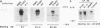Abstract
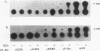
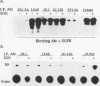
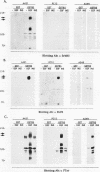
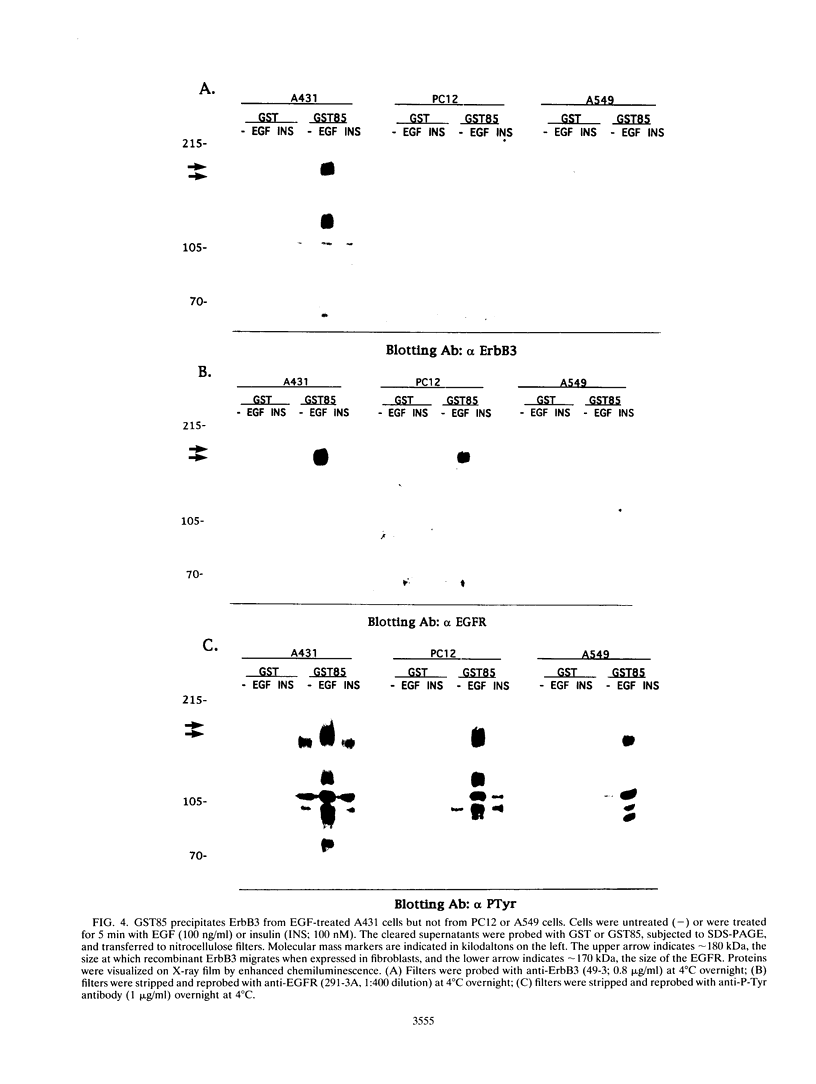
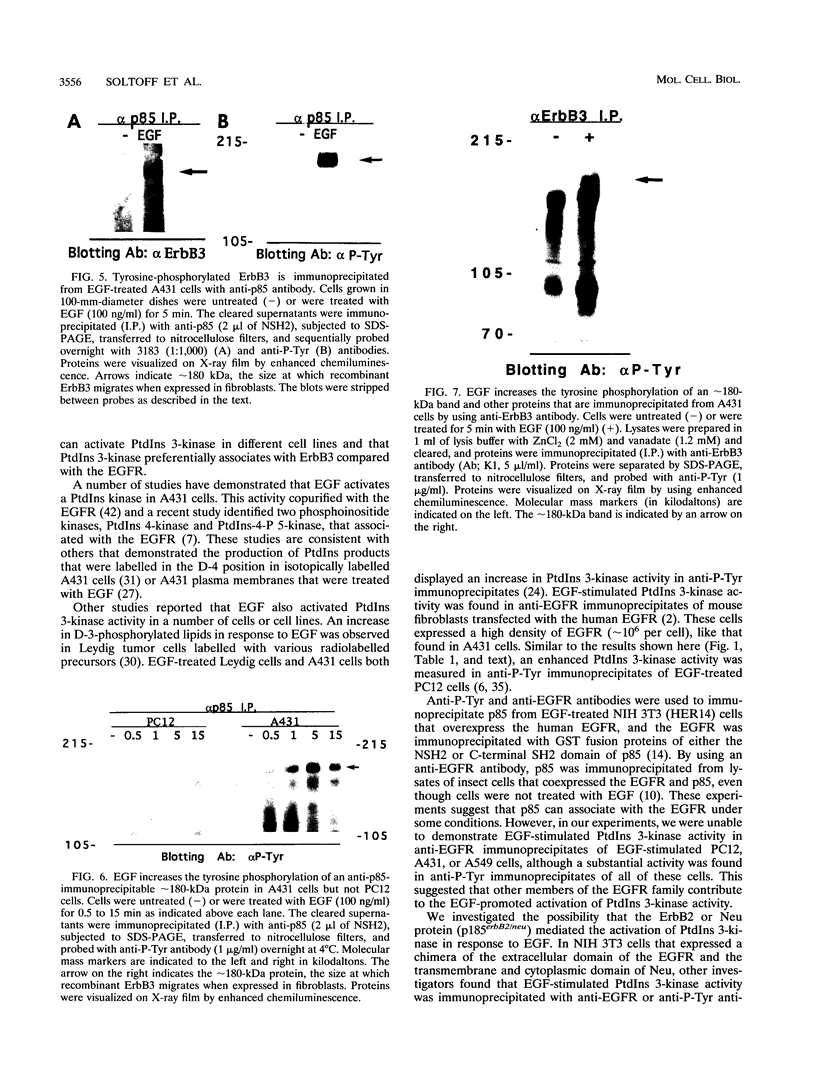
Conflicting results concerning the ability of the epidermal growth factor (EGF) receptor to associate with and/or activate phosphatidylinositol (PtdIns) 3-kinase have been published. Despite the ability of EGF to stimulate the production of PtdIns 3-kinase products and to cause the appearance of PtdIns 3-kinase activity in antiphosphotyrosine immunoprecipitates in several cell lines, we did not detect EGF-stimulated PtdIns 3-kinase activity in anti-EGF receptor immunoprecipitates. This result is consistent with the lack of a phosphorylated Tyr-X-X-Met motif, the p85 Src homology 2 (SH2) domain recognition sequence, in this receptor sequence. The EGF receptor homolog, ErbB2 protein, also lacks this motif. However, the ErbB3 protein has seven repeats of the Tyr-X-X-Met motif in the carboxy-terminal unique domain. Here we show that in A431 cells, which express both the EGF receptor and ErbB3, PtdIns 3-kinase coprecipitates with the ErbB3 protein (p180erbB3) in response to EGF. p180erbB3 is also shown to be tyrosine phosphorylated in response to EGF. In contrast, a different mechanism for the activation of PtdIns 3-kinase in response to EGF occurs in certain cells (PC12 and A549 cells). Thus, we show for the first time that ErbB3 can mediate EGF responses in cells expressing both ErbB3 and the EGF receptor.
Full text
PDF


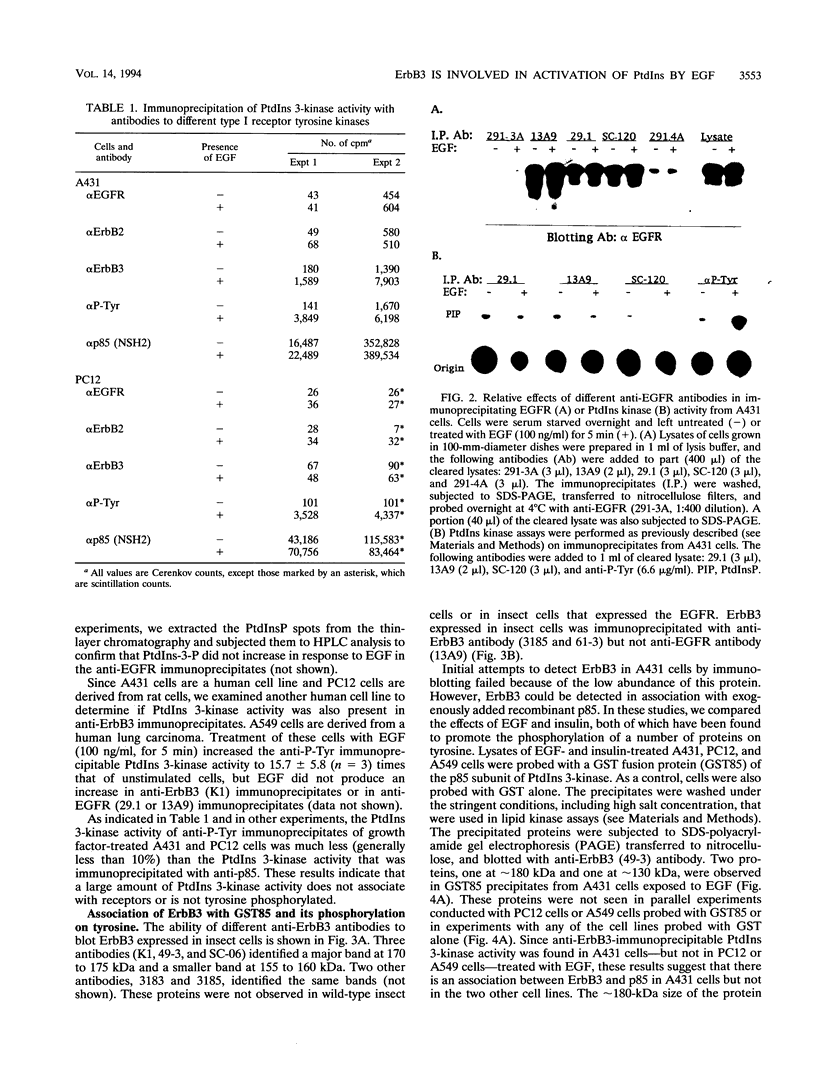
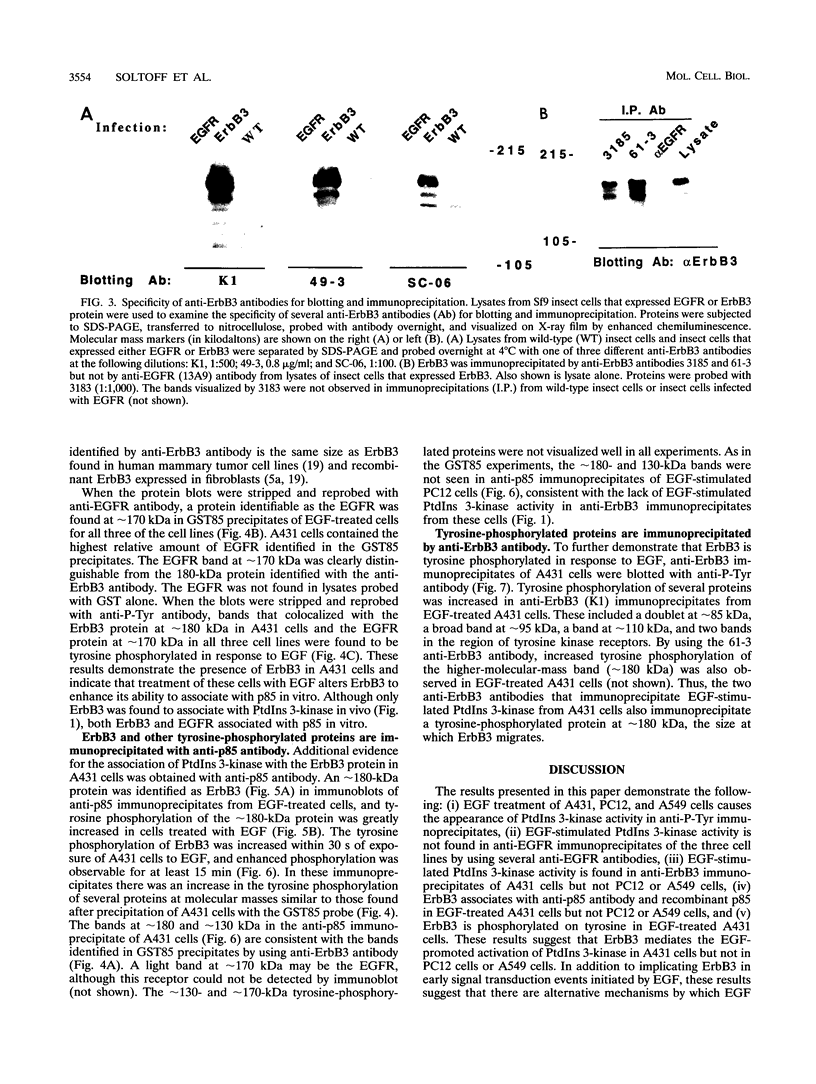


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Bjorge J. D., Chan T. O., Antczak M., Kung H. J., Fujita D. J. Activated type I phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc Natl Acad Sci U S A. 1990 May;87(10):3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Carter A. N., Downes C. P. Phosphatidylinositol 3-kinase is activated by nerve growth factor and epidermal growth factor in PC12 cells. J Biol Chem. 1992 Jul 25;267(21):14563–14567. [PubMed] [Google Scholar]
