Significance
A popular hypothesis to explain dosage compensation of the X chromosome in male Drosophila is that a histone acetylase is brought to the chromosome by the MSL complex and increases H4 lysine16 acetylation, which mediates the increased expression. We investigated the properties of the MSL complex with a series of specific gene-targeting and global gene-expression experiments. The data indicate that the MSL complex does not mediate dosage compensation directly, but rather, its activity overrides the high level of histone acetylation and counteracts the potential overexpression of X-linked genes to achieve the proper twofold up-regulation in males.
Abstract
Dosage compensation is achieved in male Drosophila by a twofold up-regulation of the single X chromosome to reach the level of the two X chromosomes in females. A popular hypothesis to explain this phenomenon is that the male-specific lethal (MSL) complex, which is present at high levels on the male X, mediates this modulation of gene expression. One member of the complex, MOF, a histone acetyltransferase, acetylates lysine 16 of histone H4 and another, MSL2, which is only expressed in males, triggers its assembly. Here, we find that when a GAL4-MOF fusion protein is targeted to an upstream-activating sequence linked to a miniwhite reporter, up-regulation occurs in females but down-regulation in males, even though in the latter the whole MSL complex is recruited to the reporter genes and produces an increased histone acetylation. The expression of a GAL4-MSL2 fusion protein does not cause dosage compensation of X and autosomal reporters in females, although its expression causes the organization of the MSL complex on the reporter genes, leading to increased histone acetylation. RNAseq analysis of global endogenous gene expression in females with ectopic expression of MSL2 to coat the X chromosomes shows no evidence of increased expression compared with normal females. These data from multiple approaches indicate that the MSL complex does not mediate dosage compensation directly, but rather its activity overrides the high level of histone acetylation and counteracts the potential overexpression of X-linked genes to achieve the proper twofold up-regulation in males.
Dosage compensation is achieved in male Drosophila by an approximate twofold up-regulation of the X chromosome to equal the two X chromosomes in females (1, 2). For several decades, it has been proposed that the components of the male-specific lethal (MSL) complex are present on the male X chromosome and produce the twofold modulation (3, 4). One member of the complex, MOF, a histone acetyltransferase, acetylates lysine 16 of histone H4 (5) and another, MSL2, which is male-specific, triggers its X chromosome assembly (6). The MSL2 protein is not expressed in females because it is blocked at translation by the female-specific sex-lethal (SXL) protein (7). Gene-expression data taken in support of the MSL hypothesis have normalized X expression to autosomal expression and found a reduced X/A ratio that was interpreted as a loss of compensation when the MSL complex was dissociated (3, 8–10). However, when gene expression is assayed phenotypically or in absolute terms, rather than as a ratio of X to autosomal expression, data from ectopic assembly of the complex in females failed to demonstrate up-regulation of the X chromosomes (11, 12) and the dissolution of the complex does not eliminate compensation (11–15). Many assayed autosomal genes were increased in expression in the maleless (mle) mutant males (11, 13, 14), providing an explanation for the reduced ratios when X values were normalized to the autosomes.
The retention of dosage compensation and elevated autosomal expression conforms to the prediction that the X monosomic state triggers an inverse dosage effect that is commonly found in aneuploids (16–23). We hypothesize that this general reaction to genomic imbalance appears to have been modified and selected for the process of dosage compensation, which could account for the twofold up-regulation in males. However, because in normal males the autosomes are similarly expressed to those of females, it was hypothesized that the MSL complex present only in males sequesters MOF from the autosomes to mute any autosomal inverse effect, and counteracts the action of the resulting very high levels of histone acetylation on the X to allow the proper twofold modulation for compensation (11, 14). To examine further the function of the MSL complex, MOF and MSL2 were targeted to reporter genes to determine their impact on gene expression and dosage compensation and a global study of gene expression was conducted in females with ectopic MSL complex on their X chromosomes.
Results
Targeting MOF to a Reporter Increases Gene Expression.
For the targeting experiments, we used transgenic flies with the GAL4-DBD (DNA binding domain) (24) fused with the protein of interest from the MSL complex (Fig. 1A). As a reporter gene, miniwhite was used with the upstream activating sequence (UAS) of GAL4 inserted 5′. Multiple insertions were recovered from transformation on the X chromosome (designated M30, M4, M54, and M76) and the autosomes (M9, M1, M5, M7, and M14). The activities of the fusion proteins were tested and confirmed to rescue loss-of-function mutations in the respective genes (Fig. S1).
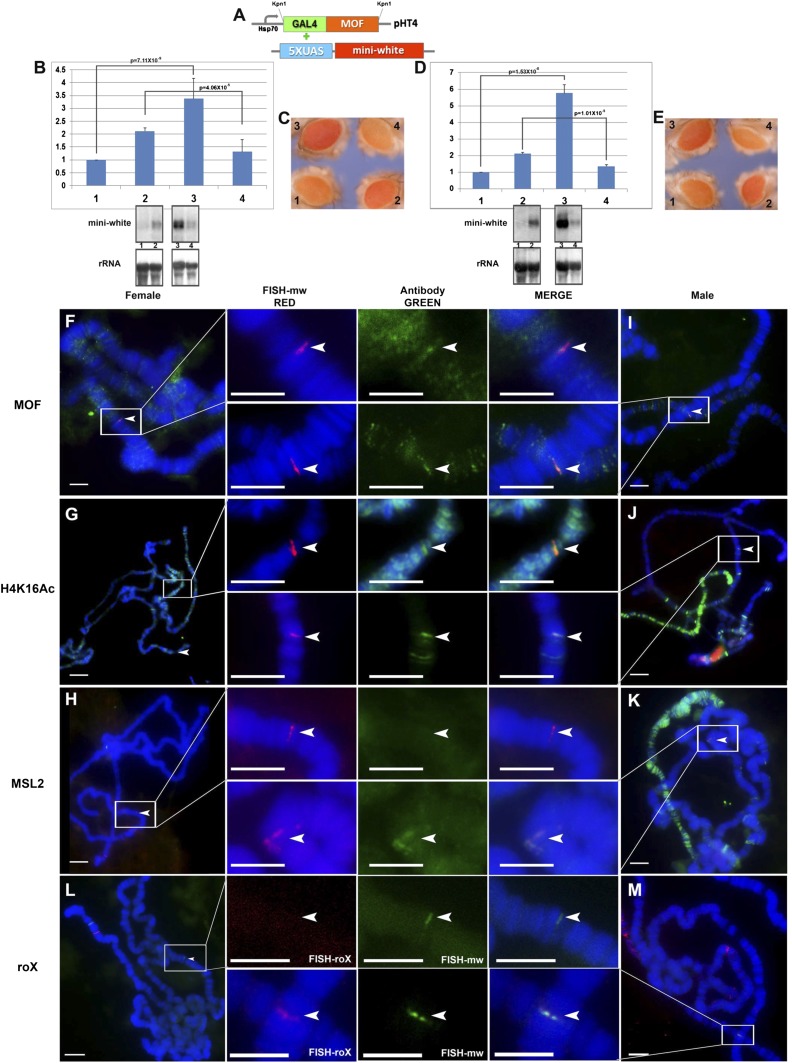
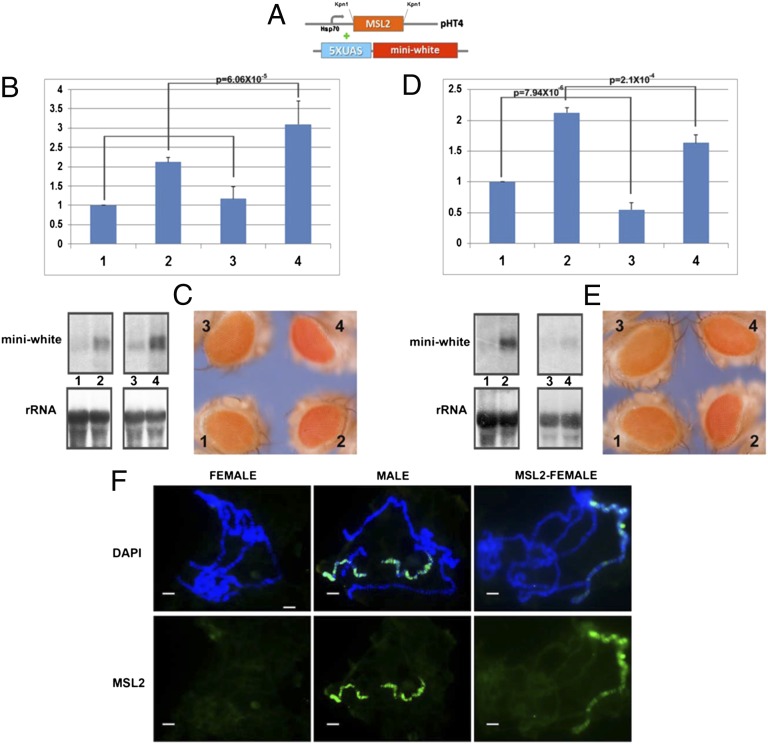
Fig. 1.
