Abstract
Memory consolidation studies, including those examining the role of the basolateral amygdala (BLA), have traditionally used techniques limited in their temporal and spatial precision. The development of optogenetics provides increased precision in the control of neuronal activity that can be used to address the temporal nature of the modulation of memory consolidation. The present experiments, therefore, investigated whether optogenetically stimulating and inhibiting BLA activity immediately after training on an inhibitory avoidance task enhances and impairs retention, respectively. The BLA of male Sprague–Dawley rats was transduced to express either ChR2(E123A) or archaerhodopsin-3 from the Halorubrum sodomense strain TP009 (ArchT). Immediately after inhibitory avoidance training, rats received optical stimulation or inhibition of the BLA, and 2 d later, rats’ retention was tested. Stimulation of ChR2(E123A)-expressing neurons in the BLA using trains of 40-Hz light pulses enhanced retention, consistent with recording studies suggesting the importance of BLA activity at this frequency. Light pulses alone given to control rats had no effect on retention. Inhibition of ArchT-expressing neurons in the BLA for 15 min, but not 1 min, significantly impaired retention. Again, illumination alone given to control rats had no effect on retention, and BLA inhibition 3 h after training had no effect. These findings provide critical evidence of the importance of specific frequency patterns of activity in the BLA during consolidation and indicate that optogenetic manipulations can be used to alter activity after a learning event to investigate the processes underlying memory consolidation.
Considerable evidence indicates that the basolateral amygdala (BLA) modulates memory consolidation for a variety of different types of learning, including spatial and cued water maze tasks (1, 2), contextual fear conditioning (3, 4), conditioned taste aversion (5), novel object recognition (6), and inhibitory avoidance (IA) (7–10). Such studies have used posttraining manipulations of BLA activity, particularly microinjections, to influence consolidation. Indeed, evidence suggests that posttraining intra-BLA drug administration enhances or impairs retention of learning, depending on the drugs’ effects on BLA activity (10–12). Although these experiments have elucidated many of the processes underlying memory consolidation (13), especially those involving the BLA (14, 15), such studies’ conclusions have necessarily been limited by the caveats associated with the techniques. Microinjections do not permit temporally precise control of neuronal activity—either in terms of controlling neuronal spiking or in limiting the drugs’ effects on BLA activity to a controllable time window—whereas electrical stimulation affects all neurons and axons within the vicinity of the electrode, including fibers of passage, and cannot be used for providing inhibition.
Recent findings highlight the need to have temporally precise control of neuronal activity, including within the BLA, in memory consolidation studies. Physiological recordings suggest that BLA activity facilitates and couples with activity in downstream structures in the gamma frequency range (35–45 Hz) across learning trials (16, 17). For example, if BLA activity is disrupted, gamma activity is reduced downstream in the striatum, further supporting the notion that the BLA is promoting synchronous oscillations of activity in both structures (16). The strength of gamma frequency coupling between structures appears to increase across learning trials, and this synchronized activity likely induces synaptic plasticity in target neurons (16, 17). Whether stimulating the BLA and/or downstream structures at similar frequencies enhances retention has been impossible to determine with pharmacological manipulations. Similarly, whether BLA activity within precise temporal parameters is necessary for normal consolidation has been difficult to examine, despite previous studies suggesting that emotionally arousing stimuli produce changes in amygdala norepinephrine levels within a temporally limited window (18, 19).
The development of optogenetics has enabled selective control of neuronal activity with millisecond temporal precision and has been used in a number of contexts to examine the influence of neuronal activity on behavior (20–22). Thus, experimental manipulations using optogenetics have significant potential to inform investigations into memory consolidation through more precise control of neuronal activity, as well as to address questions regarding the temporal aspects of BLA activity after learning that influence consolidation. However, no study has used optogenetics to modulate neuronal activity during the posttraining period, leaving unaddressed whether such manipulations would influence consolidation and under what conditions they would do so. Therefore, the present experiments examined whether posttraining stimulation and inhibition of BLA neurons modulates the consolidation of IA learning, using optical activation of either a ChR2-E123T accelerated (ChETA) version of the cation channel channelrhodopsin-2 [ChR2(E123A)] that permits high-frequency stimulation of neurons (23, 24) or the inhibitory outward proton pump archaerhodopsin-3 from Halorubrum sodomense strain TP009 (ArchT) (25), respectively. In particular, these experiments investigated whether stimulating BLA glutamatergic neurons at specific frequencies after IA training enhances consolidation and whether inhibiting BLA activity for different lengths of time impairs consolidation.