- Cochet C., Filhol O., Payrastre B., Hunter T., Gill G. N. Interaction between the epidermal growth factor receptor and phosphoinositide kinases. J Biol Chem. 1991 Jan 5;266(1):637–644. [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Fedi P., Pierce J. H., di Fiore P. P., Kraus M. H. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994 Jan;14(1):492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout I., Dhand R., Panayotou G., Fry M. J., Hiles I., Otsu M., Waterfield M. D. Expression and characterization of the p85 subunit of the phosphatidylinositol 3-kinase complex and a related p85 beta protein by using the baculovirus expression system. Biochem J. 1992 Dec 1;288(Pt 2):395–405. doi: 10.1042/bj2880395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hu P., Margolis B., Skolnik E. Y., Lammers R., Ullrich A., Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. R., Bird G. S., Obie J. F., Thastrup O., Putney J. W., Jr Role of inositol (1,4,5)trisphosphate in epidermal growth factor-induced Ca2+ signaling in A431 cells. Mol Pharmacol. 1991 Aug;40(2):254–262. [PubMed] [Google Scholar]
- Johnson G. R., Kannan B., Shoyab M., Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993 Feb 5;268(4):2924–2931. [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B. Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185-kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low density membrane vesicle. J Biol Chem. 1993 Feb 25;268(6):4391–4398. [PubMed] [Google Scholar]
- King C. R., Borrello I., Bellot F., Comoglio P., Schlessinger J. Egf binding to its receptor triggers a rapid tyrosine phosphorylation of the erbB-2 protein in the mammary tumor cell line SK-BR-3. EMBO J. 1988 Jun;7(6):1647–1651. doi: 10.1002/j.1460-2075.1988.tb02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Fedi P., Starks V., Muraro R., Aaronson S. A. Demonstration of ligand-dependent signaling by the erbB-3 tyrosine kinase and its constitutive activation in human breast tumor cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2900–2904. doi: 10.1073/pnas.90.7.