Abstract
Leptin is an adipocyte-derived hormone that controls food intake and reproductive and immune functions in rodents. In uncontrolled human studies, low leptin levels are associated with impaired immune responses and reduced T-cell counts; however, the effects of leptin replacement on the adaptive immune system have not yet been reported in the context of randomized, controlled studies and/or in conditions of chronic acquired leptin deficiency. To address these questions, we performed a randomized, double-blinded, placebo-controlled trial of recombinant methionyl-human leptin (metreleptin) administration in replacement doses in women experiencing the female triad (hypothalamic amenorrhea) with acquired chronic hypoleptinemia induced by negative energy balance. Metreleptin restored both CD4+ T-cell counts and their in vitro proliferative responses in these women. These changes were accompanied by a transcriptional signature in which genes relevant to cell survival and hormonal response were up-regulated, and apoptosis genes were down-regulated in circulating immune cells. We also observed that signaling pathways involved in cell growth/survival/proliferation, such as the STAT3, AMPK, mTOR, ERK1/2, and Akt pathways, were activated directly by acute in vivo metreleptin administration in peripheral blood mononuclear cells and CD4+ T-cells both from subjects with chronic hypoleptinemia and from normoleptinemic, lean female subjects. Our data show that metreleptin administration, in doses that normalize circulating leptin levels, induces transcriptional changes, activates intracellular signaling pathways, and restores CD4+ T-cell counts. Thus, metreleptin may prove to be a safe and effective therapy for selective CD4+ T-cell immune reconstitution in hypoleptinemic states such as tuberculosis and HIV infection in which CD4+ T cells are reduced.
Keywords: CD4 cells, metabolism, nutritional status
Leptin is an adipocyte-derived hormone that conveys information on energy availability and whose circulating levels are proportionate to the amount of adipose tissue present (1). The functional long form of the leptin receptor (LepRb) is expressed in the hypothalamus where it regulates energy homeostasis and neuroendocrine function. It also is expressed in cells of the innate and adaptive immune system where leptin exerts key regulatory functions (2). On the basis of studies in rodents and observational and uncontrolled studies in a limited number of human subjects with congenital leptin deficiency, leptin has been proposed to act as a signal that conveys information on energy availability to the immune system. The immune system, like the neuroendocrine system, requires an adequate supply of energy for optimal functioning (3, 4). Evidence of leptin’s importance can be found in animal studies in which mice lacking either leptin or LepRb show defects in cell-mediated proinflammatory T-helper 1 (Th1)-type immune responses (3, 4). Leptin stimulates in vitro the activation of monocytes from healthy humans in terms of reactive oxygen species production and chemotaxis in polymorphonuclear cells (2). Children with congenital leptin deficiency have reduced lymphocyte subpopulation numbers and show an increased risk for infection-related deaths during childhood. In these children, the T-cell CD4+ fraction and the T-cell receptor (TCR)–specific proliferative responses are reduced as compared with the general population, and anti-CD3 and purified protein derivative (PPD) recall antigens stimulations were greatly impaired in these subjects (5). Very recent observational data show that impaired leptin signaling, secondary to a specific leptin-receptor polymorphism, is associated with reduced mucosal immunity against amebiasis in children (6). A direct effect of recombinant methionyl-human leptin (metreleptin) replacement to correct immunophenotypic changes, specifically increasing circulating naive CD4+CD45RA+ T-cell numbers and reversing impaired T-cell proliferation/cytokine release in response to TCR stimulation, has been observed in an extremely small, uncontrolled pilot study of children with congenital leptin deficiency (5). Treatment with metreleptin in these individuals also led to a switch from an anti-inflammatory Th2 cytokine secretion pattern to a predominantly proinflammatory Th1 phenotype.
Complete congenital leptin deficiency is an extremely rare condition. It has been described in only two families with high rates of consanguinity and in an additional two individuals with sporadic mutations of the leptin gene (1). Acquired leptin deficiency that develops in adulthood [as seen in states of negative energy balance such as malnutrition, HIV, or hypothalamic amenorrhea (HA)] is much more common than complete congenital leptin deficiency and occurs frequently when the balance between inadequate food intake and/or excessive exercise results in a negative energy balance (1). For example, acquired leptin deficiency in its extreme phenotype is commonly seen in patients with anorexia nervosa (7) and also in strenuously exercising athletes, accounting for more than 30% of the amenorrhea seen in women of reproductive age (8). Acquired leptin deficiency also is seen in ∼20% of HIV+ subjects with highly active antiretroviral treatment (HAART)-induced lipodystrophy and metabolic syndrome (9, 10) and in many cachectic patients. However, little is known about the effects of low leptin levels (considered “relative” leptin deficiency, as seen in HA and HIV) on immune function. Preliminary evidence from a short-term, open-label study showed that leptin replacement activated the TNF system in women with HA (11). To investigate further and more rigorously the effects of leptin replacement in chronic acquired leptin deficiency on the adaptive immune system, we conducted a randomized, double-blind, placebo-controlled study of metreleptin treatment in subjects with acquired leptin deficiency and HA, treating the subjects with either placebo or replacement doses of leptin for 36 wk. Then we assessed cellular and molecular pathways in detail to dissect the precise effect of metreleptin on immune function in this condition of acquired hypoleptinemia.
Results
Baseline characteristics of both the leptin- and placebo-treated subjects are presented in Table 1. There was no significant difference between the HA groups with regards to age, weight, body mass index (BMI), or leptin levels. These parameters also were compared with a third group of women with homogeneous age and normal body weight as controls (see below). Metreleptin therapy restored menstruation and, by inference, reproductive hormones in four of eight subjects (vs. one of six placebo-treated subjects) after 12 wk, in five of eight subjects (vs. one of six placebo-treated subjects) after 24 wk, and in five of eight subjects (vs. two of six placebo-treated subjects) after 36 wk of therapy (7).
Table 1.
Baseline characteristics of all enrolled subjects
| Characteristic | Baseline in subjects receiving placebo (n = 6) |
Baseline in subjects receiving metreleptin (n = 8) |
Healthy controls (n = 13) |
|||
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Age (y) | 25 ± 4.1 | 18–31 | 26 ± 4.7 | 19–34 | 26 ± 1.4 | 24–28 |
| BMI (kg/m2) | 20.1 ± 2.1 | 18.5–23.8 | 21.1 ± 1.8 | 18.5–23.8 | 19.5 ± 1.8 | 17–22 |
| Leptin (ng/mL) | 2.70 ± 1.8 | 1.05–6.7 | 3.64 ± 1.6 | 1.69–7.1 | 4.73 ± 2.27 | 2.3–7.8 |
No significant differences were found among the three groups with regards to age, BMI, or leptin levels.
Efficacy of Metreleptin in Increasing CD4+ T-Cell Number in HA Subjects.
