Abstract
Grooming, a common behavior in animals, serves the important function of removing foreign materials from body surfaces. When antennal grooming was prevented in the American cockroach, Periplaneta americana, field emission gun scanning electron microscopy images revealed that an unstructured substance accumulated on nongroomed antennae, covering sensillar pores, but not on groomed antennae of the same individuals. Gas chromatography analysis of antennal extracts showed that over a 24-h period nongroomed antennae accumulated three to four times more cuticular hydrocarbons than groomed antennae. Moreover, nongroomed antennae accumulated significantly more environmental contaminants from surfaces (stearic acid) and from air (geranyl acetate) than groomed antennae. We hypothesized that the accumulation of excess native cuticular hydrocarbons on the antennae would impair olfactory reception. Electroantennogram experiments and single-sensillum recordings supported this hypothesis: antennae that were prevented from being groomed were significantly less responsive than groomed antennae to the sex pheromone component periplanone-B, as well as to the general odorants geranyl acetate and hexanol. We therefore conclude that antennal grooming removes excess native cuticular lipids and foreign chemicals that physically and/or chemically interfere with olfaction, and thus maintains the olfactory acuity of the antennae. Similar experimental manipulations of the German cockroach (Blattella germanica), carpenter ant (Camponotus pennsylvanicus), and the housefly (Musca domestica), which use different modes of antennal grooming, support the hypothesis that antennal grooming serves a similar function in a wide range of insect taxa.
Insects, like most animals, groom themselves regularly (1), but the diverse functions of self-grooming have been scarcely investigated. Major proposed functions of grooming are to remove debris (2, 3), parasitoids (4), and pathogens (5). It has also been shown that grooming can be evoked by irritant chemicals (6, 7), but grooming-facilitated removal of environmental chemicals has not been demonstrated. Mutual grooming (allogrooming), likewise, can remove pathogens, especially from nestmates in social insects (8, 9). Self-grooming also serves to redistribute antimicrobial substances over the body surface (10), and a similar function has been suggested for redistribution of cuticular lipids (2).
Paradoxically, however, insects meticulously self-groom, especially their sensory appendages (e.g., antennae), even in clean environments free of pathogens and dust. Although it is intuitively evident that sensory organs should be regularly groomed to keep them responsive to the environment, and the mechanics of these behaviors have been comprehensively described in various insect species (2, 11–17), the composition of the materials removed by regular grooming has not been analyzed and the adaptive outcomes of this behavior have not been investigated.
We observed that antennae of the American cockroach, Periplaneta americana (Linnaeus) (Blattodea, Blattidae), that were immobilized for electrophysiological studies, accumulated a shiny substance on their surface (18). We hypothesized that grooming might serve to remove these excess native secretions and compared groomed and nongroomed antennae of male P. americana cockroaches using field emission gun scanning electron microscopy (FEG SEM). We also investigated the chemical nature of the accumulated material and the relationship between antennal grooming and the amount of this material on the antennae of four species representing three insect orders with different means of antennal grooming. In the American cockroach, and the German cockroach, Blattella germanica (L.) (Blattodea, Blattellidae), a foreleg serves the contralateral antenna to the mouth, which cleans the antenna from base to tip (15, 16). In the carpenter ant, Camponotus pennsylvanicus (De Geer) (Hymenoptera, Formicidae), a specialized structure on the foreleg—the basitarsal brush—grooms the ipsilateral antenna, and then the basitarsal brush is orally groomed (17). The house fly, Musca domestica (L.) (Diptera, Muscidae), does not use its mouth for antennal grooming, but instead sweeps the forelegs, and rarely the middle legs, over the head and cleans head appendages (19). By preventing antennal grooming we now demonstrate that large amounts of cuticular hydrocarbons (CHCs) accumulate on nongroomed antennae of all four species. We further tested whether grooming also removes environmental contaminants from the antennae, and using electroantennogram (EAG) and single-sensillum recordings (SSRs) we assessed whether grooming enhances the olfactory acuity of the antennae of P. americana. Our results support the notion that self-grooming physically removes excess native cuticular lipids as well as extrinsic chemicals from olfactory sensilla and thus maintains the insect’s olfactory acuity to all odorants.
Results
Grooming Cleans Olfactory Sensilla on the Antenna.
