In PNAS, Sardi et al. describe that viral vector-mediated increase in glucocerebrosidase enzyme (GCase) activity can reverse synuclein-related pathological features and improve behavioral function in the D409V mouse model of Gaucher disease (GD) (1). This important study has several implications for our understanding of the pathogenesis of Parkinson disease (PD) and contributes toward the rationale for investigating the modulation of GCase activity as a treatment for PD.
GD is an autosomal recessive disease caused by mutations in the glucocerebrosidase (GBA) gene (2). Mutations are usually associated with a significant reduction in GCase activity. However, the mechanisms by which the GBA mutations result in GD remain unknown and are thought to include the effects of reduced GCase and lysosomal activity, accumulation of toxic substrates, and arrest of misfolded mutant protein in the endoplasmic reticulum (ER) with unfolded protein response and enhanced ER-associated degradation (3).
The major motor features of PD are the result of the loss of dopamine neurons in the substantia nigra (SN) pars compacta of the midbrain. However, other neurotransmitter systems are involved and are associated with nonmotor problems, including cognitive decline, autonomic dysfunction, sleep disorders, and other such problems. Some of these nonmotor symptoms manifest before motor problems emerge and therefore precede the diagnosis of PD (4). There are several genetic causes of familial PD, although these currently account for <20% of cases. SNCA (α-synuclein) is considered central to the pathogenesis of PD in which neuronal degeneration is accompanied by SNCA-rich Lewy body formation. Mutations, multiplications, or increased transcription of the SNCA gene are associated with the development of PD. Current treatments for PD are symptomatic and developing interventions that can slow or stop the progression of the disease is a major unmet need.
The relevance of GD to PD comes from the observation that GD patients and heterozygous GBA mutation carriers are at a significantly increased risk for the development of PD (5). The precise risk for GD patients developing PD is not known, but has been variously estimated as 20- to 30-fold (6, 7). Conversely, 5–10% of PD patients have GBA mutations, making them numerically the most important risk factor for the disease identified to date. PD associated with GBA mutations (GBA-PD) is clinically, pathologically, and pharmacologically indistinguishable from idiopathic “sporadic” PD (8), although GBA-PD has a slightly earlier onset (∼5 y) and more frequent cognitive dysfunction (5).
Recent studies demonstrating a reciprocal relationship between SNCA and GCase are of considerable importance to our understanding of the pathogenesis of GBA-PD and idiopathic PD (9, 10), and highlight the relevance of the work by Sardi and colleagues in developing this association as a therapeutic target (1).
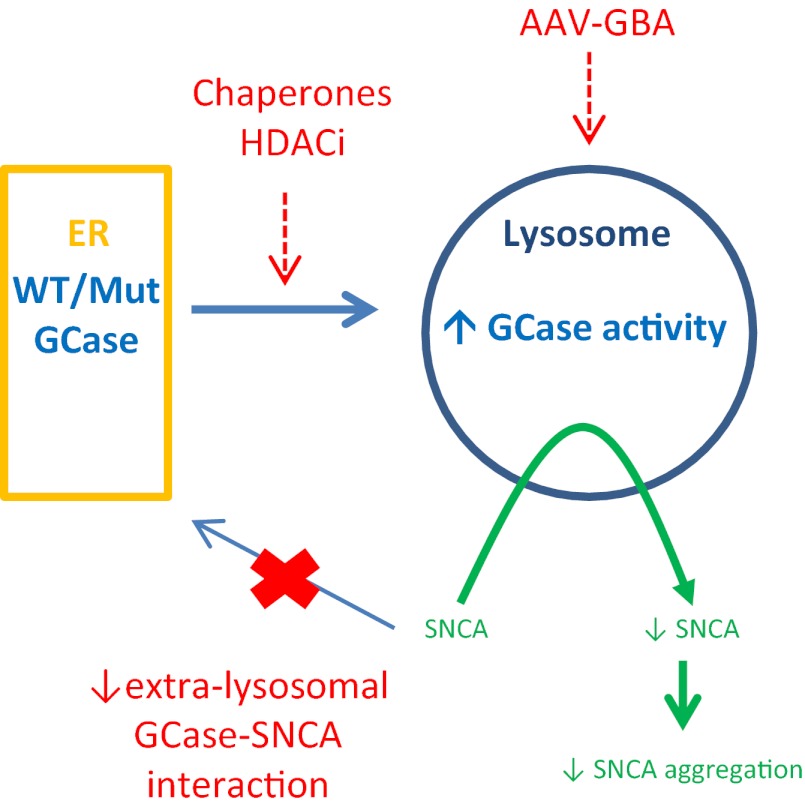