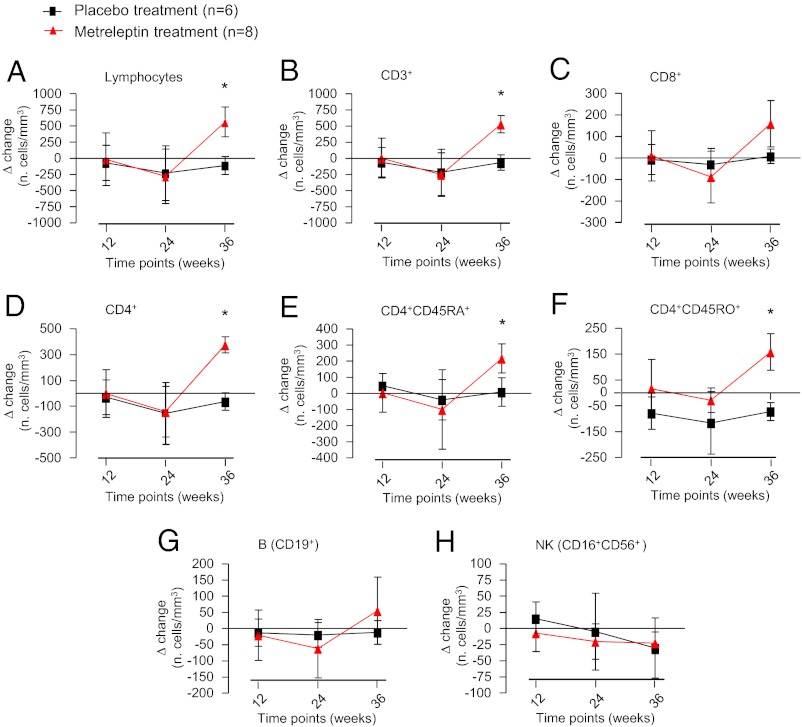
We first studied whether leptin treatment could affect the immune phenotype of HA subjects. We initially compared the total number of lymphocytes and several subpopulations in the HA subjects (n = 14) and matched them with normal control subjects (n = 13) at baseline. The HA subjects had a significantly lower number of total lymphocytes, B cells, and natural killer (NK) cells. The difference in the populations of CD4+ and CD8+ T cells followed the same trend, but the difference was not statistically significant (Fig. S1). After 36 wk of metreleptin administration in replacement doses, the lymphocyte subpopulations of CD3+ and CD4+ cells increased in terms of Δ change in the number of cells/mm3 over time [calculated by the absolute number of cells/mm3 at week of treatment minus the number of cells/mm3 at baseline (time 0); P < 0.05] and in terms of absolute cell number over time compared with normal controls (Fig. 1 and Fig. S1). B-cell and NK-cell populations did not change significantly. Within the CD3+ T-cell population, we observed a notable increase in both the naive and memory CD4+ cells (expressing CD4+CD45RA+ and CD4+CD45RO+ markers, respectively) (Fig. 1 and Fig. S1).
Fig. 1.
Effects of metreleptin on immune phenotype. Administration of metreleptin for 36 wk in replacement doses induced a significant increase in terms of Δ change in the number of cells over time [calculated as the absolute number of cells/mm3 at week of treatment minus the number of cells/mm3 at baseline (time 0)] in total lymphocytes and CD3+, CD4+, CD4+CD45RA+ naive, and CD4+CD45RO+ memory cells. *P < 0.05 in A, B, and D–F. No significant effect was observed on CD8+ (C), B (G), and NK cells (H) respectively.
Partial Efficacy of Metreleptin in Restoring T-Cell Proliferation in HA Subjects.
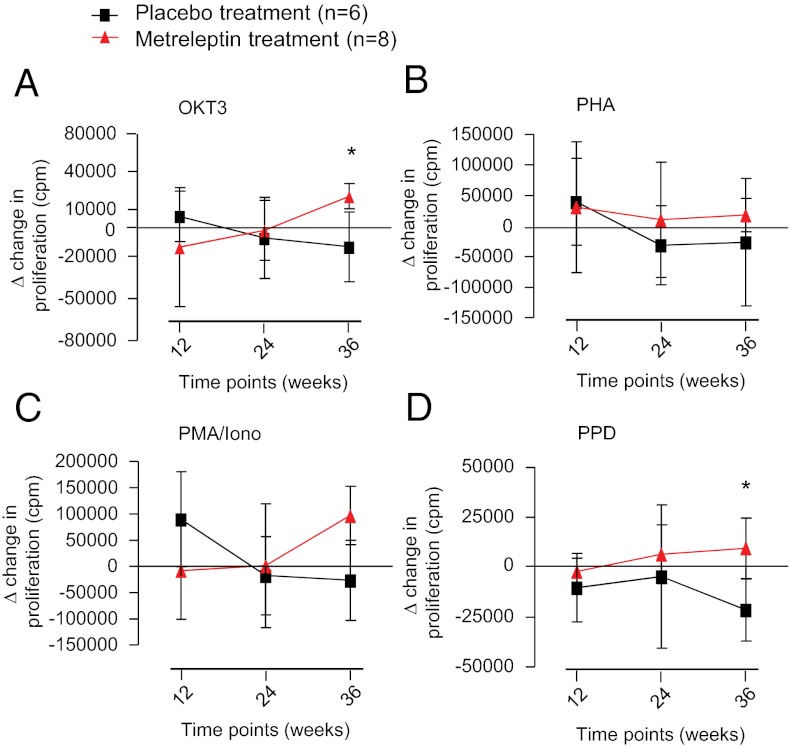
We next evaluated the effect of metreleptin treatment on the subjects’ peripheral blood mononuclear cell (PBMC) proliferative profile. We stimulated PBMCs isolated from subjects’ blood, using either physiologic T-cell–specific stimuli (OKT3 mAb or recall antigen PPD) or using polyclonal unspecific stimuli (phytohemagglutinin or phorbol 12-myristate 13-acetate plus ionomycin) to analyze the proliferative potential specifically of T cells within the PBMC fraction. We first evaluated the impact of HA on in vitro T-cell proliferative responses and found a significant reduction in proliferation in HA subjects as compared with normal subjects (Fig. S2), particularly in TCR-specific stimulations such as OKT3 (polyclonal) and PPD (antigen specific) (P < 0.05) (Fig. S2). Furthermore, using in vitro T-cell assays, we evaluated the effect of metreleptin treatment in the HA subjects over time and observed a significant increase in proliferation at week 36 by OKT3 and PPD stimulation (P < 0.05) in metreleptin-treated patients as compared with placebo-treated patients (Fig. 2).
Fig. 2.
Effects of metreleptin on PBMC proliferation. After 36 wk of metreleptin administration in replacement doses, the proliferative response to T-cell–specific OKT3 (polyclonal) (A) and PPD (antigen-specific) (D) stimulation was increased significantly in metreleptin-treated patients vs. placebo-treated patients in terms of Δ change in proliferation over time [calculated as the cpm at week of treatment minus the number cpm at baseline (time 0)]. *P < 0.05. This effect was not observed during strong unspecific stimulations such as PHA (B) and PMA/Iono (C).
Immune Changes Induced by Metreleptin Are Not Associated with Significant Changes in Serum Hormonal Patterns, Survival Cytokines, or Metabolic/Inflammatory Parameters.
We next assessed the impact of metreleptin treatment on levels of hormones and cytokines that potentially are responsible for altered immune cell survival. We observed no difference in leptin-binding protein, cortisol, adrenocorticotropic hormone (ACTH), or insulin (Fig. S3). We also investigated the impact of metreleptin treatment on circulating survival cytokines such as IL-2, IL-7, and IL-15. Again, no significant difference was detected in the three groups (Fig. S3). Finally, no difference was observed in the levels of serum metabolic/inflammatory parameters such as CD40L, soluble TNF receptors (sTNFR1 and sTNFR2), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), myeloperoxidase (MPO), soluble intercellular adhesion molecule-1, resistin, or C-reactive protein (Fig. S4). It is possible, however, that the lack of significance may result from the relatively small number of participants enrolled in this study.
Specific Transcriptional Signature Induced by Metreleptin in PBMCs from HA Subjects.