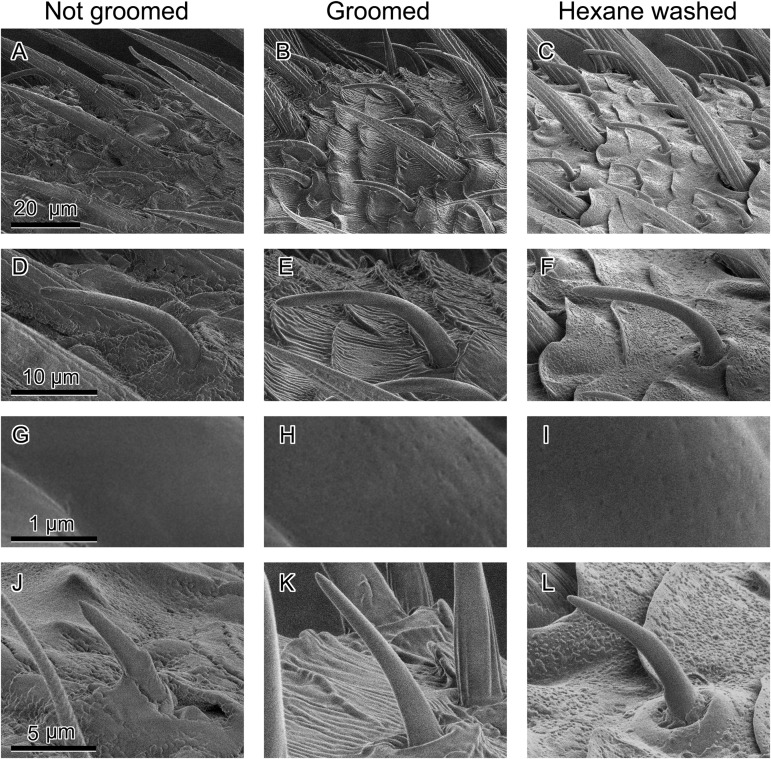
The FEG SEM technique allows imaging at high resolution with minimal sputter coating, while at the same time, minimizing the electrical charging caused by the nonconductive cuticular lipids, the outermost layer of the cuticle. FEG SEM revealed significant accumulation of unstructured material on antennae that were prevented from being groomed for 24 h (Fig. 1A), but not on the contralateral groomed antennae of male P. americana (Fig. 1B). This material is readily removed with a 2-min hexane extraction of the antenna (Fig. 1C). The olfactory units of the insect antenna are various sensilla with numerous cuticular pores that guide odorants to the olfactory receptor neurons. To assess whether the accumulated material affected the olfactory pores, we focused on the single-walled sensilla type B, known to detect the major female-produced sex pheromone component periplanone-B (20); these sensilla (Fig. 1 D–F) were also easily distinguishable morphologically from other olfactory sensilla. The accumulated material completely covered the sensillar bases and pores on all of the swB sensilla we observed on nongroomed antennae (Fig. 1G). On the other hand, a grooved material coating was visible on groomed antennae (Fig. 1 B and E), and the sensillar pores were clearly discernible on swB sensilla (Fig. 1H). Hexane removed this material (Fig. 1 C and F), and the sensillar pores were clearly visible, as in the groomed antennae (Fig. 1I). Other types of olfactory sensilla were similarly affected by grooming, as shown for single-walled type A (swA) olfactory sensilla (Fig. 1 J–L).
Fig. 1.
Accumulation of material on nongroomed antennae, covering olfactory pores. Field emission gun scanning electron microscope images of nongroomed, groomed, and hexane-washed antennae of male P. americana. Large amounts of unstructured material completely cover the surface of nongroomed antennae, including the bases of all sensilla (A), groomed antennae have grooved material on their surface (B), whereas this material is completely removed from the surface of hexane-washed antennae (C). Images in the second row (D–F) show pheromone-sensitive sensilla, morphological type swB (20), whereas the third row shows high-magnification images of the same sensilla around the middle of their shaft. Sensillar pores are completely covered and not visible on the nongroomed antenna (G), but they are clearly visible on sensilla of the groomed antenna (H) of the same insect, as well as on hexane-washed antennae (I). The last row of images shows hexanol-sensitive single-walled type A (swA) olfactory sensilla on the nongroomed (J), groomed (K), and hexane-washed (L) antennae.
Grooming Removes Excess Antennal Cuticular Hydrocarbons.
To identify and quantify the material on nongroomed and groomed antennae, we used two independent approaches to prevent antennal grooming in P. americana males. In the first approach, grooming of one antenna was prevented by a rigid ring glued to the head, whereas the other antenna of the same male could be groomed and served as control (Movie S1). We confirmed that antennal grooming was indeed prevented by stimulating both antennae with an irritant compound, formic acid; all grooming attempts of the restrained antennae were unsuccessful in four cockroaches, whereas the control antennae of the same individuals were immediately groomed. The second approach was to glue the mouthparts, thus preventing both antennae from being groomed.
Because the FEG SEM results implicated hexane-soluble material, we suspected that CHCs might be involved. Indeed, gas chromatography (GC) of antenna extracts confirmed that significantly more CHCs accumulated on restrained and nongroomed P. americana antennae than on groomed antennae (Fig. 2 B and C); however, the GC profiles of groomed and nongroomed antennae were the same (Fig. 2A). Within 24 h, the restrained antennae accumulated 3.8 times more CHCs than the control antennae of the same individuals (Student’s paired t test, t = 8.309, P = 0.0002) (Fig. 2B). Glue-treated antennae of control cockroaches that were also exposed to CO2 and ice anesthesia contained the same amount of CHCs as the untreated antennae (P = 0.696) (Fig. 2B), confirming that the accumulation of CHCs on the restrained antennae was due solely to lack of grooming. Similarly, the antennae of cockroaches with glued mouthparts accumulated 3.5 times more CHCs in 24 h than the antennae of untreated cockroaches (Student’s unpaired t test, t = 18.423, P < 0.0001) (Fig. 2C).
Fig. 2.