GAL4-MOF fusion protein combined with reporter genes. (A) Constructs used in this study. GAL4-DBD fused with the MOF gene inserted in the expression plasmid pHT4 with the Hsp70 promoter; the 5× UAS sequences are followed by the miniwhite reporter. (B and C) Expression levels of the X-linked reporter gene, M30, with GAL4-MOF fusion protein. Samples: 1, M30 female (n = 6); 2, M30 male (n = 6); 3, M30+GAL4-MOF(2 copies) female (n = 4); 4, M30+GAL4-MOF(2) male (n = 4). Two copies of GAL4-MOF are present. n, number of replicates for each sample. Error: SD of the independent replicates. A t test is applied to each comparison and P values are shown. Normal female levels are set at 1.0. (B) Target gene (miniwhite), the upper bands are from P32-labeled white probe and endogenous control gene (rRNA) from Northern blot. (C) Comparisons of eye colors (miniwhite) from the individual reporter stocks (M30) and the combined stocks including GAL4-MOF fusion protein and M30. (D and E) The expression levels of the autosomal reporter gene, M9, with GAL4-MOF fusion protein. Samples: 1, M9 female (n = 5); 2, M9 male (n = 5); 3, M9+GAL4-MOF(2 copies) female (n = 4); 4, M9+GAL4-MOF(2) male (n = 4). Two copies of GAL4-MOF are present. n, number of replicates. Error: SD of independent replicates. A t test is applied to each comparison and P value is shown. (D) Target gene (miniwhite), upper bands are from P32-labeled white probe and endogenous control gene (rRNA) from Northern blot. (E) Comparisons of eye colors (miniwhite reporter) from the individual reporter stocks (M9) and the combined stocks with GAL4-MOF fusion protein and M9. (F–K) Immunolocalization and FISH of polytene chromosomes from third instar larvae of females (F–H) and males (I–K) in the M9 reporter. The red channel (arrowhead) is the signal from FISH; the green channel (arrowhead) is the signal from antibody. (F and I) Signals of miniwhite (red) from FISH and protein MOF (green) from immunolocalization. (G–J) Signals of miniwhite (red) from FISH and protein H4K16Ac (green) from antibody. Females have H4K16Ac on all chromosomes. (H–K) Signals of miniwhite (red) from FISH and protein MSL2 (green) from antibody. (L and M) Cytological RNA FISH signals of the roX RNA (red signals) and miniwhite (green signals) in females (L) and males (M). Heterozygous reporters (half bands) were used to distinguish them from potential endogenous signals. (Scale bars, 10 μm.)
First, GAL4-MOF was combined with the reporters as MOF is a histone acetyltransferase that specifically acetylates histone H4 at lysine 16 (5). We assumed that targeting GAL4-MOF to the reporters would cause an up-regulation in transgenic flies because H4Lys16Ac is typically a mark of activated chromatin (25). By analyzing the RNA level via Northern blot (Fig. 1B), we found the transcripts of the reporter were significantly increased in females ∼3.5-fold with two copies of the GAL4-MOF transgene compared with normal females. However, a reduced expression of the reporter gene was observed in males. Because we used miniwhite as our reporter gene in a background of a white deletion, the phenotypic differences of eye pigments confirmed this effect (Fig. 1C). The use of a phenotypic validation confirms the molecular results on an absolute level rather than relative measurement, which has obscured previous studies, as noted above. To test the generality of this response, we combined all of the other heterozygous X-linked reporters with one copy of the GAL4-MOF transgene (Fig. S2) and with additional GAL4-MOF copy numbers (Fig. S3). Collectively, the results confirm that the GAL4-MOF construct induces a higher expression of X-linked reporters in females, and a decrease in males. Furthermore, these phenotypic changes become stronger with the increase of the dose of GAL4-MOF (Fig. S3). Using two copies of the transgene, GAL4-MOF was also targeted to an autosomal reporter, M9 (Fig. 1 D and E, and Fig. S4) and others (Fig. S4). Based on the Northern blot analysis (Fig. 1D) and the comparison of eye colors (Fig. 1E), these transgenes also exhibited an up-regulation in females, in this case almost sixfold, and a reduction in males. Because females and males differ by the absence or presence of the MSL complex, respectively (6), we presumed that the presence of the complete complex in males produced the repressive response. To test this hypothesis, we performed immunolocalizations for various MSL complex components on the lines with GAL4-MOF constructs combined with M9 (Fig. 1 F–K). The protein MOF (Fig. 1F) was cytologically detected on the reporter genes, as well as enriched histone 4 lysine 16 acetylation in both sexes (Fig. 1 G and J), confirming the activity of MOF on the reporters. Indeed, the MSL2 protein was colocalized at the reporter in males (Fig. 1K) and not in females (Fig. 1H). The FISH signals from the noncoding roX RNAs also colocalized with MSL2 in males (Fig. 1M). These results indicate that targeting MOF brings the whole MSL complex to reporters in males. Targeting a GAL4-mof1 fusion, which contains a MOF mutant protein with only 10% activity as normal (26), produces less increase in females but still a reduction of expression in males (Fig. S5).
The above results demonstrate that the MSL complex is organized at the positions of the reporter genes in males by the targeting of the GAL4-MOF fusion protein. Together with the reporter expression studies, the data suggest that the effect of the accumulated MOF and enriched H4Lys16Ac is counteracted by other components of the MSL complex.
Targeting of MSL2.