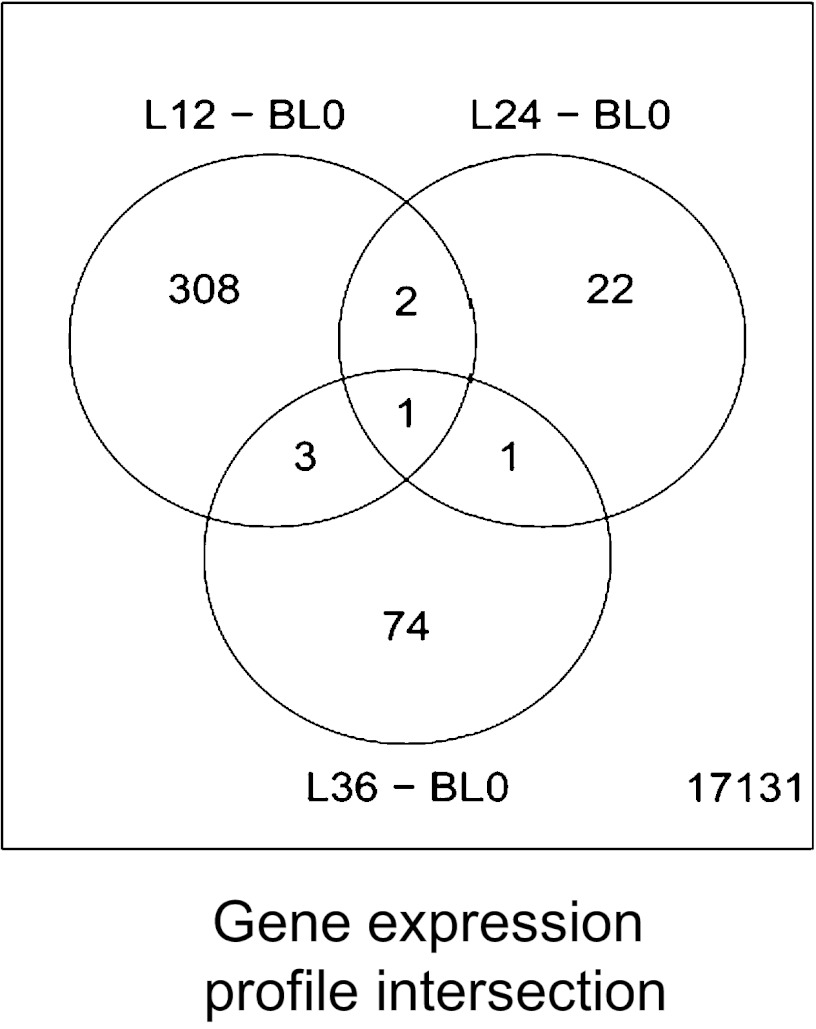
Given the profound effects of metreleptin on immune cell reconstitution and proliferation, we next evaluated the modulation of gene expression in PBMCs from patients at the different time points during metreleptin treatment (weeks 12, 24, and 36). When comparing the transcriptional profiles at week 12 of treatment vs. baseline (n = 14), we observed 314 differentially expressed genes. Of those, 177 were up-regulated, and 137 were down-regulated compared with baseline. Likewise, at weeks 24 (n = 16) and 36 (n = 11), only 26 and 79 genes changed, respectively, compared with baseline (Fig. 3). Of these, 13 genes were up-regulated and 13 were down-regulated at week 24; 50 genes were up-regulated and 29 were down-regulated at week 36 (Fig. 3). Changes in gene expression at week 12 are summarized in Tables 2 and 3; changes at weeks 24 and 36 are shown in Tables S1 and S2, respectively.
Fig. 3.
Transcriptional signature induced by metreleptin in PBMCs from HA subjects. Diagram of gene-expression profile at weeks 12, 24, and 36 of treatment with metreleptin vs. baseline. The total number of genes analyzed was 17,131. There were more differentially expressed genes at week 12 (308) than at week 24 (22) or week 36 (74) of treatment. L12 -24-36, Leptin treatment week 12, 24, and 36 respectively; BL0, base line week 0.
Table 2.
Genes up-regulated in response to metreleptin treatment at week 12 vs. baseline
| Gene symbol | Gene assignment | Gene ID | P value | Fold change |
| ADAM23 | ADAM metallopeptidase domain 23 | 8745 | 0.00657402 | 0.64445533 |
| Paep | Progestogen-associated endometrial protein | 5047 | 0.00268326 | 0.62717751 |
| SELE | Selectin E | 6401 | 0.0076583 | 0.56464262 |
| Zfp42 | Zinc finger protein 42 homolog (mouse) | 132625 | 0.00391605 | 0.54934091 |
| Vcam1 | Vascular cell adhesion molecule 1 | 7412 | 0.00908406 | 0.53175772 |
| ASB12 | Ankyrin repeat and SOCS box-containing 12 | 142689 | 0.00853215 | 0.52964533 |
| Tgm5 | Transglutaminase 5 | 9333 | 0.0037842 | 0.50489131 |
| MMP10 | Matrix metallopeptidase 10 (stromelysin 2) | 4319 | 0.00792751 | 0.49938345 |
| FSHB | Follicle stimulating hormone, beta polypeptide | 2488 | 0.00645861 | 0.46332061 |
| NTF3 | Neurotrophin 3 | 4908 | 0.00791533 | 0.45733451 |
| CYP1A1 | Cytochrome P450, family 1, subfamily A, polypeptide 1 | 1543 | 0.00851326 | 0.4530755 |
| actn3 | Actn3 | 89 | 0.00447152 | 0.42328933 |
| TUBGCP4 | Tubulin, gamma complex associated protein 4 | 27229 | 0.00366037 | 0.42254891 |
| LRRK1 | Leucine-rich repeat kinase 1 | 79705 | 0.00171516 | 0.38451621 |
| TREM2 | Triggering receptor expressed on myeloid cells 2 | 54209 | 0.00797656 | 0.37922294 |
| Il7 | Interleukin 7 | 3574 | 0.00912747 | 0.37264077 |
| EFEMP1 | EGF-containing fibulin-like extracellular matrix protein 1 | 2202 | 0.00731755 | 0.36643077 |
| ATP2C2 | ATPase, Ca++ transporting, type 2C, member 2 | 9914 | 0.00339537 | 0.36473719 |
| TM4SF4 | Transmembrane 4 L six family member 4 | 7104 | 0.00051904 | 0.36139 |
| Lama2 | Laminin, alpha 2 | 3908 | 0.00654983 | 0.35533335 |
| TRPM3 | Transient receptor potential cation channel, subfamily M, member 3 | 80036 | 0.00950655 | 0.33836606 |
| FUT3 | Fucosyltransferase 3 (galactoside 3 (4)-L-fucosyltransferase, Lewis blood group) | 2525 | 0.00877468 | 0.32655352 |
| Apobec1 | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1 | 339 | 0.00504257 | 0.32323031 |
| tcf7l1 | Transcription factor 7-like 1 (T-cell specific, HMG-box) | 83439 | 0.00841957 | 0.31810237 |
| FOXD3 | Forkhead box D3 | 27022 | 0.00455988 | 0.31602 |
| Gpr20 | G protein-coupled receptor 20 | 2843 | 0.00566617 | 0.30375888 |
| Hdac10 | Histone deacetylase 10 | 83933 | 0.00432499 | 0.28392 |
| Krt32 | Keratin 32 | 3882 | 0.00276204 | 0.26272288 |
| CD1E | CD1e molecule | 913 | 0.00678964 | 0.24852336 |
Table 3.