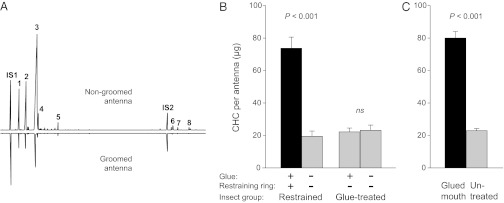
Cuticular hydrocarbons (CHCs) accumulate on nongroomed antennae of male P. americana. Gas chromatographic CHC profiles of restrained (nongroomed) and control (groomed) antennae of a male P. americana (A). Peaks are as reported by Jackson (21) and Saïd et al. (22): n-pentacosane (A, 1), 3-methylpentacosane (A, 2), (Z,Z)-6,9-heptacosadiene (A, 3), n-heptacosane (A, 4), n-nonacosane (A, 5), (Z)-15-hentetracontene (A, 6), 13-methylhentetracontane (A, 7), and tritetracontadiene (A, 8). Internal standards n-tetracosane (IS1) and n-tetracontane (IS2) were used to quantify CHCs on the antennae. Amounts of CHCs (mean ± SEM) on nongroomed and groomed antennae (B and C). In glue-control insects (n = 7) one antenna was treated with glue that did not interfere with grooming; in the “restrained” group grooming of one antenna was prevented while the other antenna could be groomed (n = 7) (B). Another set of cockroaches was either untreated (n = 7) or their mouthparts were glued to completely prevent antennal grooming (n = 7) (C). Significant differences are indicated between nongroomed and groomed antennae of the same individuals (Student’s paired t test) and between mouthparts-glued and untreated cockroaches (Student’s unpaired t test); ns, not significant.
Antennal grooming is highly efficient at removing accumulated CHCs. We prevented antennal grooming in male P. americana for 24 h and then stimulated a single act of antennal grooming. This single passage of the antenna through the mouth removed 35% of the CHCs from the antenna (Fig. S1). Nevertheless, the antenna still contained three times as much CHCs as the normal groomed antenna of the same cockroach (Student’s paired t test, t = 5.910, P = 0.0004), showing that, whereas grooming is a highly efficient mechanism for removing excess CHCs from the antennae, multiple grooming events are required for normal antennal maintenance, as seen in freely behaving insects.
Generalization to Other Insects.
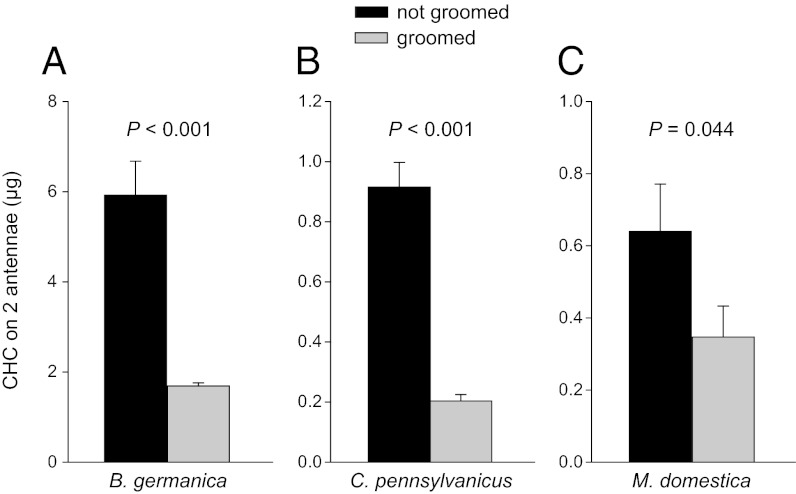
We extended our observations from P. americana to B. germanica (B.g.), a related cockroach species in a different family (Blattellidae) and two evolutionarily more distant insect species, the carpenter ant C. pennsylvanicus (C.p.) and the housefly M. domestica (M.d.). As in P. americana, when the antennae of these three species were prevented from being groomed—by gluing the mouthparts of the cockroach or by removing the ant’s and fly’s forelegs—they accumulated 3.5, 4.5, and 1.8 times more CHCs, respectively, than the corresponding control insects (Student’s unpaired t tests, B.g.: t = 8.403, P < 0.0001; C.p.: t = 8.398, P < 0.0001; and M.d.: t = 1.873, P = 0.044) (Fig. 3). In these insects, as in P. americana, nongroomed antennae had similar CHC profiles as groomed antennae.
Fig. 3.
Species representing three insect orders accumulate excess CHCs on the antennae when grooming is prevented. Accumulation of CHCs on nongroomed and groomed antennae of B. germanica adult male cockroaches (n = 10) (A), C. pennsylvanicus worker ants (n = 10) (B), and M. domestica adult male flies (n = 7–9) (C). Mean ± SEM are shown, and significant differences between respective treatments are indicated (Student’s unpaired t test).
Antennal Grooming Removes Environmental Chemicals.