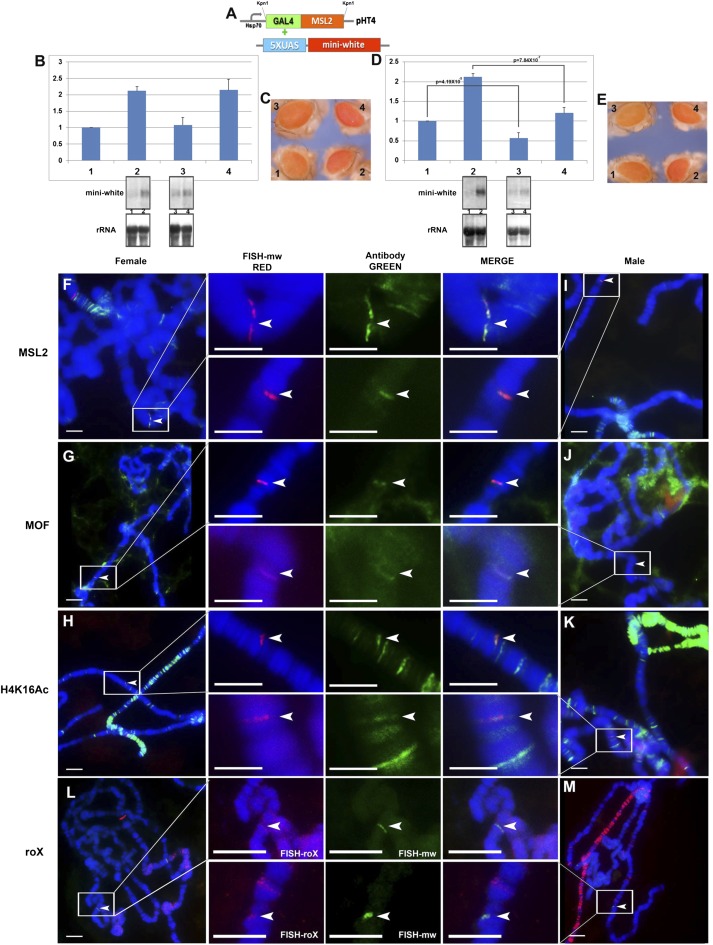
The key question for different explanations of dosage compensation is whether the MSL complex mediates dosage compensation directly. To explore this function of the MSL complex, we combined a GAL4-MSL2 construct with the same reporters (Fig. 2A). This construct is engineered to allow expression in females and, therefore, when this transgene is present, the MSL complex is organized on the female X chromosomes. If the MSL complex were the direct inducer of dosage compensation, the reporter in females with ectopically expressed GAL4-MSL2 protein should be up-regulated to the level of normal males. However, the transcript levels of the reporters M30 (Fig. 2B) and M9 (Fig. 2D), were not increased in GAL4-MSL2 females compared with normal females, and in fact for M9 the levels were significantly reduced. To fulfill the criterion of dosage compensation, one copy of the targeted reporter in females should be equivalent to the normal males, which was not the case (Fig. 2 C and E). These results were generalized to other X (Fig. S2) and autosomal reporters (Fig. S4). The immunolocalizations of various MSL complex components were compared between females and males (Fig. 2 F and K). We found MSL2 (Fig. 2 F and I), MOF (Fig. 2 G and J), histone modification H4K16Ac (Fig. 2 H and K), and the roX RNAs (Fig. 2 I and M) were all colocalized at the loci of the reporters in females and males, illustrating that the targeting brings the whole complex to the reporters and is functional. These results demonstrate that despite the fact that the complete functional MSL complex was organized on the reporters, no dosage compensation was induced.
Fig. 2.
GAL4-MSL2 fusion protein combined with reporter genes. (A) Constructs used in GAL4-UAS system. GAL4-DBD fused with MSL2 protein is inserted in the expression plasmid pHT4 with the Hsp70 promoter; the 5× UAS sequences is followed by the miniwhite reporter gene. (B and C) Expression levels of the X-linked reporter gene, M30, with GAL4-MSL2 fusion protein. Samples: 1, M30 female (n = 6); 2, M30 male (n = 6); 3, M30+GAL4-MSL2(1 copy) female (n = 4); 4, M30+GAL4-MSL2(1) male (n = 4). One copy of GAL4-MSL2 is present. n, number of replicates for each sample. Error: SD of independent replicates. A t test is applied to each comparison and P value is shown. Normal female levels are set at 1.0. (B) Target gene (miniwhite), the upper bands are from P32-labeled white probe and endogenous control gene (rRNA) from Northern blot. (C) Comparisons of eye colors (miniwhite) from the individual reporter stocks (M30) and the combined stocks including GAL4-MSL2 fusion protein and M30. (D and E) Expression levels of the autosomal reporter gene, M9, with GAL4-MSL2 fusion protein. Samples: 1, M9 female (n = 5); 2, M9 male (n = 5); 3, M9+GAL4-MSL2(1 copy) female (n = 4); 4, M9+GAL4-MSL2(1) male (n = 4). One copy of GAL4-MSL2 is present. n, number of replicates for each sample. Error: the SD of the independent replicates. A t test is applied to each comparison and P value is shown. Normal female levels are set at 1.0. (D) Target gene (miniwhite), the upper bands are from the P32-labeled white probe and endogenous control gene (rRNA) from Northern blot. (E) Comparisons of eye colors (miniwhite) from the individual reporter stocks (M9) and the combined stocks with GAL4-MSL2 fusion protein and M9. (F–K) Immunolocalization and FISH of polytene chromosomes from third instar larvae of females (F–H) and males (I–K) with M9 reporter. The red channel (arrowhead) is the signal from FISH; the green channel (arrowhead) is the signal from antibody. (F and I) The signals of miniwhite (red) from FISH and protein MSL2 (green) from antibody. (G–J) The signals of miniwhite (red) from FISH and protein MOF (green) from antibody. (H–K) The signals of miniwhite (red) from FISH and protein H4K16Ac (green) from antibody. (L and M) The cytological RNA FISH signals of the roX RNA (red signals) and miniwhite (green signals) in females (L) and males (M). Heterozygous reporters were used to distinguish them from potential endogenous signals. (Scale bars, 10 μm.)