Genes down-regulated in response to metreleptin treatment at week 12 vs. baseline
| Gene symbol | Gene assignment | Gene ID | P value | Fold change |
| CCNI | Cyclin I | 10983 | 0.00817352 | −0.2289351 |
| TGOLN2 | Transgolgi network protein 2 | 10618 | 0.00415695 | −0.2934237 |
| Psmd11 | Proteasome (prosome, macropain) 26S subunit, non-ATPase, 11 | 5717 | 0.00992041 | −0.3663301 |
| Polr2c | Polymerase (RNA) II (DNA directed) polypeptide C, 33kDa | 5432 | 0.00923576 | −0.3997785 |
| Terf2ip | Telomeric repeat binding factor 2, interacting protein | 54386 | 0.00972071 | −0.4122286 |
| Ergic3 | ERGIC and golgi 3 | 51614 | 0.00748465 | −0.4385879 |
| NDUFS4 | NADH dehydrogenase (ubiquinone) Fe-S protein 4, 18kDa (NADH-coenzyme Q reductase) | 4724 | 0.00853818 | −0.5093644 |
| TUBA1C | Tubulin, alpha 1c | 84790 | 0.00458926 | −0.5157148 |
| TRIAP1 | TP53 regulated inhibitor of apoptosis 1 | 51499 | 0.00476182 | −0.5386698 |
| ARID1B | AT rich interactive domain 1B (SWI1-like) | 57492 | 0.00238552 | −0.5438516 |
| Mad2l1bp | MAD2L1 binding protein | 9587 | 0.00620568 | −0.544755 |
| LOC100133398 | Selenoprotein T; similar to Selenoprotein T | 51714 | 0.00627308 | −0.5606207 |
| SNRNP27 | Small nuclear ribonucleoprotein 27kDa (U4/U6.U5) | 11017 | 0.0028898 | −0.6324866 |
| BCL10 | B-cell CLL/lymphoma 10; hypothetical LOC646626 | 8915 | 0.0045983 | −0.6407714 |
| arfgap3 | ADP ribosylation factor GTPase activating protein 3 | 26286 | 0.00881985 | −0.6912068 |
| Abt1 | Activator of basal transcription 1 | 29777 | 0.00359616 | −0.7326123 |
| rbm38 | RNA-binding motif protein 38 | 55544 | 0.0085592 | −0.816973 |
| TBPL1 | TBP-like 1 | 9519 | 0.00175630 | −0.9431134 |
Using the Database for Annotation, Visualization and Integrated Discovery (DAVID; National Institute of Allergy and Infectious Diseases, National Institutes of Health) functional pathway annotation tools, we analyzed the full pathways at weeks 12, 24, and 36 (Datasets S1, S2, and S3, respectively). The genes affected at week 12 of treatment included those encoding for proteins involved in the regulation (33 genes) and induction (21 genes) of transcription, cell/biological adhesion (17 genes), biological proteolytic pathways (ubiquitin conjugation) (13 genes), negative regulation of macromolecule metabolic process (17 genes), vacuole formation (eight genes), and protein (17 genes) and macromolecule (20 genes) catabolic process. Among the genes that were affected significantly, metreleptin treatment induced the transcripts involved in cell survival and development such as the cytokines IL-7 and neurotrophin-3 (NT-3) factor in PBMCs at week 12. Both IL-7 and NT-3 are involved in lymphocyte survival, their effector functions, and neural development (12, 13). Conversely, genes involved in cell death/apoptosis, such as the B-cell chronic lymphocytic leukemia/lymphoma 10 (BCL-10) and TP53-regulator of apoptosis-1 (TRIAP-1) genes, were down-modulated significantly, thus confirming an antiapoptotic/prosurvival effect of leptin (14–16). The transcription factor 7–like-1 (TCF7L1), known to be a transcriptional activator involved in T-lymphocyte differentiation and necessary for survival of CD4+ and CD8+ immature thymocytes (17), was up-regulated. In parallel with these changes, genes involved in cell adhesion and cell–cell/cell–matrix interactions, such as ADAM-metallopeptidase-23 (ADAM-23), actinin-3, vascular adhesion molecule-1 (VCAM-1), laminin-α2, and selectin-E (relevant in fertilization, muscle development, and neurogenesis), also were up-regulated (18), confirming the induction of adhesiveness and activation of immune cells by leptin previously observed in vitro (3).
Additionally, some hormones important in maintaining reproductive functions, such as FSH and progestogen-associated endometrial protein (Paep), were up-regulated by metreleptin treatment (7). Notably, in addition to its important role in reproductive function, FSH has relevant immune-modulating effects (19). These effects suggest that leptin, by inducing FSH up-regulation, also could be involved in immunomodulatory processes. Moreover, at week 12 we observed up-regulation of genes involved in the control of immune tolerance, such as the forkhead box-D3 (FOXD3) gene (20), the CD1e molecule [which is necessary for the presentation of lipid antigens to T cells (21)], and the triggering receptor expressed on myeloid cells-2 (TREM-2), which may have a role in chronic inflammation and may stimulate production inflammatory chemokines and cytokines (22).
It is interesting that at week 24 (Table S1) the transcription of the aryl-hydrocarbon receptor nuclear translocator (ARNT) was up-regulated. ARNT is a key component in the elimination of dioxins, well known to be involved in autoimmune disease susceptibility, generation of Th17 and regulatory T (Treg) cells, and protection from infections (23, 24). The BCL-2–like-11 protein, which is an apoptosis facilitator, and the cannabinoid receptor 2 (Cnr2), which is known to inhibit macrophage-induced inflammation, were both down-regulated (25). Finally, we also observed the down-modulation of the TGF-β receptor-associated protein-1 (TGFBRAP-1), which plays a role in the TGF-β/activin signaling pathways known to be involved in the control of immune responses and down-modulation of inflammation.
At week 36 (Table S2), we observed up-regulation of the receptor for the cytotoxic ligand TRAIL, TNF-R-10c. This receptor lacks a cytoplasmic death domain and hence is not capable of inducing apoptosis; rather, it protects against TRAIL-mediated apoptosis by competing with TRAIL-R1 and -R2 for binding to the ligand. The hormone gastrin also was up-regulated, whereas both interleukin enhancer-binding factor-2 (ILF-2) and the growth factor-independent 1 (GFI-1) transcription repressor were down-regulated (26, 27). The following factors are involved directly and indirectly in lymphocyte survival and function: (i) gastrin, in addition to its effect on acid secretion by gastric cells, has hematopoietic and proinflammatory activities (28); (ii) ILF-2 encodes for a nuclear factor of activated T cells (NFAT) required for T-cell expression of the IL-2 gene (29); and (iii) GFI-1 encodes a nuclear zinc finger protein that functions as a transcriptional repressor (30). GFI-1 plays a role in diverse developmental contexts, including hematopoiesis and oncogenesis (30, 31). Finally, the significant changes in gene expression seen at week 12 vs. baseline in the metreleptin-treated group were validated by real-time PCR (Fig. S5).
Acute in Vivo Metreleptin Signaling in PBMCs and in Vitro Signaling on CD4+ T Cells from Lean, Normoleptinemic Subjects.
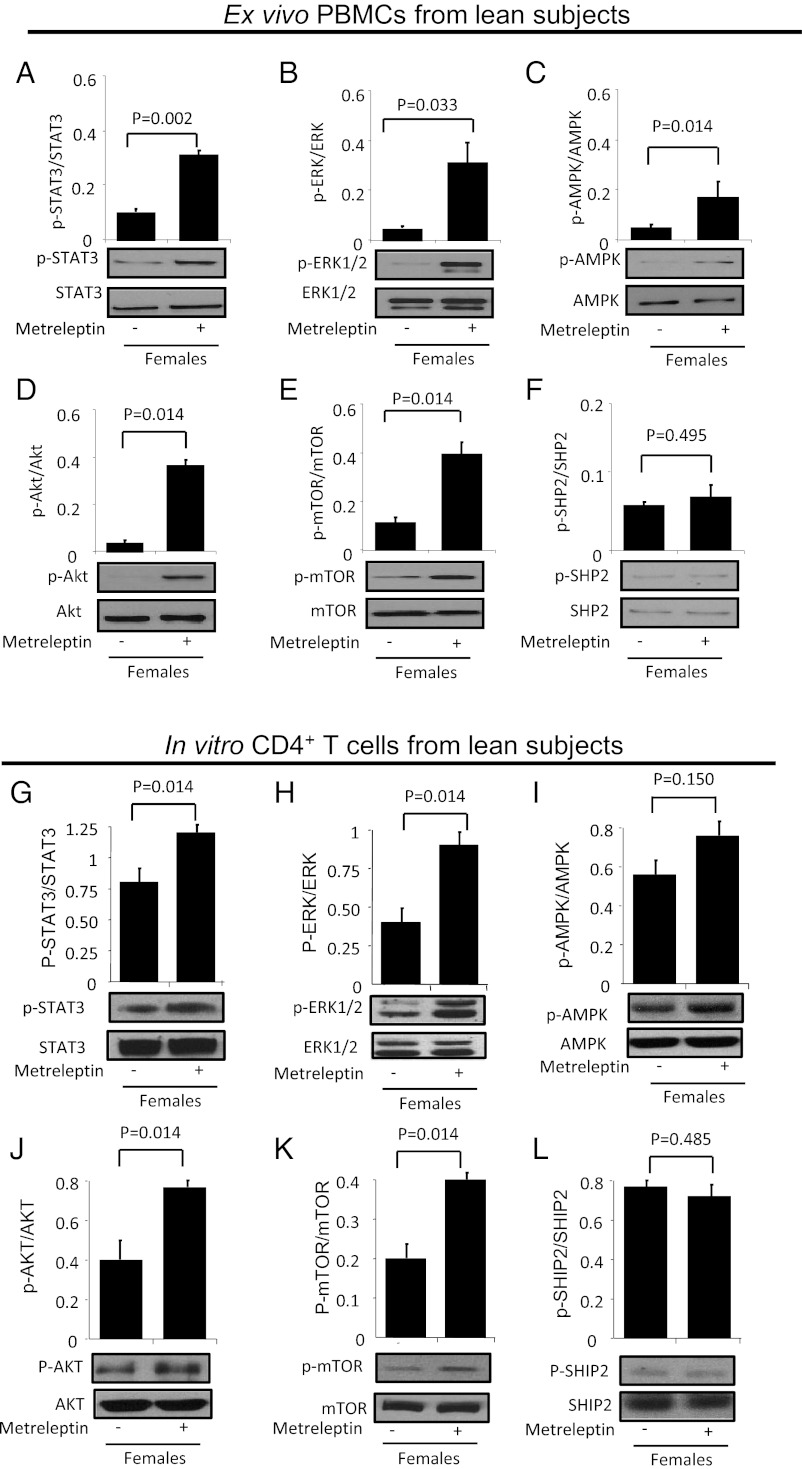
We next investigated the effects of acute in vivo metreleptin administration on signaling in both PBMCs and CD4+ T cells from lean subjects. Similar to the transcriptional signature induced by metreleptin in PBMCs taken from subjects with HA, the signaling pathways involved in cell growth/survival/proliferation, such as the STAT3, AMPK, mTOR, ERK1/2, and Akt pathways, were activated directly by acute in vivo metreleptin administration in PBMCs (Fig. 4 A–F) and by acute in vitro stimulation with metreleptin in isolated CD4+ T cells from lean subjects (Fig. 4 G–L). In contrast, acute in vivo metreleptin administration did not regulate SHP2 activation either in PBMCs or in isolated CD4+ T cells, probably because induction of LepR signaling without simultaneous TCR engagement cannot induce high counterregulatory loops mediated by SHP2.