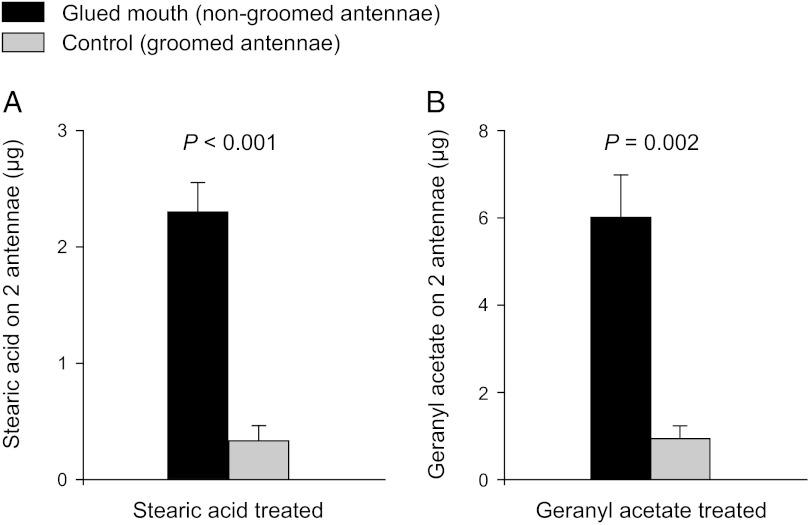
We hypothesized that antennal grooming might also serve to remove foreign chemicals acquired from the environment. Cockroaches were exposed to stearic acid, a relatively nonvolatile chemical that was applied on the inner surface of experimental jars, and to the headspace of the volatile chemical geranyl acetate. Both compounds accumulated in significantly greater amounts on nongroomed antennae of cockroaches with glued mouthparts than on groomed antennae of the control cockroaches; seven times more stearic acid (Student’s unpaired t test, t = 6.893, P < 0.0001) and six times more geranyl acetate (t = 4.964, P = 0.0016) were found on nongroomed than on groomed antennae (Fig. 4 A and B). As in earlier experiments, native CHCs also accumulated significantly more on nongroomed than on groomed antennae (Fig. S2).
Fig. 4.
Environmental chemicals accumulate on nongroomed antennae of male P. americana. Amounts of stearic acid (A) and geranyl acetate (B) (mean ± SEM) are presented. Mouthparts-glued (n = 8) and control (n = 7) cockroaches were kept in stearic acid-coated canning jars for 24 h (A) or in jars with geranyl acetate in the headspace (B) (n = 7). Significant differences are indicated between nongroomed and groomed antennae of mouthparts-glued and control cockroaches, respectively (Student’s unpaired t test).
Antennal Grooming Enhances the Sensitivity of the Peripheral Olfactory System.
Because the FEG SEM images revealed that sensillar pores are only exposed on groomed antennae but not on nongroomed antennae, we investigated the responsiveness of the olfactory system of groomed and nongroomed antennae of male P. americana. First, we conducted EAG dose–response studies to determine whether excess CHCs could impair olfactory sensitivity at the whole antenna level. Groomed antennae were significantly more responsive to the sex pheromone than the nongroomed antennae of the same individuals; the median effective concentration (EC50) was 2.6 ng (95% fiducial limits 2.24, 3.10) and 15.9 ng (13.53, 18.64), respectively, and responses of the two antennae from the same individuals were significantly different at both 1 and 10 ng periplanone-B (Student’s paired t test, P < 0.05) (Fig. 5A). In contrast, EAG responses of the glue-treated and untreated antennae of control cockroaches (both antennae groomed) broadly overlapped, showing no significant differences in sensitivity to the pheromone [P > 0.05; EC50 1.4 ng (1.20, 1.65) and 2.0 ng (1.70, 2.43), respectively] (Fig. 5B). Similarly, control groomed antennae were significantly more responsive to 10 and 100 µg of geranyl acetate than the nongroomed antennae of the same individuals (Student’s paired t test, P < 0.05) (Fig. 5C), whereas there was no significant difference in the EAG responses of the control glue-treated and untreated antennae at any geranyl acetate dose (Fig. 5D). We obtained similar results with hexanol: EAG responses of groomed antennae were almost twice the amplitude of nongroomed antennae (Student’s unpaired t test, t = 5.897, P < 0.0001) (Fig. 5E).
Fig. 5.
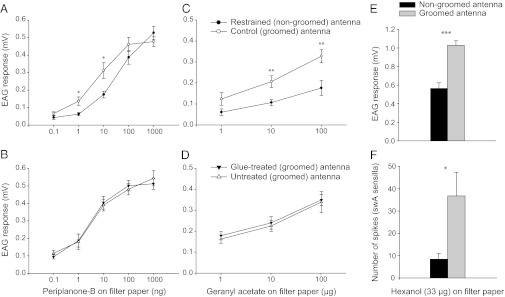
Antennal grooming enhances the sensitivity of the olfactory system. Electroantennogram (EAG) dose–response results of male P. americana antennae stimulated with periplanone-B (n = 8) (A and B) and geranyl acetate (n = 6) (C and D). In the experimental group one antenna of each cockroach was prevented from being groomed (A and C). In the control group both antennae were allowed to be groomed, with one antenna being sham glue treated (B and D). Results of EAG recordings (E) and single sensillum recordings (SSRs) with hexanol-sensitive swA sensilla (F) on groomed (n = 12 EAG, 9 SSR) and nongroomed antennae (n = 11 EAG, 10 SSR) of male P. americana. The absolute EAG responses are presented in A–D, whereas in E and F the responses to the mineral oil control (vehicle) were subtracted from the respective sensillum responses to hexanol. Mean ± SEM are shown. Differences in the responses of pairs of antennae from the same individuals (A–D) are indicated for each dose (Student’s paired t test), and differences in the responses between treatment groups (E and F) are also indicated (Student’s unpaired t test): *P < 0.05, **P < 0.01, ***P < 0.001.