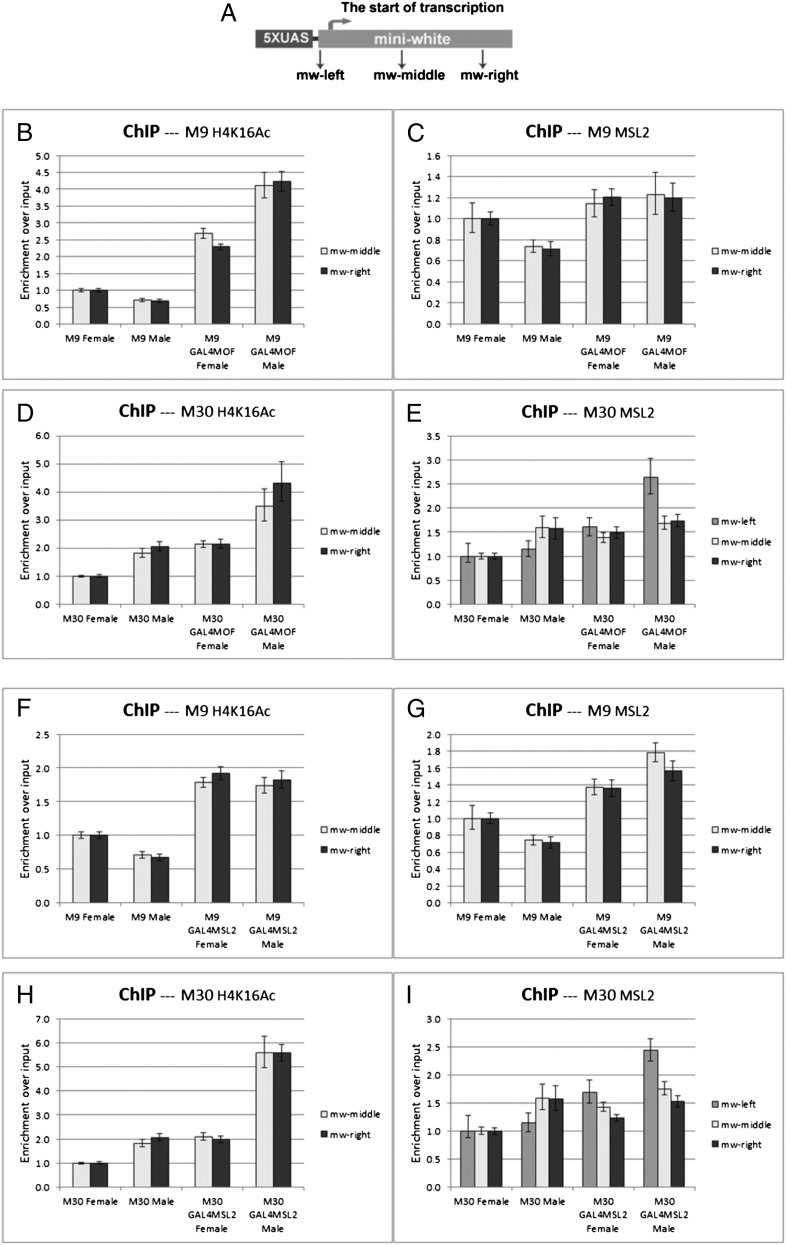
To confirm further the binding pattern of the MSL complex on the reporter genes, we used ChIP on the combined GAL4-fusion reporter lines. The presence of H4K16Ac and MSL2 was measured on different target sequences (Fig. 3A) of the reporter genes by ChIP followed by relative quantitative-PCR. When the GAL4-MOF fusion protein was present, the levels of H4K16Ac (Fig. 3 B and D) were elevated on both assayed sequences with M9 (autosomal) and M30 (X chromosomal), illustrating its ability to catalyze histone acetylation across the length of the gene. With GAL4-MOF targeting, the MSL2 protein (Fig. 3 C and E) was only significantly increased in males, the sex in which it is expressed.
Fig. 3.
ChIP analysis of GAL4 fusion proteins with the X-linked (M30) and autosomal reporter (M9). (A) Three different amplicons along the miniwhite reporter were tested by the primers (arrows) in these ChIP experiments: mw-left, mw-middle and mw-right. TSS, transcription start site. Two copies of the targeting construct are present in each case. (B–E) GAL4-MOF fusion protein. (B and D) The levels of H4K16 acetylation on the autosomal M9 (B) and the X linked M30 (D). (C and E) The levels of MSL2 protein on M9 (C) and M30 (E). GAL4-MOF fusion protein causes an increased H4K16Ac in females and males and an elevated level of MSL2 protein in males. Normal female levels are set at 1.0. (F–I) GAL4-MSL2 fusion protein. (F and H) The levels of H4K16 acetylation on the autosomal M9 (F) and the X-linked M30 (H). (G and I) The levels of MSL2 protein on M9 (G) and M30 (I). The results indicate that targeting GAL4-MSL2 protein induces a higher level of H4K16Ac despite no impact on expression (Fig. 2). The real-time data were analyzed with Applied Biosystems 7300 system SDS software with calculation of the 95% confidence interval. Errors shown: positive represents the maximum relative quantification (RQ); negative represents the minimal RQ.
With the GAL4-MSL2 construct, H4K16Ac acetylation was increased on all target sequences with both reporters in females (Fig. 3 F and H). The MSL2 protein was significantly elevated on the 5′ target sequence (mw-left) of the reporter gene (Fig. 3 G and I). The MSL complex is targeted outside the assayed sequences; however, local spreading of the complex (27) explains the presence of the complex on the target and the increase of acetylation across both targets (Fig. 3 F and H). Consistent with the cytological results, the whole MSL complex was organized on these reporters in males when GAL4-MOF was targeted and in females and males when GAL4-MSL2 was targeted.
Prestel et al. (28) have reported results of targeting a GAL4-MOF fusion protein to single inserts of two different reporters that are organized similarly to each other but distinctly from the nine inserts described above. The authors report related results in that there is a greater expression in females than in males with targeting. However, in contrast to the results reported above, there is some increase in the targeted male compared with the control male, which we have confirmed phenotypically for the miniwhite gene present in their two constructs. The difference in behavior of constructs when targeted by GAL4-MOF in males is unknown, but how the quantities of GAL4-MOF present in the nonspecific lethal (NSL) complex (29) or other contexts versus those assembled with the MSL2 complex behave on the reporters might be considered. We examined these two published insertions when targeted with GAL4-MSL2. For these autosomal inserts there is no twofold increase of gene expression phenotypically and on the RNA level, and indeed there is a reduction in expression in males despite the fact that histone acetylation is increased across the reporter (Fig. S6). In this test, all three types of miniwhite reporters fail to exhibit dosage compensation in association with the MSL complex despite the confirmation of its enzymatic function.
Effect of the Addition of the MSL Complex on MOF Targeting.
It was previously shown that in the mle mutant, in which the MSL complex is unable to assemble, most of the assayed X chromosomal genes retain dosage compensation when the expression is determined phenotypically or as an absolute measurement rather than an X-to-autosomal ratio (11–15). In addition, dosage compensation also occurs in the male germ line (30), partially in early embryos before sequestration of the MSL complex on the male X (31) and in triple X metafemales (22), even though there is no MSL complex present in these circumstances, illustrating that compensation occurs without the MSL complex. Moreover, when the SXL protein, which is normally expressed only in females to repress the translation of the MSL2 protein (7), is removed, the accumulated MSL complex on the X chromosomes did not produce any difference in X-linked gene expression (12, 14, 15). To compare these results to the targeting experiments, the effect of GAL4-MOF on the reporters was assayed by addition of ectopically expressed MSL complex.