Results
Optical Activation of ChR2(E123A) in the BLA Enhances Retention of IA Learning.
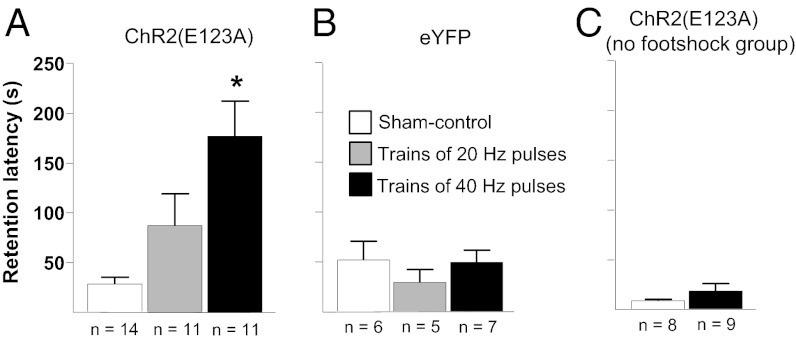
Rats underwent IA training and, immediately afterward, received optical stimulation of ChR2(E123A)-expressing BLA neurons. The ChR2(E123A) construct used is a ChETA variant of the light-activated cation channel ChR2 with fast off-kinetics that permits optical stimulation up to 200 Hz (23, 24). We stimulated the BLA with trains of light pulses (2-s trains, given every 10 s) given at 20 or 40 Hz (10-ms pulse duration, 473-nm light) over 15 min. Rats’ retention was assessed 2 d later, and their latencies to enter the shock chamber were recorded and are shown in Fig. 1. Fig. 1A shows the latencies of sham-control rats and rats that were given trains of either 20- or 40-Hz light pulses for 15 min immediately after IA training. A one-way ANOVA revealed a significant effect of stimulation [F(2,33) = 8.695; P < 0.001]. Those rats that received trains of 40-Hz light pulses had significantly higher latencies to enter the shock compartment than those of the sham-control rats (P < 0.05), whereas the latencies of those rats receiving trains of 20-Hz stimulation were not significantly different from either of the other two groups. To ensure that the light pulses alone were not responsible for the enhanced retention, a control experiment was conducted in which the BLA was transduced with enhanced YFP (eYFP) alone. Similarly, to ensure that stimulating BLA neurons alone was not creating a memory or inducing aversion to the dark compartment, another group of rats with their BLA transduced with ChR2(E123A) underwent IA training but with no footshock. Neither eYFP-expressing (Fig. 1B) nor no-shock (Fig. 1C) control rats exhibited significant latency differences compared with their respective 40-Hz groups (P > 0.05 in both cases).
Fig. 1.
Retention effects of optical stimulation of ChR2(E123A)-transduced BLA neurons immediately after IA training. (A) Enhanced retention of rats given posttraining optical stimulation of the BLA, via activation of ChR2(E123A). Shown are the mean latencies, in seconds (±SEM), to enter the shock compartment during the retention test as well as the n of each group underneath each bar. *P < 0.05, compared with sham control. (B) No effect on retention of posttraining light pulses given to eYFP-expressing control rats. (C) No effect of posttraining optical stimulation of the BLA on retention in ChR2(E123A)-expressing rats that did not receive a footshock during training.
Optical Activation of ArchT in the BLA Impairs Retention of IA Learning.