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Issing W., Miki T., Popescu N. C., Aaronson S. A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera G. L., Rittenhouse S. E. Human platelets form 3-phosphorylated phosphoinositides in response to alpha-thrombin, U46619, or GTP gamma S. J Biol Chem. 1990 Apr 5;265(10):5345–5348. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Miller E. S., Ascoli M. Anti-phosphotyrosine immunoprecipitation of phosphatidylinositol 3' kinase activity in different cell types after exposure to epidermal growth factor. Biochem Biophys Res Commun. 1990 Nov 30;173(1):289–295. doi: 10.1016/s0006-291x(05)81055-1. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Lapetina E. G. Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J Biol Chem. 1990 Feb 15;265(5):2441–2445. [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Payrastre B., Plantavid M., Breton M., Chambaz E., Chap H. Relationship between phosphoinositide kinase activities and protein tyrosine phosphorylation in plasma membranes from A431 cells. Biochem J. 1990 Dec 15;272(3):665–670. doi: 10.1042/bj2720665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Lamprecht R., Ben-Levy R., Tzahar E., Yarden Y. Regulated coupling of the Neu receptor to phosphatidylinositol 3'-kinase and its release by oncogenic activation. J Biol Chem. 1992 Jun 15;267(17):12266–12274. [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., de Laat S. W. Epidermal growth factor-activated calcium and potassium channels. J Biol Chem. 1991 Oct 25;266(30):19938–19944. [PubMed] [Google Scholar]