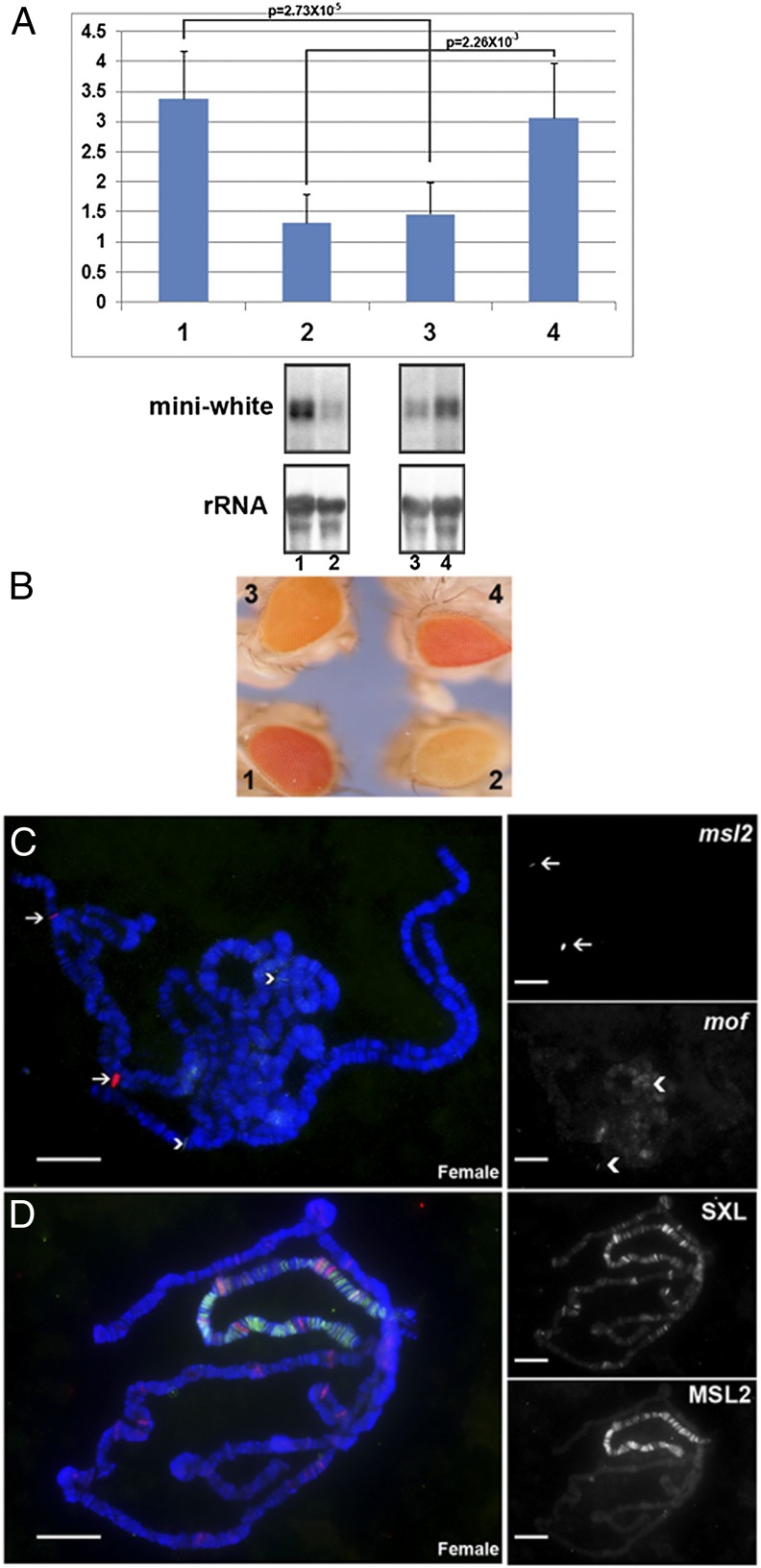
To examine the inhibitory function of the MSL complex, we tested the effect of ectopically expressing the MSL2 protein without the GAL4-DBD in the X-linked M30;GAL4-MOF line. We found that the expression changes caused by GAL4-MOF were reversed both on the RNA level (Fig. 4A) and phenotypically (Fig. 4B), confirming that the RNA level determinations reflect the absolute expression. Immunolocalization and FISH were applied to confirm the genotype of this combination stock, which showed two signals of msl2 and mof (Fig. 4C) from FISH: one is endogenous and the other is the transgenic signal. The ectopic expression of MSL2 in females was also confirmed, as illustrated by association of the MSL complex with the X chromosomes (Fig. 4D).
Fig. 4.
Counteracting function of the MSL complex on histone acetylation. Ectopically expressed MSL2 protein in combination with GAL4-MOF fusion construct and the X-linked reporter M30. (A) Expression levels of the X-linked reporter gene, M30 in each sample. Samples: 1, M30+GAL4-MOF(2 copies) female (n = 4); 2, M30+GAL4-MOF(2) male (n = 4); 3, M30+GAL4-MOF(2)+MSL2(2) female (n = 3); 4, M30+GAL4-MOF(2)+MSL2(2) male (n = 3). When present, two copies of GAL4-MOF and MSL2 are represented, respectively. n, number of replicates for each sample. Error: SD of independent replicates. A t test is applied to each comparison and P value is shown. Normal females levels are set at 1.0 (not in histogram). (B) Comparisons of eye colors (miniwhite reporter) of M30+GAL4-MOF(2) stock with the combined stock M30+GAL4-MOF(2)+MSL2(2). (C) FISH of the polytene chromosomes from the combined [M30+GAL4-MOF(2)+MSL2(2)] female larvae. The red channel is from the signal of msl2 genes (arrow); the green channel is from signal of mof genes (arrowhead). (D) Immunolocalization on the polytene chromosomes from the combined [M30+GAL4-MOF(2)+MSL2(2)] female larvae. Red channel (Upper Right) is the signal of protein SXL confirming a female nucleus; green channel (Lower Right) is the signal of protein MSL2. (Scale bars, 10 μm.)
The addition of MSL2 to females produces a situation in some sense analogous to males in that GAL4-MOF is capable of attracting the whole MSL complex to the reporter. In terms of its impact on gene expression, a similar result is obtained as in males, namely that it reverses the overexpression seen with GAL4-MOF alone. In contrast, the expression in males is increased. In this case, there is preexisting MSL complex on the X chromosome. A similar result in males occurs on the reporter alone with ectopic MSL2 expression (see below) but there is no effect in females. The basis for the increased expression of the X-linked reporter in males is unknown but a possible explanation, among others, is that overexpression of MSL2 subunits has a dominant-negative effect on the counter activity of the complex on histone acetylation. On the other hand, with the hypothesis that the MSL complex mediates dosage compensation, it might be hypothesized that overexpression of MSL2 alone fosters enhanced expression; this interpretation, however, is in contrast to the effects found in this study with all or none association of the MSL complex.
No Increased Expression of X-Linked Reporters from Ectopic Expression of MSL2 in Females.
To test this conclusion in another way, the ectopically expressed MSL2 without the GAL4 BD was combined with the X and autosomal reporters. In this case, the MSL complex is present along the length of the X chromosomes (Fig. 5) and the reporters are not specifically targeted. Northern analysis and eye color determinations showed that there is no increase in expression of the X-linked reporter in females that now have the X chromosome associated with the MSL complex (Fig. 5). Males that already have MSL complex association did show a slight increase in agreement with the results described above. The autosomal reporter exhibits a significant reduction of expression in both males and females, particularly in the latter, which is consistent with previous results (11). This result might reflect the depletion of MOF from the autosomes, as previously noted (11), although MOF is also involved in the NSL complex (29), specifically with MBD-R2 (28). There is still little known concerning how the sequestration of the MSL complex impacts the NSL complex function or how global these autosomal effects might be. The molecular effects were validated phenotypically (Fig. 5). Similar results were obtained with other X and autosomal reporters (Fig. S7), including the fact that no single-copy X-linked reporter in females was converted by ectopic MSL2 expression to the eye color intensity of a single copy in males, which is the result that would have been indicative of inducing dosage compensation.