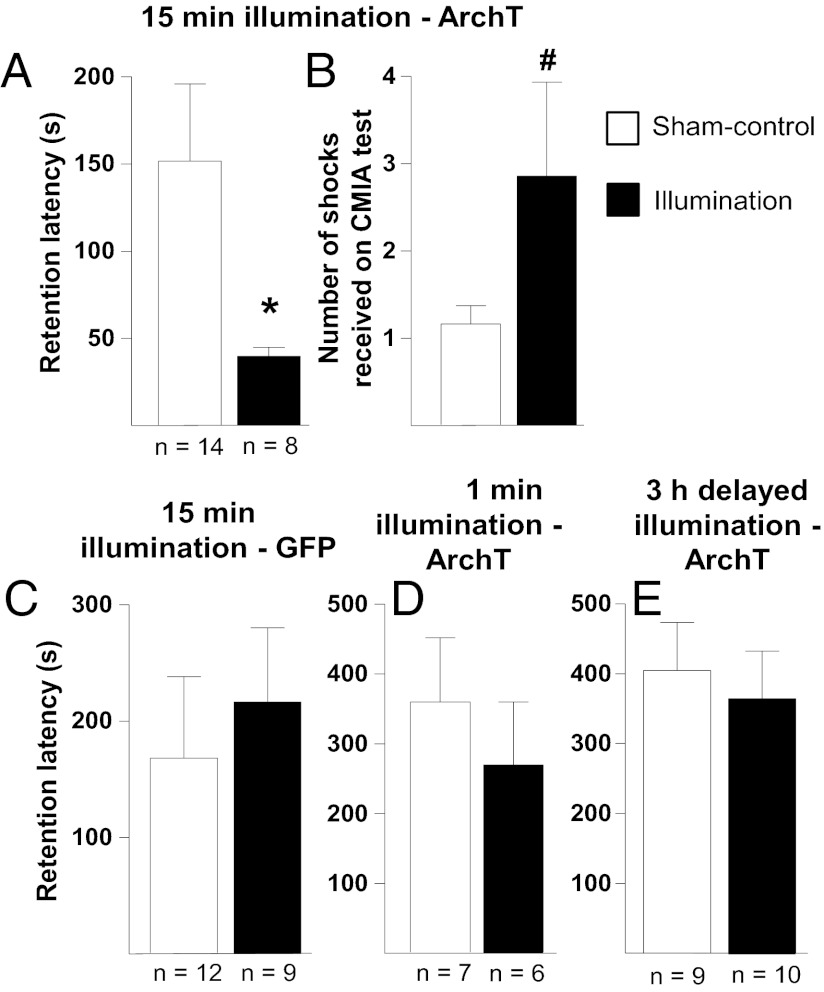
Rats underwent IA training and received immediate posttraining optical inhibition of ArchT-transduced neurons in the BLA. ArchT is an outward proton pump that, when activated by light, hyperpolarizes neurons and thereby inhibits neuronal activity (25). Activation of ArchT was provided by continuous illumination with 561-nm light. As described above, we assessed retention latencies 2 d later. The latencies of rats that received 15 min of immediate posttraining BLA inhibition through activation of ArchT are shown in Fig. 2A and are significantly different from those of sham-control rats [t(20) = 1.884; P < 0.05]. Thus, BLA inhibition for 15 min immediately after IA training impaired retention. For this experiment, an additional measure of retention was also used. After their retention latencies were recorded, rats underwent continuous multiple-trial IA (CMIA) training, in which rats received a continuous footshock upon entering the shock chamber (3, 26). The number of footshocks required for a rat to learn to stay in the start chamber for 200 s was then used as an index of retention for the original IA learning. Fig. 2B shows that those rats that had previously received optical inhibition of the BLA required more footshocks to learn to stay out of the shock chamber compared with sham-control rats, as a t test revealed a trend toward a significant difference in the number of footshocks between the two groups [t(20) = 1.971; P < 0.07]. As with the ChR2(E123A) experiments, to ensure that illumination alone was not producing the impaired retention, the BLA of another group of rats was transduced with GFP alone. Fig. 2C shows the latencies of rats expressing GFP alone in the BLA that were given 15 min of illumination immediately after training, and no significant difference between the sham-control and illumination groups was found (P > 0.05). To determine whether a shorter period of inhibition would also impair retention, a group of rats received 1 min of posttraining BLA inhibition. Fig. 2D shows the latencies of those rats. No significant difference between the latencies of the sham-control rats and those receiving 1 min of illumination was found (P > 0.05). To determine whether such inhibition produced long-lasting changes in the BLA that could have been responsible for the impaired retention, another group of rats received 15 min of posttraining inhibition of the BLA 3 h after training. Fig. 2E shows the latencies of those rats. No significant difference between the latencies of the sham-control and illumination groups was found (P > 0.05).
Fig. 2.
Retention effects of optical inhibition of ArchT-transduced BLA neurons immediately after IA training. (A) Impaired retention of rats given immediate posttraining optical inhibition of the BLA, via activation of ArchT. Shown are the mean latencies, in seconds (±SEM), to enter the shock compartment during the retention test as well as the n of each group underneath each bar. *P < 0.05, compared with sham control. (B) Impaired retention of the same rats from A, as assessed by CMIA training that immediately followed recording of retention latencies from A. #P < 0.07, compared with sham control. (C) No effect of 15 min of illumination on retention in GFP-expressing control rats, following the same parameters as those used for the experiments in A. (D) No effect of 1 min of posttraining optical inhibition of the BLA on retention. (E) No effect of 15 min of optical inhibition of the BLA, given 3 h after training, on subsequent retention.
Histology.