We next investigated the effect of CHC accumulation on the responsiveness of olfactory receptor neurons in individual sensilla: hexanol-sensitive swA sensilla (Fig. 1 J and K) on nongroomed and groomed antennae of male P. americana were tested. Cockroaches were restrained individually in small boxes for 24 h so they could not groom their antennae. Some cockroaches in this treatment were released into clean jars and allowed to groom their antennae three times before being prepared for electrophysiological recordings. Olfactory sensilla on groomed antennae were significantly more responsive to hexanol (Student’s unpaired t test, t = 2.609, P = 0.0283), generating nearly five times more spikes than sensilla on nongroomed antennae (Fig. 5F).
Discussion
We have shown that antennal grooming serves to mitigate the efflux of cuticular lipids to the epicuticular surface of the antenna. Disruption of this behavior results in the accumulation of large amounts of native CHCs not only on the antennal surface but also on the surface of olfactory sensilla, which interferes with olfaction. Because nongroomed P. americana antennae were less responsive than groomed antennae to both classes of odorants, sex pheromone and general odorants, we conclude that disruption of grooming interferes with general olfaction. Moreover, our observations with four phylogenetically diverse species indicate that this hitherto unknown role for grooming is common to a wide diversity of insects, encompassing species that groom their antennae directly with the mouth (e.g., cockroaches), with the forelegs (e.g., flies), or with specialized structures on the forelegs and subsequently the mouth (e.g., ants).
Cuticular hydrocarbons are often the predominant compounds on the epicuticular surface of insects. They prevent water loss from the cuticular surface (23) and likely serve the same function on the antennal surface, which has an extraordinarily large area punctuated with numerous sensilla, membranes, and cuticular pores. CHCs also function as barriers to pathogen penetration, and in many insects they serve as species-, sex-, and nestmate-recognition signals, which are commonly deposited onto the antennal surface, the anterior-most appendage of most insects (24). It is not clear, however, whether native CHCs also have specific functions in sensory reception on the antennal surface. It has been postulated that antennal CHCs might serve as an “olfactory lens,” possibly guiding odorants to the sensillar pores (25), but empirical support for this notion is lacking. Our results suggest that the fine coordination of antennal grooming mitigates the large efflux of CHCs, preventing the sensillar pores from being completely buried under the CHC layer.
It is unknown what mechanisms regulate the transfer of CHCs to the epicuticular surface. Our observations in four insect species that large amounts of CHCs quickly accumulate on antennae that were prevented from being groomed suggest a relatively rapid efflux of CHCs onto the antennal surface. Moreover, the profile of the excess CHCs that accumulated on the antennae was similar to the CHCs on groomed antennae. Therefore, it appears that the antennal epicuticular surface is continuously supplied with CHCs, as is the rest of the body. The adaptive significance of a continuous deposition of CHCs is also enigmatic. It may be related to the characteristic waterproofing functions of CHCs on epicuticular surfaces and also to the aforementioned proposed sensory function of CHCs in facilitating odorant delivery into the sensillar pores (25). When excess CHCs accumulate on olfactory sensilla the delivery of odorants through the pores may be hindered, compromising olfaction. The coordinated actions of recurrent CHC deposition and frequent grooming may achieve an optimal CHC layer on the sensillar surface that both facilitates olfaction and prevents desiccation.
An often proposed but never-tested hypothesis is that antennal grooming serves to remove environmental chemical contaminants from the epicuticular surface. Our results not only empirically demonstrate that antennal grooming efficiently removes foreign chemicals from the antennae, but also highlight the importance of this behavior in light of the efflux of native CHCs. CHCs readily adsorb and solubilize environmental chemicals, and the continuous CHC deposition on the antennal surface may further entrap extrinsic chemicals and interfere with olfaction and gustation. Odorants absorbed in the CHC layer might also stimulate receptor neurons long after the odor has dissipated, decreasing the time resolution of olfactory stimuli. The physical removal of CHCs through grooming may maintain a fresh layer of CHCs on the epicuticular surface, as well as a fresh and reliable chemical signal free of extrinsic contaminants.
Our results provide compelling support for a critical role of self-grooming in the regulation of CHC homeostasis. When grooming is disrupted or prevented, large amounts of excess CHCs accumulate on sensory structures, significantly impeding olfactory reception. In intact insects, on the other hand, species-typical grooming behaviors effectively remove the excess CHCs together with environmental chemicals and sustain the acuity of olfactory sensilla. A comprehensive survey of the differential accumulation of CHCs on different body parts of insects, coupled with observations of differential grooming of their various sensory appendages, should yield clues about the multiplicity of functions that CHCs play in arthropod evolution.
Materials and Methods
Chemicals.
Hydrocarbon standards n-tetracosane, n-hexacosane, n-dotriacontane, and n-tetracontane (all 99%), and geranyl acetate (98%) were purchased from Sigma-Aldrich. Hexanol (hexan-1-ol) was from Merck and mineral oil (oleum vaselini, P 71.273.2) was from Tver Pharmaceutical. Periplanone-B (98%) was a gift from d-CON (Parsippany, NJ) from a synthesis by Dr. Timothy L. Macdonald (University of Virginia, Charlottesville, VA). Omnisolv High Purity Solvent hexane (EMD Chemicals) was used for extractions.