Fig. 5.
Expression levels of the reporter genes with ectopic expression of MSL2. (A) Constructs used in UAS system. The MSL2 protein without GAL4 is inserted in the expression plasmid pHT4 with the Hsp70 promoter; the miniwhite reporter gene is also shown. (B and C) Expression levels of the X-linked reporter gene, M30, with ectopically expressed MSL2. Samples: 1, M30 female (n = 6); 2, M30 male (n = 6); 3, M30+MSL2(1 copy) female (n = 3); 4, M30+MSL2(1) male (n = 3). When present, one copy of MSL2 is represented. n, number of replicates for each sample. Error: SD of independent replicates. A t test is applied to each comparison and P value is shown. Normal female levels are set at 1.0. (B) Target gene (miniwhite), the upper bands are from P32-labeled white probe and the lower is rRNA control from Northern blots. (C) Comparisons of eye colors (miniwhite) from the individual reporter stocks (M30) and the combined stocks of MSL2 and M30. (D and E) Expression levels of the autosomal reporter gene, M9, with ectopically expressed MSL2. Samples: 1, M9 female (n = 5); 2, M9 male (n = 5); 3, M9+MSL2(1 copy) female (n = 3); 4, M9+MSL2(1) male (n = 3). When present, one copy of MSL2 is represented. n, number of replicates for each sample. Error: the SD of the independent replicates. A t test is applied to each comparison and P value is shown. Normal female levels are set at 1.0. (D) Target gene (miniwhite), the upper bands are from P32-labeled white probe and lower is rRNA control from Northern blots. (E) Comparisons of eye colors (miniwhite) from the individual reporter stocks (M9) and the combined stocks of MSL2 and M9. (F) Detection of ectopically expressed MSL2 protein in females. The cytological location of normal MSL2 protein in females (negative), males (positive), and MSL2-expressing females. The blue channel represents the signals from DAPI and the green from MSL2 immunolocalization. (Scale bars, 10 μm.)
No Effect on Global Endogenous X-Linked Gene Expression by Ectopic MSL Complex Association.
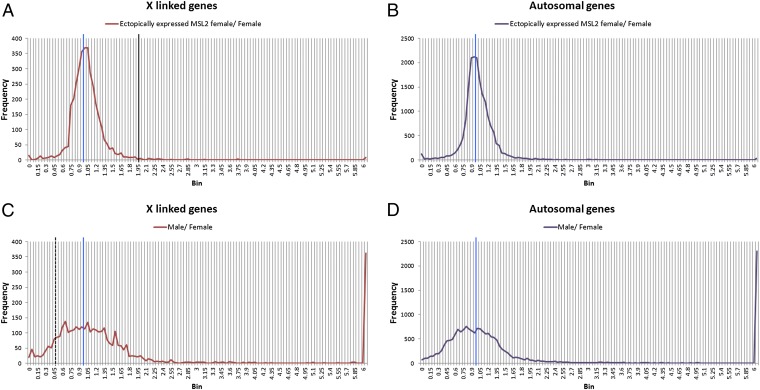
To determine if the targeted reporters and ectopic MSL2 expression studies on the reporters were representative of endogenous genes, an RNAseq experiment was conducted using a line that expresses MSL2 strongly (H83M2) (6). Adult females with this ectopic expression were compared with normal adult females. Normal adult males and females from the same progeny were also analyzed. Gene reads from three biological replicates were averaged and a ratio-distribution analysis conducted. The ratios of ectopic MSL2 female to normal female average number of reads for each X-linked and autosomally expressed gene isoform were plotted in bins with 0.05 increments (Fig. 6). This type of analysis does not normalize X-linked genes to autosomal expression but provides strong power to reveal trends because hundreds of datapoints (all expressed isoforms) per bin contribute to the distribution. The results reveal that the X chromosome distribution is sharply centered around a ratio of 1.0, although a minor shoulder of reduced expression is present. The prediction from the hypothesis that the MSL complex mediates dosage compensation would be a distribution surrounding 2.0. There is no evidence that a substantial subset of X-linked genes exhibits or approaches this behavior. The distribution of male/female X-chromosome ratios is more broadly centered around 1.0, as would be expected from a comparison of different sexes. There is a range of ratios from overcompensation to near 0.5, the value of no compensation. This result confirmed that endogenous genes on the X chromosome are not increased in expression twofold by association with the MSL complex.
Fig. 6.
Global endogenous gene expression ratios in ectopically expressed MSL2 females compared with normal females. The ectopically expressed MSL2 females were obtained using a P-element msl2 construct with a miniwhite transformation marker, [(w+) H83M2-6I] (6). The global expression pattern of these females [y w;(w+) H83M2-6I/+;+/+], normal females (y w;+/+; TM3/+) and males (y w;+/+;TM3/+) were obtained by mRNA-sequencing and analyzed by comparing the ratio distributions to each other. (A and B) Ratio distributions of expressed isoforms from the ectopically expressed MSL2 females were compared with normal females, separated into the X-linked genes (A) and autosomal genes (B). The comparisons of normal males with females are shown separately with X linked genes (C) and autosomal genes (D). The blue line represents the ratio “1.0” (no change); the black line marks the ratio “2.0” (the ratio for acquisition of dosage compensation in MSL2 females); the black dash line shows the ratio “0.5” (no dosage compensation in males). The spike at the far right represents genes with large differences of expression between the sexes. Three biological replicates were performed for each genotype.
Discussion
In the multiprong approach taken here, we find no evidence that the MSL complex will cause a twofold up-regulation of associated genes that would suggest that it mediates dosage compensation directly. Instead, the overall evidence suggests that the MSL complex has an activity that overrides the impact of histone acetylation on gene expression. Typically, histone acetylation is associated with increased expression, which led to the original suggestion that the MSL complex was responsible for compensation. Indeed, with targeting of MOF alone, there are elevated levels of acetylation of H4Lys16 but under circumstances in which the whole complex is present and brought to reporters or the X chromosome as a whole, there is no evidence of an increase of gene expression. Adding the necessary component for complex assembly, namely MSL2, to the genotype with MOF targeting to the reporters reverses the up-regulation.
It should be pointed out that the reporters on the X chromosome are already dosage-compensated in males before targeting. Thus, targeting them in an assay for induction of dosage compensation might be considered uninformative. Indeed, a potential interpretation of the reduced expression of the reporter with GAL4-MOF targeting is that normal dosage compensation is disrupted. However, autosomal reporters in males have no preexisting MSL complex and behave in a similar manner. We do not feel that the preexisting association of the MSL complex with the X reporters complicates our interpretation that the complex does not mediate dosage compensation. We conclude this because in all circumstances in which reporters or endogenous genes transition from no association to MSL association [X and autosomal reporters in females; autosomal reporters in males, and X endogenous genes in females; previously in Sex lethal mutants (12)], there is no evidence for an increased expression despite the increase in histone acetylation.
A result that has been interpreted in support of the MSL complex mediating dosage compensation is that silenced miniwhite transgenes become expressed when the MSL complex spreads from roX transgenes (32, 33). This change in gene expression proceeds from a null state to nearly normal pigment levels. Thus, the change in expression is infinity rather than the approximately twofold change characteristic of dosage compensation. This phenomenon is therefore likely to represent suppression of gene silencing rather than dosage compensation.
