+CD25+ splenocytes from both TCR transgenic and non-TCR transgenic mice expressing either I-Ab or I-Ab-g7 had superior antidiabetogenic activity than those derived from their non–I-A transgenic counterparts. Although a control CD11c promoter-driven I-Ag7 transgenic mouse was not made (it would not have been possible to differentiate transgenic from endogenous I-Ag7, hence assess levels of transgene expression), the outcome of these experiments cannot be accounted for by systemic I-Ag7 overexpression. Unlike the case in NOD mice expressing high levels of an I-Ag7 transgene in all professional APC types, which displayed significant reductions in the percentages of B-cells and a reduced incidence of diabetes (23), the transgenic mice described herein express normal levels of total I-Ag7 and relatively low levels of the transgenes only on CD11c+ cells, and have normal numbers of B-cells. Nevertheless, we cannot completely rule out effects of transgene expression on some of the read-outs.
Collectively, these data demonstrate that DCs play a central role in the antidiabetogenic activity of at least certain protective class II alleles; that these molecules afford T1D resistance by promoting the deletion of MHC-promiscuous autoreactive thymocytes and their differentiation into autoregulatory T-cells; and that residues around β-chain position 57 control the magnitude of this process, presumably by modifying the avidity of the TCR–MHC interaction (Fig. S6). Because the MHC promiscuity of the 4.1-TCR is peptide-dependent (16) and residues 57, 61, and 67 in I-Ag7 interact with bound peptides (30), the I-Ab-g7 replacement likely resulted in a conformational change in I-Ab capable of altering the repertoire of bound peptides and the affinity with which it is engaged by the 4.1-TCR. Additionally, these results demonstrate that β-chain polymorphisms residing outside of positions 56–67, such as residue 26, lying in the P4 pocket (31), also play a role in the antidiabetogenic activity of protective MHC class II molecules.
Whatever the structural underpinnings, these results support the hypothesis that MHC-promiscuous autoreactive TCRs like the 4.1-TCR play a key role in the development of T1D, and that antidiabetogenic MHC class II polymorphisms harness these TCRs intrinsic MHC-promiscuity to generate autoregulatory T cells. We note that the MHC-promiscuous nature (i.e., the ability to recognize different MHC molecules) of the 4.1-TCR has also been documented for diabetogenic MHC class I-restricted TCRs (32) and a human encephalitogenic TCR (33). Although we cannot exclude the possibility that protective MHC class II molecules select for Tregs that afford increased diabetes protection by competing with effectors of the same antigenic specificity, we think that this phenomenon linking MHC promiscuity with pathogenic autoreactivity (15) is more related to how pathogenic autoreactive TCRs engage cognate pMHC than to the nature of their autoantigenic targets. Several autoreactive TCRs have been noted to bind cognate pMHCs with a different topology than foreign antigen-specific TCRs, or to recognize peptides that only partially occupy the MHC peptide-binding groove (34, 35). Polymorphic MHC residues on protective MHC class II may compensate for the paucity of molecular contacts between TCR and peptide and disease-promoting MHC. This interpretation would help explain how a structurally diverse repertoire of class II molecules, binding a diverse array of peptides, can afford dominant resistance to an autoimmune disease, the development of which requires expression of a completely different class II molecule, I-Ag7, and which is effected by a diverse repertoire of autoreactive T-cell specificities. Furthermore, this hypothesis accords with the fact that the MHC promiscuity of the 4.1-TCR and the DQB1-linked resistance to murine and human T1D both map to residues around β-chain position 57 (5, 6, 17).
Materials and Methods
Mice.
C57BL/6, NOD/LtJ, NOD.scid, and NOD.I-Aβb−/− mice were from the Jackson Laboratory. RAG2+ and RAG2– 4.1-NOD mice have been described previously (14, 15). I-Aαb and I-Aβb or I-Aβb-g7 cDNAs were cloned downstream of the CD11c promoter and coinjected into NOD or 129 oocytes, to produce transgenic mice, which were then backcrossed to NOD.Ltj mice at least 10 times. B6.K14P-I-Aβb and B6.CD11cP-I-Aβb transgenic mice (a gift from T. Laufer, University of Pennsylvania, Philadelphia, PA) were backcrossed to NOD mice for at least 10 generations.
Peptides.
The peptides NRP-A7 (KYNKANAFL) (36), and 2.5mimetope (37) were purchased from Mimotopes Pty Ltd.
Flow Cytometry.
Cell suspensions were stained with Abs or streptavidin diluted 1:100 in FACS buffer [PBS containing 1% (vol/vol) FBS and 0.05% NaN3], washed, fixed in 1% (vol/vol) PFA in PBS and analyzed by flow cytometry. Intracellular staining of Foxp3, Helios, and Nur77 were performed using the intracellular staining buffer kit from eBioscience. AnnexinV/Propidium iodide staining was performed according to the manufacturer’s recommendations.