Post mortem analysis of GBA-PD brains demonstrated a significant reduction in GCase activity, most pronounced in the SN, a site of major degeneration in PD (10). Of particular interest was the finding that GCase activity was reduced in the SN in PD brains without GBA mutations, thereby confirming the relevance of GCase function to the wider PD population. Several groups have demonstrated in cell and animal models that knockdown of GBA, overexpression of mutant GBA, or inhibition by condruitol β-epoxide causes accumulation of SNCA (9, 11–13). Conversely, increased SNCA causes a decrease in GCase protein and activity in cells (9, 10). This finding is further confirmed by Sardi et al. (1) in the A53T SNCA mutant mouse brain. The mechanisms that underlie the interaction of GCase and SNCA are not known, but must explain the reciprocal effects evident with the respective wild-type proteins, as well as their mutant forms. There is evidence to support a direct interaction of GCase with SNCA at membranes, perhaps within the lysosome itself (14). SNCA also appears to impair the transport of GCase from ER to the lysosome (9, 10). Normally GCase is synthesized on ER-bound polyribosomes, translocated into the ER and subjected to ER “quality control” for correct folding, and then transported to the lysosome. GBA mutations commonly associated with PD cause GCase to fold abnormally and become arrested in the ER, whereupon it is degraded by the ubiquitin proteasomal system, resulting in decreased GCase protein resident in the lysosome (3). The impaired lysosomal function that would result from GBA mutations, or impairment of wild-type GCase transport by SNCA, would in turn reduce SNCA turnover, given that it is predominantly metabolized through chaperone-mediated autophagy. It is notable that there is evidence of defective chaperone-mediated autophagy in the PD brain (15). The interaction of GCase with SNCA forms the basis for the hypothesis that augmenting GCase activity may favorably modify SNCA metabolism to reduce its accumulation or aggregation, and thus help ameliorate this component of PD pathogenesis (Fig. 1).
Fig. 1.
The glucocerebrosidase/SNCA axis, potential targets for therapeutic intervention for PD. SNCA interacts with both wild-type (WT) and mutant (Mut) GCase, and modulation of this may serve to reduce SNCA concentrations and delay or reverse PD-related pathology. Strategies to increase lysosomal GCase or to decrease extralysosomal GCase–SNCA interactions would be anticipated to reduce SNCA concentrations. Chaperones, histone deacetylase inhibitors [HDACi), or increased expression of GCase by, for example, gene therapy (AAV-GBA) may be examples of such strategies.
The data from Sardi et al. (1) show that increasing GCase activity by adeno-associated viral vector (AAV) delivery of the enzyme into the brain of their GD mouse model can reduce the accumulation of substrate, SNCA, tau, and ubiquitin to varying degrees. This reduction was associated with an improvement in the memory defect in this model, whether given before or after the protein accumulations had become established. The implication is that modulation of GCase activity may be beneficial before or after diagnosis of PD and, if effective in modifying the pathogenesis of the disease, such treatment might prevent onset in asymptomatic individuals and slow progression in those already affected.
Treatments for GD include enzyme-replacement infusion therapy or substrate-reduction therapy by inhibition of glucosylceramide synthase, but do not cross the blood brain barrier. Interest has more recently focused on strategies to enhance GCase activity and lysosomal function using small-molecule chaperones (16). Several converging lines of evidence suggest that this may be successful and of relevance to both GD and PD, as well as to other SNCA-related disorders, such as dementia with Lewy bodies.