Electrophysiology and histology results verified robust influence of ChETA and ArchT on BLA neuronal activity. Fig. 3A shows the activity of a BLA neuron in a ChR2(E123A)-transduced rat in response to trains of 20- and 40-Hz light pulses, indicating that the light pulses, at the behaviorally effective parameters, were able to drive BLA neuronal spiking. Fig. 3B shows the inhibition of spontaneous activity of a BLA neuron in an ArchT-transduced rat in response to 1 and 15 min of illumination. Fig. 3C shows a diagram of the BLA with 20 randomly selected representative optical fiber termination sites. Optical fiber ends were intentionally placed dorsal to the BLA to allow for illumination of the structure ventral to the inserted optical fiber (i.e., the BLA), as evidence indicates that the parameters used in this study (Materials and Methods) produce a spherical shape of light ∼1 mm in diameter centered 0.5 mm away from the fiber tip (24). Fig. 3 D and E show representative images of the BLA expressing ChR2(E123A) and ArchT, respectively, as visualized by using immunocytochemical staining procedures. In both images, the BLA is densely labeled, as a result of opsin expression in the cells and surrounding neuropil. In addition, moderate staining is visible outside the BLA as a result of opsin expression in BLA axons that either terminate in those regions or pass through them (22). Any animals that did not have at least one-third of the BLA expressing GFP/eYFP were excluded (n = 6). More than 90% of rats showed staining throughout >90% of the BLA.
Fig. 3.
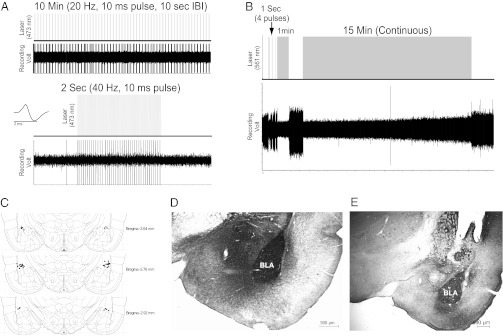
Electrophysiology and histology results. (A) Representative trace showing spike responses of a ChR2(E123A)-transduced BLA neuron to light pulses (10-ms duration, 473-nm wavelength) at either 20 Hz (Upper) or 40 Hz (Lower), given over 2 s. Inset shows representative action potential wave form. (B) Representative trace showing spontaneous activity of an ArchT-transduced BLA neuron with or without light pulses of varying duration (561-nm wavelength). (C) Diagram of estimated fiber-optic track termination points in 20 randomly selected rats. (D) Anti-eYFP immunocytochemical staining from a ChR2(E123A)-transduced rat. The BLA is densely labeled, whereas adjacent areas, including the CeA, show moderate staining from labeled axons. (E) Anti-GFP immunocytochemical staining from an ArchT-transduced rat (image taken at lower magnification than D). The BLA is densely labeled, although stained fibers can be seen in surrounding regions, especially the CeA, representing ArchT-expressing axons originating in the BLA. In addition, E shows the damage from the cannula immediately dorsal to the BLA.
Discussion
The present results demonstrate that there is a critical frequency as well as period of BLA activity that modulates memory consolidation and provide evidence that posttraining optogenetic manipulations of neuronal activity modulate memory consolidation. In particular, the ChR2(E123A) findings are unique in that activity in the glutamatergic cells, the putative projection neurons of the BLA, was selectively controlled immediately after training to modulate memory consolidation, a remarkable advance that will enable additional investigations into the causal nature of the relationship between neural activity at specific frequencies and the resulting changes in memory strength and plasticity.
Posttraining optical stimulation of BLA glutamatergic neurons, using trains of light pulses in the gamma frequency range (40 Hz in the present experiments), enhanced retention of IA learning. Stimulation with trains of 20-Hz pulses did not significantly enhance retention. These findings are consistent with previous recording work examining BLA activity across learning trials in which BLA-generated oscillation activity in the gamma frequency range becomes increasingly coupled with activity in downstream structures, including the striatum and rhinal cortices (16, 17). Increased oscillation power between the BLA and these learning-associated structures correlates with increased behavioral measures of late-stage learning, suggesting that synchrony in these structures is a key driver of memory facilitation (16, 17). Moreover, across auditory associative learning trials, increases in gamma oscillation activity in the auditory cortex are positively correlated with behavioral outputs of learning, similar to those mentioned above, as well as significant changes in receptive field frequencies in A1 (27). Gamma activity then can predict not only the strength of memory for a particular stimulus association but also the plasticity associated with that learning. However, these studies, by virtue of being recording studies, were correlative in nature, and causal validation was not possible, making the current study a critical unique expansion both in our understanding of memory consolidation processes and in our ability to perform experiments addressing the causal relationships between activity and plasticity/memory. With the use of the ChETA mutant ChR2(E123A), the present study was able to drive BLA glutamatergic neurons at the relevant frequency and determine that such activity patterns enhance retention of learning. Whether the enhanced retention due to driving BLA activity at this frequency occurs through coordinated activity in downstream structures is an intriguing issue that can now be addressed in future experiments using the present study’s approach.