In Vivo CFSE Dilution.
Pure CFSE-labeled T-cells (5 × 106) were injected intravenously into NOD or transgenic hosts, and spleens, MLNs, and PLNs were collected for flow cytometry at day 7 postinjection.
In Vivo Treg Recruitment.
CFSE-stained NOD (CFSEhigh) and NOD.CD11cP-I-Aβb or NOD.CD11cP-I-Aβb-g7 (CFSElow) splenic Tregs were injected into NOD hosts and their localization quantified by flow cytometry 4 d postinjection.
Functional in Vitro T-Cell Assays.
The 8.3-CD8+ cells (2 × 104) or BDC2.5-CD4+ cells (4 × 104) were cocultured with NRP-A7 or 2.5mimetope peptide-pulsed DCs (104), respectively. 4.1-CD4+ T-cells (4 × 104) were cocultured with dissociated islet cells (2 × 105, containing autoantigen-loaded APCs). The cultures were examined for thymidine incorporation and cytokine secretion. For polyclonal T-cell stimulation, we used anti-CD3/CD28 beads.
In Vitro Treg Suppression Assay.
CD4+CD25+ or CD4+CD25– T-cells were activated with plate-bound anti-CD3 mAb (3 μg/mL) and IL-2 (18 ng/mL) and cocultured at different ratios with responder 8.3-CD8+ T-cells (2 × 104) and γ-irradiated NOD splenocytes (105) in the presence of 1 μg/mL NRP-A7 for 48 h. Culture supernatants were collected for IFN-γ determination by ELISA.
Analysis of Treg TCR-α and TCR-β Repertoire by PCR.
CD4+CD25+ Tregs and CD4+CD25– T-cells were isolated from splenocytes and PLN cells by FACS and used as a source of mRNA using the RNeasy minikit (Qiagen). cDNA was synthesized by oligo dT-primed reverse transcription using M-MLV RT (Invitrogen) followed by two rounds of PCR amplication using TCR-Cα or -Cβ and Vα- and Vβ-chain–specific primers, as previously described (29).
In Vivo Treg Suppression Assay.
CD4+CD25+ Tregs (2 × 105) were injected intravenously into 5–6 wk-old NOD.scid females. At 24 h post-Treg infusion, the hosts were transfused with purified CD4+ and CD8+ T-cells (2 × 107) isolated from the spleens of prediabetic 7–9-wk old NOD female mice. Mice were monitored weekly for development of glycosuria for at least 100 d.
Insulitis Scoring.
Scoring of insulitic lesions was performed as previously described (38).
Supplementary Material
Acknowledgments
We thank J. Luces and R. Barasi for technical assistance; L. Kennedy and L. Robertson for flow cytometry; P. Dickie for transgene microinjection into NOD oocytes; T. Laufer for K14-I-Aβb and CD11c-I-Aβb B6 mice; and J. McKean for providing anti-IAb hybridomas. This work was funded by grants from the Canadian Institutes of Health Research, the Ramón y Cajal Reintegration Program, and Ministerio de Economia y Competitividad of Spain; a studentship from Alberta Innovates–Health Solutions (S. Tsai); and a studentship from the AXA Research Fund (X.C.-C.). P. Serra is an investigator of the Ramón y Cajal program. P. Santamaria is a Scientist of the Alberta Innovates–Health Solutions and scholar of the Juvenile Diabetes Research Foundation and Instituto de Investigaciones Sanitarias Carlos III. The Julia McFarlane Diabetes Research Centre was supported by the Diabetes Association (Foothills) and the Canadian Diabetes Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211391110/-/DCSupplemental.