Fig. 4.
Acute in vivo metreleptin-induced signaling in PBMCs and in vitro in CD4+ T cells from lean, normoleptinemic subjects. (A–F) Ex vivo Western blotting analyses of PBMCs from lean females upon in vivo metreleptin stimulation. (G–L) The same analyses were performed on isolated, highly purified CD4+ T cells stimulated in vitro with metreleptin for 1 h. Similar results were obtained with PBMCs in vivo and isolated CD4+ T cells in vitro. All data were analyzed using the Student t test. Values are means ± SD; n = 3.
Discussion
Prior evidence suggests that a link exists between energy status and immunity, as well as between energy status and metabolic and neuroendocrine function (32). The adipocyte-derived hormone leptin has been suggested to be involved in this process (32). Indeed, leptin acts as a signal to the brain that conveys the amount of energy stored in fat and/or acute changes in energy availability within humans. It has been proposed that leptin acts similarly as a signal that relays energy availability to immune cells (1). Although extensive experimental evidence in leptin-deficient mice and humans suggests a link between circulating leptin levels and immune function/homeostasis, little is known about the ability of leptin to affect immune homeostasis in normal individuals and in conditions of relative leptin deficiency in humans. With this study we provide evidence that, relative to normoleptinemic women, women suffering from HA and concurrent chronic acquired relative leptin deficiency have a reduced T-cell count, especially in the CD4+ T-cell subset, and have reduced T-cell proliferative capacity. We provide further evidence that administering metreleptin to these hypoleptinemic women in replacement doses not only restores their CD4+ T-cell count but also partially restores these cells’ proliferative potential. This placebo-controlled, randomized study suggests that relative leptin deficiency is associated with reduced number and function of T cells in humans and that metreleptin treatment restores immune cell (specifically CD4+ T-cell) population, size, and function. One limitation of this study is the relatively small sample size, which calls into question this study’s ability to detect a statistically significant effect; however, the results discussed herein achieved statistical significance despite our small sample size, indicating that leptin exerts powerful regulatory effects on immune function. Future studies with greater enrollment numbers are needed to confirm and expand the results for which statistical significance was not achieved; in this respect, our data may be used for power calculations and sample-size estimates for future, large, phase III clinical trials.
The capacity of metreleptin to increase CD4+ T-cell numbers, and thus augment the immune-reconstitution process, could have great impact in settings in which CD4+ T cells are reduced, such as in states of chronic energy deficiency including tuberculosis, HIV infection, anorexia nervosa, or exposure to radiation (33, 34). In the context of immune reconstitution of the CD4+ T-cell compartment, the results obtained are highly encouraging. Indeed, the normal range of CD4+ T cells in adult humans generally varies from 750–1,200 cells/mm3 (35). In placebo-treated HA subjects we observed a trend from a low degree lymphopenia to frank lymphopenia over time: The basal average CD4+ T-cell count of these subjects was 721 ± 363 cells/mm3; this cell count became 428 ± 176 cells/mm3 at week 36. This progressive decline in the CD4+ T-cell count was significant and resembled the decline in CD4+ T counts seen in the progression of other immunocompromised conditions, such as HIV infection. Although the two study groups (placebo and metreleptin) had similar basal CD4+ T-cell counts (721 ± 363 and 775 ± 234 CD4+ T cells/mm3, respectively), metreleptin treatment was able to increase subjects’ CD4+ cell count from 775 ± 234 at baseline to 1,152 ± 227 cells/mm3 at week 36; the effect of metreleptin treatment on CD4+ T-cells was even more apparent when we compared the CD4+ T-cell counts of placebo-treated vs. metreleptin-treated subjects at week 36 (428 ± 176 cells for placebo-treated subjects vs. 1,152 ± 227 cells/mm3 for metreleptin-treated subjects). This observed increase in the CD4+ T-cell count as a result of leptin treatment is dramatic when compared with the average increase in CD4+ T-cell count observed during HAART: An increase from 50 to 150 CD4+ T cells/mm3 per year (35) is considered the best possible upper limit of CD4+ T-cell restoration in HIV-infected individuals undergoing HAART, but in this study we observed an average 35–40% increase in CD4+ T-cell count over ∼36 wk. Moreover, the time needed to achieve this 35–40% increase in CD4+ T-cell count is consistent with the average time required for immune reconstitution during HAART treatment, which generally takes about 25–30 wk (35).
A specific gene signature was observed at the transcriptional level in the PBMCs of metreleptin-treated subjects: Genes involved in cell survival, hormonal response, and cell adhesion were up-regulated, and genes involved in cell death/apoptosis were down-regulated. These observations confirm that leptin administration promotes the expression of genes involved in pathways critical to cell survival, proliferation, and migration through transcriptional regulation (3, 36). These observations also help explain leptin’s marked ability to improve CD4+ T-cell survival and to promote immune reconstitution, perhaps suggesting an important role for leptin in the context of susceptibility to autoimmune disease (2). We have shown previously that leptin may moderate susceptibility to autoimmune disease through its effects on the survival of Th1/Th17 cells and the inhibition of proliferation of Treg cells (37). In the context of this observed effect on Th1/Th17 cell survival, the current finding that 24 wk of metreleptin administration induces up-regulation of ARNT expression is an interesting outcome. Aryl-hydrocarbon receptors are transcription factors known to enhance Th17 responses (23, 24). Therefore, the ability of leptin to induce transcription of ARNT must be considered a critical issue in possible adverse effects and breaks in self-tolerance during treatment, increasing the risk of inflammation and autoimmunity. These data also are in agreement with our finding that the expression of all factors directly or indirectly related with inflammation, immune response, and autoimmune disease susceptibility (including CD1e, FOXD3, and TREM-2) was up-regulated as a result of metreleptin treatment.