In addition to the results presented here, there are other aspects of dosage compensation that the MSL hypothesis fails to accommodate. First of all, in addition to males, there is also dosage compensation in triple X metafemales that possess three X chromosomes with diploid autosomes (34). In this case each copy of X-linked genes must be reduced in expression to about two-thirds of the level found in females rather than the twofold increase for males. The MSL hypothesis does not address this issue and, given that there is no MSL complex in metafemales (22), it is inadequate to do so. Second, dosage compensation not only occurs in diploid males but also in triploid flies that have only two or one X chromosome compared with the three present in triploid females (35–37). The level of change to account for compensation per gene copy to equal the total triploid female expression is different in each case, as well as distinct from that of diploid males. The MSL hypothesis does not suggest a means by which different degrees of modulation are achieved based on chromosomal dosage.
Furthermore, in previous work we have failed to observe a loss of compensation in a sampling of endogenous genes or transgenes in mle mutants in which there is no MSL complex associated with the single X chromosome when the expression values were calculated in absolute terms. We have found this result to be the case not only in larvae (11, 13) but also in embryos (14). In these cases, the dissolution of the MSL complex from the male X chromosome does not cause a loss of compensation.
A few studies have been conducted to examine gene expression when the MSL complex has been disrupted (3, 8–10). These studies have all made the assumption that there would be no change of autosomal gene expression and have used the autosomes as the standard with which to normalize X-linked gene expression. We have previously noted (11, 13, 14, 38) that when the data are examined for absolute expression, the values are consistent with no generalized loss of compensation and increases of autosomal expression. Such normalization will drive down the ratio of X to autosomal expression, and thus was the basis for the conclusion that a loss of compensation occurred. When viewed in this way, there is no conflict with our data, which can be visualized (14) on the absolute level and which illustrate there is no generalized loss of compensation in the absence of the MSL complex. Similarly, there is no conflict with the results that targeting the MSL complex produces no up-regulation.
If no evidence can be garnered in support of the hypothesis that the MSL complex mediates a twofold increase in expression when associated with genes, a different process must be sought to explain the up-regulation of the male X chromosome. It has previously been suggested that an inverse dosage effect (16, 17, 19, 21, 23, 39, 40), empirically observed as the negative correlation between target gene expression and chromosomal dosage, produced by the single dose of the X chromosome in males, might be expected to increase the expression of both the X chromosome and the autosomes. Modeling the inverse effect suggests that it will result from how an altered stoichiometry of members of multisubunit regulatory complexes affects the assembly of the whole (41). The X chromosome might have evolved to use this natural consequence of aneuploidy to achieve compensation. Considering the gene expression trends in mle males with one X chromosome and no MSL complex (11–15) compared with normal females, and then also with metafemales with three X chromosomes (22, 42, 43), there is a generalized inversely affected autosomal expression as well as compensation of the X in the three genotypes. We hypothesize that to mute the potential autosomal overexpression in normal males, the formation of the MSL complex has evolved to sequester MOF from the autosomes to the X chromosome (11, 12, 14). However, this sequestration would tend to overexpress the X, as experimentally verified in the present study by MOF targeting, so an additional activity evolved to override the high level of histone acetylation generated from the tethered MOF, as also verified in the present work. This override would allow the appropriate twofold up-regulation of the single X chromosome to achieve dosage compensation. Although many parameters remain to be defined (39), the results reported herein indicate that the MSL complex is not the direct mediator of X chromosome dosage compensation.
Materials and Methods
Northern Blot.
Total RNA was isolated and separated on 1.5% (wt/vol) formaldehyde-agarose gels. The transferred membranes were cross-linked and hybridized with antisense RNA probes (white and rRNA) under the conditions as previously described (11, 13, 22). Subsequently, three washes at 75 °C were applied to the membranes. The exposed signals were measured using the Fujifilm Image Gauge V 3.3 program. Expression constructs are shown in Fig. S8.
Immunolocalization and FISH.
The salivary glands from third-instar larvae were dissected, fixed, and processed (11, 14). FISH probes were labeled by nick translation with Texas red (red) or Alexa Fluor 488-dUTP (green) and applied to slides. The samples were stored at 55 °C overnight after hybridization, washed using prewarmed 2× SSC and dried in the dark (44), and then mounted with Vectashield mounting medium containing DAPI.
ChIP.
Samples were homogenized with buffer A1 and formaldehyde using a Potter homogenizer, then a Douncer (45). Glycine solution was added to the cross-linked mixture solution. The samples were then centrifuged, washed three times with buffer A1, and once with lysis buffer without SDS. The final pellets were dissolved in lysis buffer with SDS and kept at 4 °C for 30 min on a rotating wheel. The samples were then sheared with a sonicator. The supernatants were washed in centricon YM-10 columns with ChIP dilution buffer. The primary antibodies of interest were added into each ChIP solution at 4 °C on a rotating wheel overnight. The antibodies were kept on the wheel for another 4 h after adding 60 μL protein G or A agarose followed by three washes. The immune complexes were eluted and reverse cross-linked. The DNA was precipitated by phenol/chloroform and washed. One microliter of the resuspended pellet was used in the real-time PCR reactions.
RNAseq.
Procedures are described in SI Materials and Methods. Sequencing data are archived under GEO accession number GSE41570 and NCBI Sequence Read Archive number SRP016112.
Supplementary Material
Acknowledgments
We thank Peter Becker for the GAL4-mof plasmid used in this study and the RC3 and RC5 stocks; Genetic Services (Cambridge, MA) for fly transformations; and Weiwu Xie for helpful advice. High-throughput sequencing services were performed at the University of Missouri DNA Core Facility. Research was supported by National Institutes of Health Grant R01GM068042.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE41570), NCBI Sequence Read Archive number SRP016112.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222542110/-/DCSupplemental.