The ChR2 mutant used here has been characterized physiologically (23); however, the present study uses it in a functional manner to selectively control neuronal output and behavior. Posttraining electrical BLA stimulation has been used to influence memory consolidation (28, 29), but those studies did not use specific frequencies of stimulation and therefore could not relate their findings to recording studies demonstrating precise temporal BLA activation during learning. Moreover, electrical stimulation studies are necessarily limited by simultaneous activation of all neurons in the region of interest (irrespective of phenotype) as well as fibers of passage. In the present findings, ChR2(E123A) expression, and thus stimulation, was limited to BLA glutamatergic cells through the use of a glutamatergic neuron-specific promoter [Calcium/calmodulin-dependent protein kinase IIα (CaMKIIα)] (22), allowing selective modulation of BLA glutamate neurons. Selective control of the projection neurons of the BLA is a crucial ability for investigating how BLA activity during the consolidation period alters plasticity and/or activity in downstream structures. The nearby central amygdala (CeA) is primarily GABAergic (30), precluding ChR2(E123A) transduction in the CeA as well as in the GABAergic intercalated cells of the amygdala (22). Although ArchT expression may have occurred in such cells, previous findings indicate that posttraining CeA microinjections do not modulate memory consolidation (9, 31, 32). Therefore, regardless of any CeA expression, it is unlikely that the current results in either set of experiments can be explained by direct optical manipulation of CeA activity, and significant transduction of nearby structures was not observed. Selective modulation of BLA was further ensured due to the fact that the center of illumination was targeted in the BLA. Based on analyses of the spread of light within brain tissue, as described in Materials and Methods, very little light affected tissue outside the BLA. Thus, with both illumination and viral transduction targeted at and mostly restricted to the BLA, it is unlikely that the observed effects in either set of experiments were due to opsin activation in nearby structures.
The ArchT results indicate that inhibiting BLA activity immediately after IA training for 15 min, but not 1 min, impaired retention. The findings establish an important advance in the ability to discover the temporal relationships between emotional arousal and the modulation of memory consolidation as well as set the stage for future experiments to use optogenetic inhibition in combination with other techniques. The data from the ArchT experiments are consistent with previous studies showing that BLA inactivation impairs retention for learning (33, 34), although it should be noted that the CAG promoter is a general cellular promoter, limiting interpretation of the findings based on specific cell type. However, these previous studies were unable to determine the temporal window in which emotional arousal led to BLA activation and the subsequent modulation of memory consolidation, despite the need to determine the initiation time and duration of BLA activity for normal memory consolidation. The present results, therefore, not only control BLA activity with the “on/off” precision of optical inhibition during memory consolidation but also demonstrate the critical nature of BLA activity within the entire 15-min posttraining period. Previous work indicates that a footshock, akin to those used in IA experiments, increases norepinephrine levels in the amygdala during the first 15 min after the shock, which then return to baseline by 30 min after the shock (18, 19). Together with such findings, the present work suggests that emotional arousal, as found with a footshock, activates the BLA for >1 min and at least 15 min after the learning event and that such activation throughout this extended time period is crucial for normal memory consolidation. As emotional arousal-driven increases in amygdala norepinephrine levels are believed to be critical in driving the strength of the memory consolidation (35, 36), it may be that norepinephrine-driven activation of the BLA occurs within this more specific temporal window, at least in terms of modulating the resulting memory consolidation. However, other work has shown increases in amygdala norepinephrine levels lasting up to 3 h after IA learning (36), despite the current work showing that 15 min of inhibition is sufficient to produce memory impairment. Future studies combining the present techniques with analysis of the pathways governing norepinephrine release may help to resolve this issue.
Of particular importance, the present studies included several crucial control experiments. Optical stimulation of rats that underwent “no-shock” training had no effect on retention, indicating that driving BLA glutamatergic neuronal activity alone did not induce aversion to the dark compartment or “create” a memory, consistent with the hypothesis that posttraining BLA manipulations modulate the consolidation of memories for events that have been acquired immediately before this time point (14). Because illumination, especially continuous illumination as with the ArchT experiments, may produce undesirable effects such as heating (24), no-opsin illumination-alone control experiments were conducted. In the present experiments, illumination alone, matching the parameters used in the main experiments, had no effect on subsequent retention, decreasing concerns about tissue damage or alterations from prolonged illumination. Moreover, the results from the delayed posttraining optical inhibition of the BLA indicate that inhibiting ArchT-expressing neurons 3 h after training (i) no longer has an effect on retention because it falls outside of the limited consolidation time window and (ii) does not produce prolonged alterations in BLA neuronal activity when illumination is suspended that could alter retention performance on day of testing.