Although we and others recently have shown that leptin administration effectively improves the metabolic profile of subjects who have HAART-associated lipodystrophy (38–41), the results of this study indicate that metreleptin administration also may be considered as a potential therapy for other immunocompromised disease states in which CD4+ T cells play a critical role. For instance, leptin therapy may help decrease the incidence of infectious disease states. One of these states is amebiasis, which is caused by a parasite responsible for the most common form of intestinal infection leading to diarrhea in less developed countries (6). Recent observational studies have demonstrated that a specific LepR polymorphism appears to impair leptin signaling and induce higher susceptibility to this parasite (5); leptin treatment, through its immunoreconstitutive effects, may help ameliorate this increased susceptibility.
Because leptin acts on immune and neuroendocrine systems in a pleiotropic manner at multiple levels, it is possible that the immunoreconstitutive effects observed in this study with metreleptin treatment may not necessarily be caused by the direct impact of leptin on CD4+ T cells but instead may be a result of leptin’s concerted actions on stress hormones or circulating cytokines that control the survival of T cells. To explore this question, we evaluated levels of IL-2, IL-7, and IL-15 (all of which are prosurvival cytokines involved in T-cell survival and thymic selection) in participant serum. We observed no significant change in the circulating levels of these cytokines during metreleptin treatment (12). Notably, however, IL-7 transcription was up-regulated in PBMCs from metreleptin-treated HA patients. This result suggests that metreleptin treatment induced expression of IL-7 selectively in immune cells and not at a systemic level. Indeed, we observed a significant up-regulation of the IL-7 in circulating cells but saw no significant change in serum levels of IL-7, perhaps because of a dilution effect resulting from circulation. Another possible explanation for this result is that changes in concentrations of circulating cytokines are relatively less relevant than their concentration at the level of the microsite (i.e., lymph nodes, thymus, or bone marrow); the high concentration reached at the microsite can elicit a dramatic local effect by prompting gene up-regulation locally and in the recirculating cells on which our microarray was performed. Indeed, we observed a significant gene up-regulation in circulating cells but no significant change in serum IL-7, possibly because of a dilution effect resulting from circulation.
We also observed that FSH transcription was increased in immune cells as a result of metreleptin treatment. Because FSH promotes both immune cell homeostasis and inflammatory response, we cannot exclude the possibility that some of the increases seen in peripheral immune-cell count/proliferation could be caused by increased FSH expression (19). Notably, stress hormones, such as cortisol and ACTH, and other hormones such as insulin did not correlate significantly with the changes in immune function reported herein. Therefore, we hypothesize that leptin’s immunoreconstitutive actions are caused partly by (i) an indirect prosurvival effect on T cells via induction of IL-7 and FSH but also by (ii) a direct effect of leptin on LepR, which is known to be expressed on T cells. Nevertheless, in another recently conducted randomized, controlled trial, we demonstrated that HA subjects treated with metreleptin recovered menstruation and regained normal levels of hormones in the gonadal, thyroid, growth hormone, and adrenal axes (42). Therefore, despite the nonsignificant associations reported herein, the distinct possibility exists that the improvement in immune cell number and function could be related, in minor part, to the normalization of the gonadal or other axes (7, 42). This hypothesis is corroborated by the increase in FSH transcript seen in circulating immune cells from metreleptin-treated HA individuals in this study.
Studies from Tschöp et al. (43) have shown that leptin-initiated neuroendocrine pathways are able functionally to coordinate the systemic immune response. More specifically, the Tschöp group observed that leptin deficiency is associated with impaired immune response and decreased survival in a murine model of sepsis, primarily as a result of impaired neutrophil function. Genetic rescue of leptin signaling exclusively and specifically within the CNS was sufficient to improve mortality and cytokine profiles in these septic mice (43), suggesting that efficient coordination of the immune response during sepsis relies on leptin-dependent neurocircuitry in the CNS to limit organ damage and prevent mortality (43). All the observations presented herein reveal the existence of a specific CNS leptin-signaling system that controls systemic immune defense in a functionally relevant manner and suggest that leptin might act in the brain to regulate peripheral immune function directly. Therefore, it is possible that the effects observed as a part of this study in women with HA also could be ascribed to a specific CNS activity whereby metreleptin partly restores immune functions in the periphery (43).
Finally, we extended findings from these studies by performing acute in vivo leptin-signaling experiments to determine whether the pathways induced by metreleptin in PBMCs and in vitro on CD4+ T cells from lean subjects are similar to or different from those induced by metreleptin in PBMCs from HA subjects. Consistent with data obtained in PBMCs from HA subjects, in which acute in vivo metreleptin administration activates signaling pathways involved in cell growth/survival/proliferation (i.e., STAT3, AMPK, ERK1/2, mTOR, and Akt), we replicated these results in highly purified CD4+ T cells from lean subjects using an in vitro method. Although we have not examined all signaling pathways potentially mediating leptin’s actions on PBMCs in vivo, we have studied signaling pathways that we believe are of critical importance. The results potentially are biologically important. The mechanisms reported herein lend themselves to future studies with an ultimate goal of identifying in vivo leptin signaling in immune cells and states in which leptin replacement could help the immune-reconstitution process.
In conclusion, this study provides data on the ability of metreleptin treatment to facilitate immune reconstitution in nonobese subjects with acquired hypoleptinemia. These data begin to construct the conceptual basis for the use of metreleptin and its agonists in hypoleptinemic conditions of negative energy balance such as HIV, tuberculosis, and other infections with reduced T-cell counts. The effects we observed herein need to be replicated in larger studies and in different disease states associated with chronic hypoleptinemia. Whether metreleptin administration can contribute to improved morbidity and mortality in these conditions remains to be shown in future studies.
Materials and Methods
Placebo-Controlled Trial of Metreleptin Replacement in Women with HA.
Eligible subjects were women, 18–35 y old, with secondary HA, defined by an absence of menstrual cycles for 6 mo or more, in the setting of strenuous exercise and/or negative relative energy balance. All had normal weight (within 15% of ideal body weight for 6 mo or more) that had not fluctuated more than ± 5 lb over the last 6 mo. All subjects had baseline serum leptin levels <5 ng/mL but were otherwise healthy, with an absence of significant coexisting medical conditions and psychiatric diseases, including depression or past or active eating disorders, as determined using a screening questionnaire, physical examination, and routine blood tests. Other causes of absent menstrual cycles were excluded, including hypothyroidism or hyperthyroidism, polycystic ovarian syndrome, hyperprolactinemia, Cushing’s syndrome, congenital adrenal hyperplasia, or primary ovarian failure. None of the subjects had been taking medications known to affect neuroendocrine, immunological, or bone-density measurements, including glucocorticoids, anti-seizure medications, thyroid hormones, or estrogens. Also excluded were women who were breastfeeding, pregnant, or planning pregnancy within 1 y and those with a known history of hypersensitivity to Escherichia coli-derived proteins. Because the effects of metreleptin on the developing fetus are not known, all subjects were required to use double-barrier methods of contraception (diaphragm with intravaginal spermicide, cervical cap, male or female condom with spermicide). All participants were tested for pregnancy at screening and at all subsequent visits.
Study Design: Placebo-Controlled, Randomized Trial of Metreleptin Replacement in Women with HA.