Small-molecule chaperones, competitive inhibitors of GCase, may bind to the misfolded GCase, correct folding such as to allow the protein to pass through the ER to the lysosome where the GCase-chaperone complex dissociates in the acidic pH and the residual GCase activity hydrolyses substrate (16). There is a substantial body of work demonstrating the efficacy of chaperones to increase GCase activity (see ref. 17 and references therein) and reduce substrate concentrations (18).
Ambroxol and isofagomine are two orally active, brain-penetrant small-molecule chaperones that have been investigated for their ability to increase mutant GCase activity in GD fibroblasts (17, 18) and in a mouse model (19). The degree to which the enzyme activity can be increased depends in part on the GBA mutation, but has been seen in the two commonest GD mutations, N370S and L444P. The chaperones also reduce the unfolded protein response and
Modulation of GCase activity may be beneficial before or after diagnosis of PD.
ER-associated degradation associated with the mutant GCase protein, and have additional benefits for cell function.
An alternative and potentially complementary strategy is the use of histone deacetylase inhibitors to reduce recognition of misfolded GCase by Hsp90β, decrease degradation, and increase residual enzyme levels. This strategy has been validated in GD patient fibroblasts (20).
The recognition of the link between GBA mutants, GCase activity, SNCA, and PD has made an important contribution to our thinking on the pathogenetic pathways in PD and dementia with Lewy bodies, and to how the interaction of GCase and SNCA may be leveraged to develop treatments that slow progression. Sardi et al.’s (1) contribution is particularly valuable in that it supports the notion that correcting GCase deficiency can directly influence SNCA and other protein metabolism in the brain in vivo both before and after accumulations have become established. This observation has special relevance to GBA mutation carriers. There is evidence that they may exhibit a sequence of nonmotor clinical features, coupled with biochemical abnormalities that may enable detection of those more at risk for converting to clinical PD. These individuals would be ideally suited to GCase restoration treatments that might prevent that conversion. However, those with established GBA-PD, on present evidence, might also benefit from such therapy. The observation of reduced GCase in non–GBA-PD makes such treatments potentially applicable to all PD patients.
Acknowledgments
The authors' work described here was supported in part by the Wellcome Trust/MRC Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson's Disease Consortium (UKPDC). We also acknowledge the support of Parkinson's UK the Kattan Trust.
Footnotes
The authors declare no conflict of interest.
See companion article on page 3537.
References
- 1.Sardi SP, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci USA. 2013;110:3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 3.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14(16):2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH, Tolosa E. Molecular and clinical prodrome of Parkinson disease: Implications for treatment. Nat Rev Neurol. 2010;6(6):309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 5.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bultron G, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33(2):167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeill A, Duran R, Hughes DA, Mehta A, Schapira AH. A clinical and family history study of Parkinson’s disease in heterozygous glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatry. 2012;83(8):853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzulli JR, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gegg ME, et al. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann Neurol. 2012;72(3):455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning-Boğ AB, Schüle B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: A biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30(6):1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Cleeter MW, et al. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int. 2013;62(1):1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen V, et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol. 2011;69(6):940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 14.Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab. 2013;108(1):56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67(12):1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman RL, et al. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3(2):101–107. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 17.Bendikov-Bar I, Maor G, Filocamo M, Horowitz M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol Dis. 2013;50(2):141–145. doi: 10.1016/j.bcmd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, et al. Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease. J Biol Chem. 2012;287(6):4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, et al. Isofagomine in vivo effects in a neuronopathic Gaucher disease mouse. PLoS ONE. 2011;6(4):e19037. doi: 10.1371/journal.pone.0019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, et al. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc Natl Acad Sci USA. 2013;110(3):966–971. doi: 10.1073/pnas.1221046110. [DOI] [PMC free article] [PubMed] [Google Scholar]