The current results open avenues of investigation into how the modulation of memory consolidation occurs (37). That BLA activity in the first 15 min immediately following an event is crucial for normal consolidation suggests that the actions of stress hormones and activation of brainstem systems, such as the noradrenergic system, likely influence the BLA throughout this time window. Future studies, therefore, can use optogenetic control of the BLA in combination with manipulations of these emotional arousal systems to determine the temporal and causal relationships among these systems during memory consolidation. Moreover, as previous work suggests that the BLA influences consolidation through projections to downstream structures (38), future use of optogenetics to control neural pathways connecting structures, via illumination of opsin-transduced axon terminals in the downstream structure (24, 39), should prove particularly informative. In particular, the causal relationship between specific frequencies of BLA activity (i.e., 35–45 Hz) and its projections to different downstream targets remains unexplored but should provide a wealth of knowledge regarding the interactions and coherence of these systems during consolidation.
Materials and Methods
Subjects.
Male Sprague–Dawley rats (200–250 g at time of first surgery; Charles River; n = 152) were used for this study. All rats were single housed in a temperature-controlled environment under a 12-h reverse light/dark cycle (lights on at 19:00) and allowed to acclimate to the vivarium at least 3 d before surgery. Food and water were available ad libitum throughout all training and testing. All procedures used were in compliance with National Institutes of Health guidelines for care of laboratory animals and approved by the University of Iowa Institutional Animal Care and Use Committee.
Surgery.
Rats were anesthetized by using ketamine HCL (10 mg/kg, i.m.) and xylazine HCl (3 mg/kg, i.m.) and placed in a stereotax (Kopf Instruments). One week after arrival, rats received virus microinjections [0.35 µL; AAV5-CaMKIIα-hChR2(E123A)-eYFP, the ChR2(E123A) control AAV5-CaMKIIα-eYFP, AAV2-CAG-ArchT-GFP, or the ArchT control AAV5-CAG-GFP; University of North Carolina Vector Core] delivered bilaterally through a 33-gauge needle into the BLA (coordinates: 2.8 mm posterior and 5.0 mm lateral to bregma and 8.6 mm ventral to skull surface). The CaMKIIα promoter limits gene expression to BLA glutamatergic cells (22), and the CAG promoter is a general cellular promoter. Injectors were left in place for 5 min to permit diffusion. Two weeks later, rats underwent a second surgery in which 20-gauge guide cannulae (Plastics One) aimed bilaterally at the BLA (2.8 mm posterior and 5.0 mm lateral to bregma and 6.5 mm ventral to the skull surface) were implanted and secured by surgical screws and dental acrylic. Obdurators cut to the length of the guide cannulae with no projection were used to maintain openness and removed only during optical stimulation/inhibition. The rats were given 1 wk to recover before behavioral training.
Optical Inhibition or Stimulation.
Optical probes were constructed by gluing an optical fiber (200-µm core, multimode, 0.37 NA) into a 24-gauge internal cannula. The fiber extended 0.5 mm beyond the cannula end. During optical stimulation/inhibition (see Behavioral Procedures for details), the probe was inserted into the previously implanted guide cannula, terminating ∼0.5 mm dorsal to the virus injection site. The fiber’s other end [fiber connector/physical contact (FC/PC) connection] was threaded through a metal leash to protect the fiber from being damaged by the rat and attached to a 2:1 splitter, allowing bilateral illumination. The splitter’s single end attached to an optical commutator. An insulated optical fiber connected the commutator to the appropriate laser source [DPSS; 300 mW, 473 nm for ChR2(E123A) or 561 nm for ArchT, with a multimode fiber coupler for an FC/PC connection; OEM Laser Systems]. Light output was adjusted to 10 mW at the fiber tip, as measured by an optical power meter. In all cases, the comparison control was a “sham-control” in which no illumination was provided. Based on measurements from mammalian brains (24), light output of 10 mW at the fiber tip will produce ∼1 mW/mm2 of light, which is the minimum amount necessary to produce opsin activation, up to 1 mm directly away from the fiber tip in a spherical shape centered 0.5 mm away from the fiber tip (20, 22, 24, 25). Based on in vivo measurements of the light output shape in mammalian brain tissue, these parameters should provide sufficient light for opsin activation in at least 0.4 mm3 of tissue (24).