Insects.
Adult male P. americana were 2–8 wk old, and adult male B. germanica were 12 d old. The cockroach colonies were maintained at 26–27 °C under a 12:12 light–dark photoperiod with access to water and dry LabDiet rat chow (5001; PMI Nutrition International). A queenright colony of carpenter ants, C. pennsylvanicus, was collected locally and major workers were used in experiments. Experimental insects were kept in groups in a climate chamber under the same conditions as above for at least a week before experiments. M. domestica adult males were 3 d old and maintained on sugar, powdered low-fat milk, and water. All experimental treatments were conducted in the insect’s scotophase (P. americana and B. germanica) or photophase (C. pennsylvanicus and M. domestica).
Prevention of Antennal Grooming.
Insects were anesthetized with CO2 and held on ice during the preparation procedure. Grooming of the antennae was prevented in P. americana in three ways: (i) the mouthparts were glued using cyanoacrylate gel, (ii) movement of one antenna was prevented by gluing a small ring cut from a pipette tip to its base and the head, or (iii) cockroaches were restrained in a box that prevented antennal grooming. In the second treatment, a similar ring was pulled over the other antenna and removed immediately to control for effects of the manipulation. In a control group of cockroaches the base of one antenna was treated with glue to account for potential detrimental effects of the glue while the other antenna remained untreated. Unilateral treatments were alternated between the right and left antennae. Another control group of cockroaches was handled as the treated insects, but the antennae were not manipulated. All experimental P. americana were placed individually into glass jars (20 cm high × 18 cm deep) that were covered with aluminum foil and kept in the climate chamber for 24 h before extraction of CHCs.
The third approach to prevent antennal grooming was used for EAG and SSR with hexanol stimulation. Two- to 4-wk-old P. americana males were restrained in a 15 × 45 × 9 mm acrylic box with only the head protruding through a 3-mm wide notch. Cockroaches were able to (and did) move their legs and antennae but were prevented from grooming the antennae. Restrained cockroaches were kept in the dark for 24 h with no food or water. To obtain cockroaches with groomed antennae, individual males were released into a 1-L glass canning jar covered with aluminum foil and allowed to groom their antennae three times before they were transferred into the electrophysiological setup. Males with nongroomed antennae were transferred from the restraining box into the electrophysiological apparatus.
To prevent grooming in B. germanica, we glued the mouthparts and individual cockroaches were maintained for 24 h in glass test tubes (2.3 cm diameter × 14 cm long). The forelegs or middle legs of C. pennsylvanicus major workers were severed and individual ants were maintained for 40 h in 4-mL Teflon-capped glass vials. Similarly, the forelegs of M. domestica males were severed and individual flies were maintained for 24 h in 4-mL Teflon-capped glass vials.
Effects of a Single Grooming Event on Antennal CHCs.
A P. americana male was placed in a 20-mL plastic tube for 24 h with only its head protruding through a parafilm cover, thus preventing the insect from grooming its head. To stimulate a single antennal grooming event, the forelegs were exposed, and the distal part of one antenna of each cockroach was momentarily touched with the tip of a Pasteur pipette that had been briefly dipped in formic acid. Immediately after a single antennal grooming event the insect was anesthetized with CO2 and the groomed and nongroomed antennae were extracted separately.
Exposure to Environmental Chemicals.
To investigate the accumulation on the antennae of a chemical acquired through antennal contact, mouthparts-glued and control male P. americana were kept individually in stearic acid-coated jars with uncoated plastic lids for 24 h. We used stearic acid because it is common in the environment as a component of fats, oils, soaps, cosmetics, and even candles; moreover, it is solid at room temperature and would therefore be acquired primarily through direct antennal contact. Canning jars (Mason jars, 473 mL) were surface coated with stearic acid solution in pentane to achieve a 10 μg/cm2 surface concentration of stearic acid. To examine the accumulation of airborne chemicals on the antennae, mouthparts-glued and control male P. americana were kept individually in similar jars containing 10 μL of geranyl acetate (∼9.1 μg), a common component of essential oils used in creams, soaps, and foods. Geranyl acetate was placed in a small aluminum tray enclosed in a plastic Petri dish with a screen lid, which prevented the cockroaches from directly contacting the chemical. The jars were covered with aluminum foil.
Extraction of CHCs and Chemical Analysis.
P. americana antennae were individually extracted for 2 min in 1 mL of hexane while still attached to the head. After evaporation of the solvent, the residue was redissolved in 100 μL of hexane containing 10 μg each of n-tetracosane and n-tetracontane as internal standards. In the experiments with stearic acid and geranyl acetate, both antennae were extracted for 2 min in 1 mL of hexane containing 10 μg each of n-tetracosane and n-tetracontane, as well as n-hexadecane, n-tetracosane, and n-tetracontane, respectively, as internal standards. Samples were evaporated to about 100 μL before analysis. Due to a relatively high limit of quantitation for stearic acid, samples of groomed antennae were evaporated further to near dryness and dissolved in 2 µL of octane. For B. germanica, C. pennsylvanicus, and M. domestica both antennae from each individual were detached and extracted in 100 µL (cockroach) or 50 µL hexane (ant and fly) containing 100 ng of n-hexacosane, 50 ng of n-dotriacontane, or 50 ng n-docosane, respectively, as internal standards. The antennal extracts were evaporated and redissolved in 2 µL octane.