- Pignataro O. P., Ascoli M. Epidermal growth factor increases the labeling of phosphatidylinositol 3,4-bisphosphate in MA-10 Leydig tumor cells. J Biol Chem. 1990 Jan 25;265(3):1718–1723. [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Plowman G. D., Culouscou J. M., Whitney G. S., Green J. M., Carlton G. W., Foy L., Neubauer M. G., Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Whitney G. S., Neubauer M. G., Green J. M., McDonald V. L., Todaro G. J., Shoyab M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent S. A., Lemoine N. R., Hughes C. M., Plowman G. D., Selden C., Gullick W. J. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene. 1992 Jul;7(7):1273–1278. [PubMed] [Google Scholar]
- Raffioni S., Bradshaw R. A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor, and nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Miralpeix M., Myers M. G., Jr, Glasheen E. M., Backer J. M., Kahn C. R., White M. F. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992 Nov 5;267(31):22662–22672. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Thompson D. M., Cochet C., Chambaz E. M., Gill G. N. Separation and characterization of a phosphatidylinositol kinase activity that co-purifies with the epidermal growth factor receptor. J Biol Chem. 1985 Jul 25;260(15):8824–8830. [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Thompson B. L., Harris A. L., Taylor P., Omann G. M., Sklar L. A. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem. 1989 Sep 15;264(26):15668–15673. [PubMed] [Google Scholar]
- Tuveson D. A., Carter R. H., Soltoff S. P., Fearon D. T. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993 May 14;260(5110):986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Suh P. G., Rhee S. G., Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
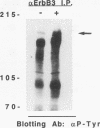
- Yoakim M., Hou W., Liu Y., Carpenter C. L., Kapeller R., Schaffhausen B. S. Interactions of polyomavirus middle T with the SH2 domains of the pp85 subunit of phosphatidylinositol-3-kinase. J Virol. 1992 Sep;66(9):5485–5491. doi: 10.1128/jvi.66.9.5485-5491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]