This protocol was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC). Clinical-quality human recombinant leptin (metreleptin, A-100, formerly known as met-Hu-leptin; phase II clinical trial) was provided by Amylin Pharmaceuticals. Metreleptin was administered in the context of an Investigator-Initiated Study protocol and under an Investigational New Drug application by C.S.M. to the Food and Drug Administration. Twenty women were enrolled and provided written informed consent to participate in a randomized, double-blind, placebo-controlled trial. Fourteen of these subjects eventually were studied for immune phenotype and function outcomes. Data from all 20 subjects were used in the microarray analysis. Subjects were randomized in a 1:1 fashion to receive either metreleptin or placebo in the form of a self-administered daily s.c. injection (given between 7:00 PM and 11:00 PM) for a period of 36 wk. Eight subjects were randomized to receive metreleptin and six to receive placebo. The initial dose of metreleptin was 0.08 mg/kg, aiming at achieving a concentration of endogenous leptin within the normal range. If participants had no clinical response, i.e., had not begun menstruating, by week 12, their doses were increased to 0.12 mg/kg and continued at this dose until week 36. Weight was monitored at monthly visits, and metreleptin doses were adjusted accordingly. Metreleptin doses were reduced by 0.04 mg/kg if the participant lost >5% of baseline weight. Participants who lost >8% of their baseline weight or were found to be <80% of ideal body weight were withdrawn from the study. All subjects also received the current standard of care for HA, including calcium (600 mg twice daily) and vitamin D (400 IU daily) supplementation. Fasting blood samples were obtained at visits to our General Clinical Research Center at baseline and every 4 wk. In all phases of the study heparinized blood samples were collected at the baseline visit and every 12 wk thereafter and were shipped to Naples, Italy, by express courier to be processed within 48 h for immune assays. Processing of PBMCs within this time frame assures their viability, according to standard immunology procedure and verified by our extensive studies in healthy controls and leptin-deficient subjects using a similar protocol (8). PBMCs also were collected at these time points at C.S.M.’s BIDMC laboratory using the same standard immunology procedure used in Italy. RNA for microarray analysis was isolated from PBMCs at the BIDMC Genomics and Proteomics Center using the manufacturer’s protocol for TRIzol reagent (Invitrogen).
Study Design: Healthy Controls.
We additionally included 13 healthy volunteer donors in this study. The healthy controls were matched for age, BMI, and leptin levels with HA patients at baseline (Table 1). None of these healthy controls had a history of endocrine disease, autoimmune disorders, or infection. The study was approved by the Beth Israel Deaconess Medical Center institutional ethics committee, and all individuals gave written informed consent. The experimental procedures for the immune phenotype analysis of peripheral blood and lymphocyte cultures and stimulation after isolation of human PBMCs were the same as those used for the HA patients.
Acute Metreleptin Signaling Study of PBMCs in Vivo and CD4+ Cells in Vitro.
Normal, healthy, lean volunteers were recruited from the community and screened at the Clinical Research Center (CRC) at BIDMC. Subjects were excluded if they had a history of any illness that might affect insulin sensitivity, use of medications that are known to influence glucose metabolism, history of anaphylaxis or anaphylactoid-like reactions, or a known hypersensitivity to E. coli-derived proteins or anesthetic agents such as Lidocaine or Novocain. All subjects provided written informed consent to participate, and the study was approved by the institutional review board at BIDMC.
Subjects were provided with take-home meals and consumed an isocaloric diet, specifically designed for each subject, for 48 h before their main study visit to ensure stable dietary intake. On the morning of the main study visit, subjects attended the CRC after a 12-h fast. For an acute in vivo metreleptin signaling study, we performed experiments with PBMCs before and after the injection of a bolus of metreleptin (0.01 mg/kg body weight for 20 min) or placebo (10 cm3 of normal saline for 20 min). Either metreleptin or placebo was administered to three lean females (BMI 22.21 ± 0.32 kg/m2), by slow i.v. injection over 1 min.
In another group of lean subjects, human CD4+ T cells were purified from PBMCs by magnetic cell separation with the Dynabeads CD4+ T Cell Kit (Invitrogen). Soon after isolation, CD4+ cells were 95–98% pure as verified by FACS analysis. Isolated CD4 cells were used for acute in vitro molecular signaling studies.
Immunophenotype.
Immunophenotypic analysis of peripheral blood was performed with an EPICS XL flow cytometer (Beckman Coulter). Triple combinations of different anti-human mAbs, e.g., FITC– and phycoerythrin (PE)–anti-CD3, PE– and PC-5–anti-CD4, PC5–anti-CD8, PE–anti-CD16, PC5–anti-CD19, PE–anti-CD25, FITC–anti-CD45, and PE–anti-CD56 (Coulter Immunotech) were used for immunofluorescence staining.
Lymphocyte Cultures and Stimulation.
Human PBMCs were isolated by stratifying 15 mL of whole blood on 5 mL of Ficoll-Paque PREMIUM (GE Healthcare) and centrifuging the solution at 1.2 × g for 20 min. Next, the lymphocyte layer was removed and washed twice by resuspending the pellet with 40 mL of serum-free RMPI medium and centrifugation at 1,700 rpm for 5 min at room temperature. Viability of cells was verified to be 85–95% by using trypan blue staining and annexin-5 binding during flow cytometry, and serum glucose levels were measured as a marker of metabolic activity. T cells were cultured in medium supplemented with 5% (vol/vol) autologous subject serum. T-cell cultures were performed in triplicate in 96-well round-bottomed plates. PBMCs (2 × 105 per well) were stimulated in parallel with 0.1 μg/mL OKT3 (Orthoclone); 2 μg/mL phytohemagglutinin (Sigma); 0.01 μM phorbol 12-myristate 13-acetate (Sigma); 0.5 μM ionomycin (Sigma); and 10 μg/mL PPD (Northern Serum Institut). T-cell viability, assessed before each proliferative assay, was 85–95%. Stimulated PBMCs were maintained in culture for 72 h, and H3 thymidine was added (0.5 μCi per well) during the last 12 h. Cells were harvested on fiberglass filters using a 96-well cell harvester (Tomtec Inc.) and were counted in a 1205 Betaplate liquid scintillation counter (Wallace). Results are expressed as mean ± SD cpm from triplicate cultures.
Cytokines and Hormonal Measurements.
The human obesity kit (Bender Medsystems GmbH) was used for quantitative detection of soluble MPO, ICAM-1, CD40L, TNFR, MCP-1, and OPG by flow cytometry (FACSCanto-BD) according to the manufacturer’s instructions. Data were analyzed using FlowCytomix Pro-2.2 Software (Bender Medsystems GmbH). For leptin, IL-2, IL-7, IL-15, and leptin receptor measurements commercially available ELISA kits from R&D Systems were used as previously described. The following hormone levels were measured using immunoassays: cortisol, IGF1, insulin, and ACTH (Immulite; Siemens Healthcare Diagnostics). Serum leptin and free leptin were measured by RIA (Millipore) with a sensitivity of 0.5 ng/mL, an intraassay coefficient of variability (CV) of 3.4–8.9%, and an interassay CV of 3.0–6.2%.
Protein Extraction and Western Blotting.
For total cell extracts, collected samples were suspended in a lysis buffer containing 20 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 0.1 mM PMSF, 0.05% aprotinin, and 0.1% Igepal and then were incubated for 30 min at 4 °C. The suspension was centrifuged for 25 min at 12,000 rpm, and the supernatant was saved as the total extract. For Western blotting, proteins were loaded in each lane. After SDS-PAGE, proteins were blotted onto nitrocellulose membranes (Schleicher & Schuell, Inc.). The membranes were blocked for 1 h in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween 20. Incubation with primary antibodies was performed overnight in TBS containing 5% nonfat dry milk and then with HRP secondary antibodies for 2 h. After incubation with antibodies, membranes were washed with TBS containing 0.1% Tween-20. Enhanced chemiluminescence was used for detection. The following antibodies were used : anti-STAT3, anti–phospho-STAT3 (Y705), anti-mTOR, anti–phospo-mTOR, anti-SHP2, anti–phospho-SHP2 (Cell Signaling Technology), anti-ERK, anti–phospho-ERK, anti-AMPK, anti–phospho-AMPK, anti-AKT, and anti–phospho-AKT (Santa Cruz). Measurement of signal intensity on nitrocellulose membranes after Western blotting with various antibodies was performed using Image J processing and analysis software (http://rsbweb.nih.gov/ij/).
Microarray Analyses.