Samples were analyzed on a DB-5 capillary column (20 m length × 0.18 mm internal diameter × 0.18 µm film thickness) in a 7890A Agilent gas chromatograph equipped with a flame ionization detector (GC-FID) and a 7683B Agilent autosampler controlled with Chemstation (Agilent Technologies). Quantification of CHCs was based on peak area relative to that of the respective internal standard. For the quantification of stearic acid and geranyl acetate calibration curves were established using n-tetracosane and n-hexadecane, respectively, as internal standards.
FEG SEM.
P. americana males were prepared by restraining one antenna with a plastic ring as described above. After 24 h, a single grooming event was stimulated in the free-moving antenna with formic acid and the cockroaches were immediately anesthetized. The antennae were detached at the base and blotted. Cut sections of each antenna were affixed in parallel with double-sided conductive carbon tape attached to an aluminum sample mount (Electron Microscopy Sciences). Samples were lightly coated with gold palladium to about 3 nm (Hummer II, Technics). FEG SEM images were obtained in high vacuum (QUANTA 3D FEG; FEI Company).
EAG and SSR.
Responses of male P. americana with plastic ring-restrained antennae (nongroomed) and glue-treated (groomed) control antennae were tested with different amounts of periplanone-B and geranyl acetate. Cockroaches were anesthetized 24 h after treatment with CO2 and a 2.5-cm section of the middle of the antenna was attached to the electrodes (0.5 mm gold wire; Sigma-Aldrich) with Spectra-360 electrode gel (Parker Labs). The electrodes connected to a preamplifier (Syntech) interfaced with a computer-operated amplifier system (IDAC4; Syntech); data acquisition and analysis were done with EAGPro software (Syntech). Dilution series of the compounds were prepared in hexane, 10 μL of test solution was applied onto a 5 × 30 mm piece of filter paper (No. 1; Whatman), the solvent was allowed to evaporate for ∼10 s and the filter strip was placed into a borosilicate Pasteur pipette. Humidified medical air provided a flow rate of 700 mL⋅min−1 through a stainless steel sample delivery tube onto the antennal preparation. To achieve a 3-mL stimulus puff, 600 mL⋅min−1 was redirected for 0.3 s through a three-way solenoid valve (Grass S44; Grass Technologies) into the pipette cartridge placed at the side-entrance hole of the sample delivery tube. Compounds were tested in a series starting with the lowest to the highest amount, with three replicate puffs for each dose. Each series started and ended with a control puff of hexane-loaded filter paper and a puff with 10 ng periplanone-B on the filter strip. The time interval between stimulations was 20–25 s. For EAG studies with hexanol, 4 µL of 1% hexanol dissolved in mineral oil (vol/vol, ∼33 µg of hexanol) was loaded onto an 8-mm diameter filter paper disk, which was placed into a dispenser (26, 27). Four microliters of mineral oil was used as a blank stimulus. Stimulations lasted 1 s at 300-s intervals.
SSR recordings were performed using intact cockroaches, as described in refs. 27, 28. The same dose of hexanol and stimulus regimen was used as in the EAG test described above. For each sensillum, the number of spikes was counted for a 2-s period including 50 ms before and 950 ms after the 1 s of stimulation. The responses of each sensillum to mineral oil were subtracted from its responses to hexanol.
Supplementary Material
Acknowledgments
We thank Rick Santangelo for maintaining the insect colonies, Wes Watson and Steve Denning for house flies, Charles Mooney for technical help in FEG SEM, and Elsa Youngsteadt for comments on the manuscript. This project was supported in part by the National Institute of Food and Agriculture, US Department of Agriculture [Awards 2009-35302-05303 (AFRI), 10-8100-1553-CA (APHIS), and 10-CA-11420004-369 (Forest Service)], the National Science Foundation (Award IOS-1052238), the Blanton J. Whitmire endowment at North Carolina State University, and the Russian Foundation of Basic Research (Grant 09-04-01042a).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212466110/-/DCSupplemental.