Behavioral Procedures.
One week after cannula implantation, rats were trained on an IA task. The apparatus was a trough-shaped box divided into two sections: one-third was made of white plastic and illuminated (30 cm), and the other two-thirds (60 cm) were black and darkened. The dark chamber had a stainless-steel bottom and was connected to a shock generator and timer, which was controlled by the experimenter. The two sides were divided by a stainless-steel door that could be retracted through the floor.
Rats were handled individually 1 min per day for 3 d before the start of IA training. During training, rats were placed in the lit side with the door fully retracted to allow free exploration. After the rat crossed into the dark compartment, the door was raised to prevent return to the lit compartment. When the rat reached the end of the dark compartment, it received a single inescapable footshock [0.5 mA, 1-s duration for ChR2(E123A) experiments; 1.0 mA, 2-s duration for ArchT experiments; different footshock intensities were used to prevent ceiling and floor effects, respectively] and was removed from the apparatus. For retention testing 48 h later, rats were placed in the lit side, with the door retracted. Rats’ latencies to cross into the dark compartment were used as the index of retention with a maximum latency of 600 s.
ChR2(E123A) experiments.
Immediately after IA training, animals that had been injected with the ChR2(E123A) virus received 15 min of optical BLA stimulation using the following parameters: 2-s trains of either 20- or 40-Hz light pulses (pulse duration = 10 ms), given every 10 s. In a control experiment, rats that had been injected with the eYFP control virus also received trains of 20- or 40-Hz light pulses. Other rats that had received the ChR2(E123A) virus underwent IA training but received no footshock during training. They also received 15 min of trains of 40-Hz pulses, identical to those of the main experiment.
ArchT experiments.
Immediately following IA training, rats that had been injected with the ArchT virus received continuous optical BLA inhibition for either 1 or 15 min. For those rats that received 15 min of illumination, immediately after retention latencies were recorded, the rats underwent CMIA training as an additional retention test, as detailed elsewhere (3). In brief, the rats received a continuous mild footshock when they crossed into the dark compartment until they returned to the light compartment. The number of footshocks administered before a rat remained in the light chamber for at least 200 s was recorded and used as an index of retention of their original IA training. Two control experiments were also conducted. Rats that had been injected with the GFP virus underwent IA training with 15 min of posttraining illumination. Another group of rats that had been injected with the ArchT virus underwent IA training but received the 15 min of optical inhibition 3 h after training.
Anesthetized optrode recordings.
For recording experiments, rats underwent surgeries as described above, except the anesthesia was 1–2% (vol/vol) isoflurane. After 3–4 wk of incubation time, rats were anesthetized with 1–2% isoflurane, and single neurons were recorded by using an optrode (200-μm optical fiber glued to a ∼8-MΩ recording electrode, with tips separated by 300–500 μm). Signals were amplified and filtered (0.3- to 3-kHz bandpass) by using a NeuroLog system (Digitimer). Single neuron spikes were discriminated, and digital pulses were led to a computer for online data collection with the use of a laboratory interface and software (CED 1401 Plus, Spike2; Cambridge Electronic Design). Isolated single neurons were tested for light responsiveness (excitation or inhibition) at a range of stimulation frequencies [1–40 Hz for ChR2(E123A) and prolonged tonic stimulation for ArchT] by using the appropriate laser light source with ∼10-mW light output. Multiple neurons per animal were tested to confirm robustness of expression (>3–4 responsive minimum).
Histology.
Animals were killed with an overdose of sodium pentobarbital (100 mg/mL; i.p.) and then perfused transcardially with PBS (pH 7.4) followed by PBS containing 4% paraformaldehyde. Brains were removed and stored at room temperature in 4% paraformaldehyde PBS for 24–48 h until sectioning. The brains were coronally sectioned (50 µm) on a vibratome and mounted onto either gelatin-subbed slides for staining or stored in antifreeze solution at −20 °C until immunohistochemical procedures began. Optical probe location was determined by examining Cresyl violet-stained sections under a light microscope according to the Paxinos and Watson atlas (40). Any rats whose optical probe terminated outside of the BLA were excluded from final analysis. Opsin expression was confirmed by using immunohistochemistry procedures as described previously (41). In brief, tissue sections were incubated in anti-GFP primary antibody solution for 48–72 h [PBS, 2% goat serum, 0.4% Triton X, rabbit 1:20,000 primary antibody (Abcam)]. Sections were then incubated for 1 h in a biotinylated anti-rabbit secondary antibody solution (K-PBS; 0.3% Triton X; goat, 1:200; Vector Labs) and incubated in an ABC kit (Vector Labs) for 1 h. Sections were developed in diaminobenzidine for ∼5–10 min before being mounted onto gelatin-subbed slides. Slides were allowed to dry before being dehydrated with reverse alcohol washes for 1 min each, soaked in Citrosolv for a minimum of 5 min, and coverslipped with DePeX (Electron Microscopy Sciences). GFP/eYFP expression was assessed by using a light microscope.