References
- 1.Muller HJ. Further studies on the nature and causes of gene mutations. Proc 6th Int Congr Genet. 1932;1:213–255. [Google Scholar]
- 2.Conrad T, Akhtar A. Dosage compensation in Drosophila melanogaster: Epigenetic fine-tuning of chromosome-wide transcription. Nat Rev Genet. 2011;13(2):123–134. doi: 10.1038/nrg3124. [DOI] [PubMed] [Google Scholar]
- 3.Belote JM, Lucchesi JC. Control of X chromosome transcription by the maleless gene in Drosophila. Nature. 1980;285(5766):573–575. doi: 10.1038/285573a0. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda MI, Kernan MJ, Kreber R, Ganetzky B, Baker BS. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell. 1991;66(5):935–947. doi: 10.1016/0092-8674(91)90439-6. [DOI] [PubMed] [Google Scholar]
- 5.Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi JC. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16(8):2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley RL, et al. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995;81(6):867–877. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelley RL, Wang J, Bell L, Kuroda MI. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature. 1997;387(6629):195–199. doi: 10.1038/387195a0. [DOI] [PubMed] [Google Scholar]
- 8.Hamada FN, Park PJ, Gordadze PR, Kuroda MI. Global regulation of X chromosomal genes by the MSL complex in Drosophila melanogaster. Genes Dev. 2005;19(19):2289–2294. doi: 10.1101/gad.1343705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng X, Meller VH. roX RNAs are required for increased expression of X-linked genes in Drosophila melanogaster males. Genetics. 2006;174(4):1859–1866. doi: 10.1534/genetics.106.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad T, Cavalli FM, Vaquerizas JM, Luscombe NM, Akhtar A. Drosophila dosage compensation involves enhanced Pol II recruitment to male X-linked promoters. Science. 2012;337(6095):742–746. doi: 10.1126/science.1221428. [DOI] [PubMed] [Google Scholar]
- 11.Bhadra U, Pal-Bhadra M, Birchler JA. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics. 1999;152(1):249–268. doi: 10.1093/genetics/152.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhadra U, Pal-Bhadra M, Birchler JA. Histone acetylation and gene expression analysis of sex lethal mutants in Drosophila. Genetics. 2000;155(2):753–763. doi: 10.1093/genetics/155.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiebert JC, Birchler JA. Effects of the maleless mutation on X and autosomal gene expression in Drosophila melanogaster. Genetics. 1994;136(3):913–926. doi: 10.1093/genetics/136.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhadra MP, Bhadra U, Kundu J, Birchler JA. Gene expression analysis of the function of the male-specific lethal complex in Drosophila. Genetics. 2005;169(4):2061–2074. doi: 10.1534/genetics.104.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal Bhadra M, Bhadra U, Birchler JA. Misregulation of sex-lethal and disruption of male-specific lethal complex localization in Drosophila species hybrids. Genetics. 2006;174(3):1151–1159. doi: 10.1534/genetics.106.060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birchler JA. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics. 1979;92(4):1211–1229. doi: 10.1093/genetics/92.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birchler JA, Newton KJ. Modulation of protein levels in chromosomal dosage series of maize: The biochemical basis of aneuploid syndromes. Genetics. 1981;99(2):247–266. doi: 10.1093/genetics/99.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birchler JA, Hiebert JC, Paigen K. Analysis of autosomal dosage compensation involving the alcohol dehydrogenase locus in Drosophila melanogaster. Genetics. 1990;124(3):679–686. [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinow L, Nguyen-Huynh AT, Birchler JA. A trans-acting regulatory gene that inversely affects the expression of the white, brown and scarlet loci in Drosophila. Genetics. 1991;129(2):463–480. doi: 10.1093/genetics/129.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabl JF, Birchler JA. Dosage dependent modifiers of white alleles in Drosophila melanogaster. Genet Res. 1993;62(1):15–22. doi: 10.1017/s0016672300031517. [DOI] [PubMed] [Google Scholar]
- 21.Guo M, Birchler JA. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science. 1994;266(5193):1999–2002. doi: 10.1126/science.266.5193.1999. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Birchler JA. Interaction study of the male specific lethal (MSL) complex and trans-acting dosage effects in metafemales of Drosophila melanogaster. Cytogenet Genome Res. 2009;124(3-4):298–311. doi: 10.1159/000218134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie W, Birchler JA. Identification of inverse regulator-a (Inr-a) as synonymous with pre-mRNA cleavage complex II protein (Pcf11) in Drosophila. G3 (Bethesda) 2012;2(6):701–706. doi: 10.1534/g3.112.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5(2):367–375. doi: 10.1016/s1097-2765(00)80431-1. [DOI] [PubMed] [Google Scholar]
- 25.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar A, Becker PB. The histone H4 acetyltransferase MOF uses a C2HC zinc finger for substrate recognition. EMBO Rep. 2001;2(2):113–118. doi: 10.1093/embo-reports/kve022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Birchler JA. Studies on the short range spreading of the male specific lethal (MSL) complex on the X chromosome in Drosophila. Cytogenet Genome Res. 2009;124(2):158–169. doi: 10.1159/000207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prestel M, Feller C, Straub T, Mitlöhner H, Becker PB. The activation potential of MOF is constrained for dosage compensation. Mol Cell. 2010;38(6):815–826. doi: 10.1016/j.molcel.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Raja SJ, et al. The nonspecific lethal complex is a transcriptional regulator in Drosophila. Mol Cell. 2010;38(6):827–841. doi: 10.1016/j.molcel.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Parisi M, et al. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science. 2003;299(5607):697–700. doi: 10.1126/science.1079190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lott SE, et al. Noncanonical compensation of zygotic X transcription in early Drosophila melanogaster development revealed through single-embryo RNA-seq. PLoS Biol. 2011;9(2):e1000590. doi: 10.1371/journal.pbio.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y, Kelley RL, Oh H, Kuroda MI, Meller VH. Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins. Science. 2002;298(5598):1620–1623. doi: 10.1126/science.1076686. [DOI] [PubMed] [Google Scholar]
- 33.Kelley RL, Kuroda MI. The Drosophila roX1 RNA gene can overcome silent chromatin by recruiting the male-specific lethal dosage compensation complex. Genetics. 2003;164(2):565–574. doi: 10.1093/genetics/164.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern C. Dosage compensation-development of a concept and new facts. Can J Genet Cytol. 1960;2(2):105–118. [Google Scholar]
- 35.Maroni G, Plaut W. Dosage compensation in Drosophila melanogaster triploids. I. Autoradiographic study. Chromosoma. 1973;40(4):361–377. doi: 10.1007/BF00399428. [DOI] [PubMed] [Google Scholar]
- 36.Lucchesi JC, Rawls RM., Jr Regulation of gene function: A comparison of X-linked enzyme activity levels in normal and intersexual triploids of Drosophila melanogaster. Genetics. 1973;73(3):459–464. doi: 10.1093/genetics/73.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucchesi JC, Belote JM, Maroni G. X-linked gene activity in metamales (XY; 3A) of Drosophila. Chromosoma. 1977;65(1):1–7. [Google Scholar]
- 38.Birchler JA, et al. Re-evaluation of the function of the male specific lethal complex in Drosophila. J Genet Genomics. 2011;38(8):327–332. doi: 10.1016/j.jgg.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Devlin RH, Holm DG, Grigliatti TA. The influence of whole-arm trisomy on gene expression in Drosophila. Genetics. 1988;118(1):87–101. doi: 10.1093/genetics/118.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birchler JA, Bhadra U, Bhadra MP, Auger DL. Dosage-dependent gene regulation in multicellular eukaryotes: Implications for dosage compensation, aneuploid syndromes, and quantitative traits. Dev Biol. 2001;234(2):275–288. doi: 10.1006/dbio.2001.0262. [DOI] [PubMed] [Google Scholar]
- 41.Veitia RA, Bottani S, Birchler JA. Cellular reactions to gene dosage imbalance: Genomic, transcriptomic and proteomic effects. Trends Genet. 2008;24(8):390–397. doi: 10.1016/j.tig.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Birchler JA, Hiebert JC, Krietzman M. Gene expression in adult metafemales of Drosophila melanogaster. Genetics. 1989;122(4):869–879. doi: 10.1093/genetics/122.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birchler JA. Expression of cis-regulatory mutations of the white locus in metafemales of Drosophila melanogaster. Genet Res. 1992;59(1):11–18. doi: 10.1017/s0016672300030123. [DOI] [PubMed] [Google Scholar]
- 44.Kato A, Lamb JC, Birchler JA. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc Natl Acad Sci USA. 2004;101(37):13554–13559. doi: 10.1073/pnas.0403659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavalli G, Paro R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell. 1998;93(4):505–518. doi: 10.1016/s0092-8674(00)81181-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.