References
- 1.Jander U. Phylogenetic analysis of grooming behaviors in Tracheata. (Translated from German) Z Tierpsychol. 1966;23(7):799–844. [PubMed] [Google Scholar]
- 2.Szebenyi AL. Cleaning behaviour in Drosophila melanogaster. Anim Behav. 1969;17(4):641–651. [Google Scholar]
- 3.Elawami IO, Dent DR. The interaction of surface and dust particle-size on the pick-up and grooming behavior of the German cockroach Blattella germanica. Entomol Exp Appl. 1995;77(1):81–87. [Google Scholar]
- 4.Vincent CM, Bertram SM. Crickets groom to avoid lethal parasitoids. Anim Behav. 2010;79(1):51–56. [Google Scholar]
- 5.Reber A, Purcell J, Buechel SD, Buri P, Chapuisat M. The expression and impact of antifungal grooming in ants. J Evol Biol. 2011;24(5):954–964. doi: 10.1111/j.1420-9101.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 6.Reingold SC, Camhi JM. A quantitative analysis of rhythmic leg movements during three different behaviors in the cockroach, Periplaneta americana. J Insect Physiol. 1977;23(11–12):1407–1420. [Google Scholar]
- 7.Bret BL, Ross MH. Behavioral responses of the German cockroach, Blatella germanica (L.) (Orthoptera: Blatellidae), to a propoxur formulation. J Econ Entomol. 1986;79(2):426–430. doi: 10.1093/jee/79.2.426. [DOI] [PubMed] [Google Scholar]
- 8.Currie CR, Stuart AE. Weeding and grooming of pathogens in agriculture by ants. Proc Biol Sci. 2001;268(1471):1033–1039. doi: 10.1098/rspb.2001.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagawa A, Yokohari F, Shimizu S. The role of antennae in removing entomopathogenic fungi from cuticle of the termite, Coptotermes formosanus. J Insect Sci. 2009:9:6. doi: 10.1673/031.009.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lusebrink I, Dettner K, Seifert K. Stenusine, an antimicrobial agent in the rove beetle genus Stenus (Coleoptera, Staphylinidae) Naturwissenschaften. 2008;95(8):751–755. doi: 10.1007/s00114-008-0374-z. [DOI] [PubMed] [Google Scholar]
- 11.Dawkins R, Dawkins M. Hierarchical organization and postural facilitation: Rules for grooming in flies. Anim Behav. 1976;24(4):739–755. [Google Scholar]
- 12.Lefebvre L. Grooming in crickets: Timing and hierarchical organization. Anim Behav. 1981;29(4):973–984. [Google Scholar]
- 13.Walker ED, Archer WE. Sequential organization of grooming behaviors of the mosquito, Aedes triseriatus. J Insect Behav. 1988;1(1):97–109. [Google Scholar]
- 14.Valentine BD. Grooming behavior in Coleoptera. The Coleopterists’. Bulletin. 1973;27(2):63–73. [Google Scholar]
- 15.Bobula Smith BJ, Valentine BD. Phylogenetic implications of grooming behavior in cockroaches (Insecta: Blattaria) Psyche (Stuttg) 1985;92:369–385. [Google Scholar]
- 16. Robinson WH (1996) Antennal grooming and movement behaviour of the German cockroach, Blattella germanica (L.). Proceedings of the Second International Conference on Urban Pests, ed Wildey KB, pp 361–369.
- 17.Farish DJ. The evolutionary implications of qualitative variation in the grooming behaviour of the Hymenoptera (Insecta) Anim Behav. 1972;20(4):662–676. doi: 10.1016/s0003-3472(72)80139-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhukovskaya MI. Modulation by octopamine of olfactory responses to nonpheromone odorants in the cockroach, Periplaneta americana L. Chem Senses. 2012;37(5):421–429. doi: 10.1093/chemse/bjr121. [DOI] [PubMed] [Google Scholar]
- 19.Barber GW, Starnes EB. The activities of house flies. J NY Entomol Soc. 1949;57(4):203–214. [Google Scholar]
- 20.Schaller D. Antennal sensory system of Periplaneta americana L.: Distribution and frequency of morphologic types of sensilla and their sex-specific changes during postembryonic development. Cell Tissue Res. 1978;191(1):121–139. doi: 10.1007/BF00223221. [DOI] [PubMed] [Google Scholar]
- 21.Jackson LL. Cuticular lipids of insects—IV. Hydrocarbons of cockroaches Periplaneta japonica and Periplaneta americana compared to other cockroach hydrocarbons. Comp Biochem Physiol. 1972;41(2):331–336. [Google Scholar]
- 22.Saïd I, Costagliola G, Leoncini I, Rivault C. Cuticular hydrocarbon profiles and aggregation in four Periplaneta species (Insecta: Dictyoptera) J Insect Physiol. 2005;51(9):995–1003. doi: 10.1016/j.jinsphys.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs AG. Lipid melting and cuticular permeability: New insights into an old problem. J Insect Physiol. 2002;48(4):391–400. doi: 10.1016/s0022-1910(02)00059-8. [DOI] [PubMed] [Google Scholar]
- 24.Blomquist GJ, Bagnères A-G. Introduction: history and overview of insect hydrocarbons. In: Blomquist GJ, Bagnères A-G, editors. Insect Hydrocarbons. Cambridge, UK: Cambridge Univ Press; 2010. pp. 3–18. [Google Scholar]
- 25.Maitani MM, Allara DL, Park KC, Lee SG, Baker TC. Moth olfactory trichoid sensilla exhibit nanoscale-level heterogeneity in surface lipid properties. Arthropod Struct Dev. 2010;39(1):1–16. doi: 10.1016/j.asd.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kapitsky SV, Gribakin FG. Electroantennogram of the American cockroach: Effect of oxygen and electrical model. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1992;170(5):651–663. doi: 10.1007/BF00199341. [DOI] [PubMed] [Google Scholar]
- 27.Zhukovskaya MI, Kapitsky SV. Activity modulation in cockroach sensillum: The role of octopamine. J Insect Physiol. 2006;52(1):76–86. doi: 10.1016/j.jinsphys.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhukovskaya MI. Aminergic regulation of pheromone sensillae in the cockroach Periplaneta americana. J Evol Biochem Physiol. 2007;43(3):318–326. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.