Statistical Analysis.
Retention latencies for all experiments were analyzed by using either a t test or a one-way ANOVA with a Bonferroni post hoc test. P < 0.05 was considered significant. All measures are expressed as mean ± SEM, and each group’s n is indicated.
Acknowledgments
We thank Janelle McNabb, Anne Curbow, Nathan Myhre, Rachel Gordon, Alexandrea Pitzer, Julia Collison, and Holly Steger for their technical assistance. This research was supported by National Institutes of Health Grants MH097111 (to R.T.L.) and DA032005 (to D.E.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91(18):8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem. 1999;71(2):232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 3.LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci. 2003;23(17):6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huff NC, Rudy JW. The amygdala modulates hippocampus-dependent context memory formation and stores cue-shock associations. Behav Neurosci. 2004;118(1):53–62. doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci. 2003;18(9):2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J Neurosci. 2008;28(26):6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106(12):4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: Involvement of noradrenergic and cholinergic systems. Eur J Neurosci. 2004;20(10):2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- 10.LaLumiere RT, Nawar EM, McGaugh JL. Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions. Learn Mem. 2005;12(3):296–301. doi: 10.1101/lm.93205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19(15):6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalumiere RT, McGaugh JL. Memory enhancement induced by post-training intrabasolateral amygdala infusions of beta-adrenergic or muscarinic agonists requires activation of dopamine receptors: Involvement of right, but not left, basolateral amygdala. Learn Mem. 2005;12(5):527–532. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 14.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier JG, Paré D. Role of amygdala oscillations in the consolidation of emotional memories. Biol Psychiatry. 2004;55(6):559–562. doi: 10.1016/j.biopsych.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12(6):801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer EP, Paz R, Paré D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci. 2007;27(35):9369–9379. doi: 10.1523/JNEUROSCI.2153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res. 1999;835(2):340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- 19.Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66(3):253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 20.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunaydin LA, et al. Ultrafast optogenetic control. Nat Neurosci. 2010;13(3):387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 24.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman-Mesches K, McGaugh JL. Muscimol injected into the right or left amygdaloid complex differentially affects retention performance following aversively motivated training. Brain Res. 1995;676(1):183–188. doi: 10.1016/0006-8993(95)00108-3. [DOI] [PubMed] [Google Scholar]
- 27.Headley DB, Weinberger NM. Gamma-band activation predicts both associative memory and cortical plasticity. J Neurosci. 2011;31(36):12748–12758. doi: 10.1523/JNEUROSCI.2528-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bass DI, Partain KN, Manns JR. Event-specific enhancement of memory via brief electrical stimulation to the basolateral complex of the amygdala in rats. Behav Neurosci. 2012;126(1):204–208. doi: 10.1037/a0026462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: Effect on memory varies with footshock level. Brain Res. 1975;86(3):509–513. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- 30.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208(4):401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- 31.Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem. 1997;67(2):176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 32.Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 1997;94(25):14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parent MB, McGaugh JL. Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Res. 1994;661(1-2):97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 34.Zanatta MS, et al. Involvement of the hippocampus, amygdala, entorhinal cortex and posterior parietal cortex in memory consolidation. Braz J Med Biol Res. 1997;30(2):235–240. doi: 10.1590/s0100-879x1997000200012. [DOI] [PubMed] [Google Scholar]
- 35.Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol Sin. 2000;21(6):481–493. [PubMed] [Google Scholar]
- 36.McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 37.Johansen JP, Wolff SB, Lüthi A, LeDoux JE. Controlling the elements: An optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry. 2012;71(12):1053–1060. doi: 10.1016/j.biopsych.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36(7):1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaLumiere RT. A new technique for controlling the brain: Optogenetics and its potential for use in research and the clinic. Brain Stimulat. 2011;4(1):1–6. doi: 10.1016/j.brs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th Ed. Amsterdam: Elsevier Academic; 2005. [Google Scholar]
- 41.Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18(1):50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]