Total RNA was isolated from PBMCs using the manufacturer’s protocol for TRIzol reagent (Invitrogen) and was purified further using RNeasy spin columns (Qiagen). RNA concentration and purity were determined from 260 nm/280 nm absorbances. RNA integrity was determined using the Agilent 2100 BioChip (Agilent Technologies). The RNA then was subjected to a single amplification run, labeled with biotin nucleotides, digested into fragments of the proper size, and hybridized to the HT-HG-U133A and HT-HG-U133B gene microarrays (Affymetrix) following a standard protocol established by the manufacturer. Hybridized chips were reacted with FITC-avidin, and raw fluorescence intensities were read with a laser scanner. Affymetrix GeneChip raw data (CEL files) were imported into R v. 2.13 and analyzed with the bioinformatics facilities present in the BioConductor packages (44). Data were quality checked with the BioConductor packages affyPLM and affy (45). Subsequently, the data were reannotated according to the updated information present in the National Center for Biotechnology Information (NCBI) Entrez Gene database (http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF/14.1.0/entrezg.asp) and preprocessed by the Robust Multi-array (RMA) algorithm (46). The quality-checked, reannotated, and preprocessed gene-expression data were screened for differentially expressed genes by a linear model including the interaction of the treatment-by-time and the subject (pairing) terms (Y ∼ treatment * time + subject + error) followed by a moderated t test for the pairwise comparisons of interest. Genes with nominal P value < 0.01 were considered to be significantly differentially expressed. The lists of differentially expressed genes then were searched for overrepresented biological themes by the DAVID annotation tool with the default parameters (47). The microarray data used in this study are available at NCBI Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rtmtdyekqooksxa&acc=GSE36990), accession no. GSE36990.
Real-Time PCR Validation.
Differentially expressed genes in microarray analyses were verified by real-time PCR using ABI7500FAST (Applied Biosystems) with a 20-μL reaction volume consisting of cDNA transcripts, primer pairs, and SYBR Green PCR Master Mix (Applied Biosystems). Quantifications were normalized to 18S in each reaction. The sequences for the primers are listed in Table S3.
Statistics.
Differences in baseline characteristics between HA and control subjects were calculated using unpaired t tests. Where data were not normally distributed, we used nonparametric Mann–Whitney U tests. The statistical software used was GraphPad InStat3 version 3.0. For the acute in vivo metreleptin signaling study, data were analyzed with the Student t test and/or one-way ANOVA followed by a post hoc test for multiple comparisons. Analyses were carried out using SPSS (version 11.5, SPSS).
Supplementary Material
Acknowledgments
We thank Claudio Procaccini, Fortunata Carbone, and Mario Galgani for critical reading of the manuscript; Jean Chan, MD, for contributions during initial phases of the study; and the nurses, technicians, and nutritionists at the Beth Israel Deaconess Medical Center General Clinical Research Center and Core Laboratory for their assistance in the conduct of the study. Amylin Pharmaceuticals supplied recombinant human leptin for this study and approved the design of the study but had no role in study design; conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The C.S.M. laboratory is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58785, DK79929, DK81913, and AG032030. Funding also was received from the National Institutes of Health–National Center for Research Grants UL1 RR025758 and M01-RR-01032; the Harvard Clinical and Translational Science Center; and the National Center for Research Resources. G.M. is supported by grants from the European Union Ideas Programme, European Research Council Starting Grant ‘‘menTORingTregs’’ 310496, Telethon-Juvenal Diabetes Research Foundation Grant GJT08004, and Fondi per la Ricerca di Base Medical Research in Italy Grant RBNE08HWLZ.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE36990).
Clinical trial registration: clinicaltrials.gov registration nos. NCT00130117 and NTC01275053.
See Author Summary on page 3225 (volume 110, number 9).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214554110/-/DCSupplemental.
References
- 1.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 3.Lord GM, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 4.Papathanassoglou E, et al. Leptin receptor expression and signaling in lymphocytes: Kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176(12):7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggal P, et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121(3):1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou SH, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA. 2011;108(16):6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JL, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA. 2006;103(22):8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy GS, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis. 2003;36(6):795–802. doi: 10.1086/367859. [DOI] [PubMed] [Google Scholar]
- 10.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: Clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88(5):1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 11.Oral EA, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91(2):621–628. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 12.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 13.Abram M, et al. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009;182(8):4705–4712. doi: 10.4049/jimmunol.0802814. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, et al. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14(2):513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- 15.Tang BL. Leptin as a neuroprotective agent. Biochem Biophys Res Commun. 2008;368(2):181–185. doi: 10.1016/j.bbrc.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, et al. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128(1):21–26. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Chen Q, Nguyen T, Yu Q, Sen JM. T cell factor-1 and β-catenin control the development of memory-like CD8 thymocytes. J Immunol. 2012;188(8):3859–3868. doi: 10.4049/jimmunol.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone F, et al. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J Reprod Immunol. 2010;85(2):172–179. doi: 10.1016/j.jri.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Alkhateeb A, Fain PR, Spritz RA. Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. J Invest Dermatol. 2005;125(2):388–391. doi: 10.1111/j.0022-202X.2005.23822.x. [DOI] [PubMed] [Google Scholar]
- 21.De Libero G, Mori L. Mechanisms of lipid-antigen generation and presentation to T cells. Trends Immunol. 2006;27(10):485–492. doi: 10.1016/j.it.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184(1-2):92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M. A toxin-sensitive receptor able to reduce immunopathology. Nat Immunol. 2010;11(9):779–781. doi: 10.1038/ni0910-779. [DOI] [PubMed] [Google Scholar]
- 24.Marshall NB, Kerkvliet NI. Dioxin and immune regulation: Emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215(8):598–605. doi: 10.1016/j.imbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ischia J, Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide: Different forms, different functions. Biofactors. 2009;35(1):69–75. doi: 10.1002/biof.10. [DOI] [PubMed] [Google Scholar]
- 27.Marcoulatos P, Koussidis G, Mamuris Z, Velissariou V, Vamvakopoulos NC. Mapping interleukin enhancer binding factor 2 gene (ILF2) to human chromosome 1 (1q11-qter and 1p11-p12) by polymerase chain reaction amplification of human-rodent somatic cell hybrid DNA templates. J Interferon Cytokine Res. 1996;16(12):1035–1038. doi: 10.1089/jir.1996.16.1035. [DOI] [PubMed] [Google Scholar]
- 28.Czepielewski RS, et al. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc Natl Acad Sci USA. 2012;109(2):547–552. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt A, et al. Human regulatory T cells rapidly suppress T cell receptor-induced Ca(2+), NF-κB, and NFAT signaling in conventional T cells. Sci Signal. 2011;4(204):ra90. doi: 10.1126/scisignal.2002179. [DOI] [PubMed] [Google Scholar]
- 30.Hock H, Orkin SH. Zinc-finger transcription factor Gfi-1: Versatile regulator of lymphocytes, neutrophils and hematopoietic stem cells. Curr Opin Hematol. 2006;13(1):1–6. doi: 10.1097/01.moh.0000190111.85284.8f. [DOI] [PubMed] [Google Scholar]
- 31.Duan Z, Horwitz M. Gfi-1 oncoproteins in hematopoiesis. Hematology. 2003;8(5):339–344. doi: 10.1080/10245330310001612116. [DOI] [PubMed] [Google Scholar]
- 32.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15(14):2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 33.van Crevel R, et al. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87(2):758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 34.Matarese G, et al. Serum leptin and CD4+ T lymphocytes in HIV+ children during highly active antiretroviral therapy. Clin Endocrinol (Oxf) 2002;57(5):643–646. doi: 10.1046/j.1365-2265.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 35.Abrams D, et al. INSIGHT-ESPRIT Study Group SILCAAT Scientific Committee Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361(16):1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galgani M, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474–7479. doi: 10.4049/jimmunol.1001674. [DOI] [PubMed] [Google Scholar]
- 37.Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev. 2012;249(1):116–134. doi: 10.1111/j.1600-065X.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91(7):2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 39.Magkos F, et al. Leptin replacement improves postprandial glycemia and insulin sensitivity in human immunodeficiency virus-infected lipoatrophic men treated with pioglitazone: A pilot study. Metabolism. 2011;60(7):1045–1049. doi: 10.1016/j.metabol.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Addy CL, et al. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88(2):627–636. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 41.Mulligan K, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94(4):1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sienkiewicz E, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60(9):1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Tschöp J, et al. CNS leptin action modulates immune response and survival in sepsis. J Neurosci. 2010;30(17):6036–6047. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 46.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 47.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]