References
- 1.Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: Theoretical and practical concerns. Nat Rev Genet. 2005;6(2):109–118. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
- 2.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 3.Nejentsev S, et al. Wellcome Trust Case Control Consortium Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450(7171):887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howson JM, Walker NM, Clayton D, Todd JA. Type 1 Diabetes Genetics Consortium Confirmation of HLA class II independent type 1 diabetes associations in the major histocompatibility complex including HLA-B and HLA-A. Diabetes Obes Metab. 2009;11(Suppl 1):31–45. doi: 10.1111/j.1463-1326.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85(3):291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 6.Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85(3):311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Shin CH, Yang SW, Park MH, Eisenbarth GS. Analysis of children with type 1 diabetes in Korea: High prevalence of specific anti-islet autoantibodies, immunogenetic similarities to Western populations with “unique” haplotypes, and lack of discrimination by aspartic acid at position 57 of DQB. Clin Immunol. 2004;113(3):318–325. doi: 10.1016/j.clim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 9.Böhme J, Schuhbaur B, Kanagawa O, Benoist C, Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990;249(4966):293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- 10.Slattery RM, et al. Prevention of diabetes in non-obese diabetic I-Ak transgenic mice. Nature. 1990;345(6277):724–726. doi: 10.1038/345724a0. [DOI] [PubMed] [Google Scholar]
- 11.Wicker LS, et al. Autoimmune syndromes in major histocompatibility complex (MHC) congenic strains of nonobese diabetic (NOD) mice. The NOD MHC is dominant for insulitis and cyclophosphamide-induced diabetes. J Exp Med. 1992;176(1):67–77. doi: 10.1084/jem.176.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer SM, Tisch R, Yang XD, McDevitt HO. An Abd transgene prevents diabetes in nonobese diabetic mice by inducing regulatory T cells. Proc Natl Acad Sci USA. 1993;90(20):9566–9570. doi: 10.1073/pnas.90.20.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parish NM, Chandler P, Quartey-Papafio R, Simpson E, Cooke A. The effect of bone marrow and thymus chimerism between non-obese diabetic (NOD) and NOD-E transgenic mice, on the expression and prevention of diabetes. Eur J Immunol. 1993;23(10):2667–2675. doi: 10.1002/eji.1830231042. [DOI] [PubMed] [Google Scholar]
- 14.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186(10):1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the major histocompatibility complex-linked resistance to autoimmunity. J Exp Med. 1997;186(7):1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt D, Amrani A, Verdaguer J, Bou S, Santamaria P. Autoantigen-independent deletion of diabetogenic CD4+ thymocytes by protective MHC class II molecules. J Immunol. 1999;162(8):4627–4636. [PubMed] [Google Scholar]
- 17.Thiessen S, Serra P, Amrani A, Verdaguer J, Santamaria P. T-cell tolerance by dendritic cells and macrophages as a mechanism for the major histocompatibility complex-linked resistance to autoimmune diabetes. Diabetes. 2002;51(2):325–338. doi: 10.2337/diabetes.51.2.325. [DOI] [PubMed] [Google Scholar]
- 18.Kanagawa O, Vaupel BA, Xu G, Unanue ER, Katz JD. Thymic positive selection and peripheral activation of islet antigen-specific T cells: Separation of two diabetogenic steps by an I-A(g7) class II MHC beta-chain mutant. J Immunol. 1998;161(9):4489–4492. [PubMed] [Google Scholar]
- 19.Lühder F, Katz J, Benoist C, Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J Exp Med. 1998;187(3):379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383(6595):81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 21.Lemos MP, Esquivel F, Scott P, Laufer TM. MHC class II expression restricted to CD8alpha+ and CD11b+ dendritic cells is sufficient for control of Leishmania major. J Exp Med. 2004;199(5):725–730. doi: 10.1084/jem.20030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck BN, et al. Characterization of cell lines expressing mutant I-Ab and I-Ak molecules allows the definition of distinct serologic epitopes on A alpha and A beta polypeptides. J Immunol. 1986;136(8):2953–2961. [PubMed] [Google Scholar]
- 23.Wherrett DK, Singer SM, McDevitt HO. Reduction in diabetes incidence in an I-Ag7 transgenic nonobese diabetic mouse line. Diabetes. 1997;46(12):1970–1974. doi: 10.2337/diab.46.12.1970. [DOI] [PubMed] [Google Scholar]
- 24.Calnan BJ, Szychowski S, Chan FK, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3(3):273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, et al. The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J Immunol. 2002;168(1):87–94. doi: 10.4049/jimmunol.168.1.87. [DOI] [PubMed] [Google Scholar]
- 26.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T, et al. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183(4):1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Dash P, et al. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J Clin Invest. 2011;121(1):288–295. doi: 10.1172/JCI44752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latek RR, et al. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12(6):699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 31.Jones EY, Fugger L, Strominger JL, Siebold C. MHC class II proteins and disease: A structural perspective. Nat Rev Immunol. 2006;6(4):271–282. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- 32.Serreze DV, et al. MHC class II molecules play a role in the selection of autoreactive class I-restricted CD8 T cells that are essential contributors to type 1 diabetes development in nonobese diabetic mice. J Immunol. 2004;172(2):871–879. doi: 10.4049/jimmunol.172.2.871. [DOI] [PubMed] [Google Scholar]
- 33.Friese MA, et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat Med. 2008;14(11):1227–1235. doi: 10.1038/nm.1881. [DOI] [PubMed] [Google Scholar]
- 34.Wucherpfennig KW, Call MJ, Deng L, Mariuzza R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr Opin Immunol. 2009;21(6):590–595. doi: 10.1016/j.coi.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity. 2010;32(4):446–456. doi: 10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8(+) T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci USA. 1999;96(16):9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stratmann T, et al. Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest. 2003;112(6):902–914. doi: 10.1172/JCI18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdaguer J, et al. Acceleration of spontaneous diabetes in TCR-beta-transgenic nonobese diabetic mice by beta-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-alpha chains. J Immunol. 1996;157(10):4726–4735. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.