Abstract
In the last two decades, the widespread application of genetic and genomic approaches has revealed a bacterial world astonishing in its ubiquity and diversity. This review examines how a growing knowledge of the vast range of animal–bacterial interactions, whether in shared ecosystems or intimate symbioses, is fundamentally altering our understanding of animal biology. Specifically, we highlight recent technological and intellectual advances that have changed our thinking about five questions: how have bacteria facilitated the origin and evolution of animals; how do animals and bacteria affect each other’s genomes; how does normal animal development depend on bacterial partners; how is homeostasis maintained between animals and their symbionts; and how can ecological approaches deepen our understanding of the multiple levels of animal–bacterial interaction. As answers to these fundamental questions emerge, all biologists will be challenged to broaden their appreciation of these interactions and to include investigations of the relationships between and among bacteria and their animal partners as we seek a better understanding of the natural world.
Keywords: bacterial roles animal origins, reciprocal effects animal–bacterial genomics, bacteria-driven development, microbiome and host physiology, nested ecosystems
Biologists have long appreciated the roles that microbes play in the two distinct disciplines of pathogenesis and ecosystem cycling. However, it was not until the late 1970s that Carl Woese and George Fox opened a new research frontier by producing sequence-based measures of phylogenic relationships, revealing the deep evolutionary history shared by all living organisms (1). This game-changing advance catalyzed a rapid development and application of molecular sequencing technologies, which allowed biologists for the first time to recognize the true diversity, ubiquity, and functional capacity of microorganisms (2). This recognition, in turn, has led to a new understanding of the biology of plants and animals, one that reflects strong interdependencies that exist between these complex multicellular organisms and their associated microbes (3).
Although the biosphere comprises many diverse taxonomic groups, our focus here is principally on the interactions between one group of microorganisms, the domain Bacteria, and one group of complex multicellular organisms, the animals. Although we chose to focus on animal–bacterial interactions, we expect the application of new technology to reveal similar trends among and between Archaea, fungi, plants, and animals. We begin by describing what we know about the evolution of animals and their interactions with bacteria and about the influence that these relationships have had on the present-day genomic makeup of the partners. We review the wealth of new data on the roles of bacteria in animal development and physiology and conclude with a discussion of the nesting of animal–bacterial relationships within their larger ecological frameworks. We argue that interactions between animals and microbes are not specialized occurrences but rather are fundamentally important aspects of animal biology from development to systems ecology.
In addition to the references of the main text of this article, we include a list of useful citations to provide the reader a broad opening to the subtopics covered in this contribution (SI Suggested Readings).
Bacteria and the Origin of Animals
Understanding how associations among bacteria and animals first evolved may reveal the foundations of ecological rules that govern such interactions today. Animals diverged from their protistan ancestors 700–800 Mya, some 3 billion years after bacterial life originated and as much as 1 billion years after the first appearance of eukaryotic cells (4) (Fig. 1). Thus, the current-day relationships of protists with bacteria, from predation to obligate and beneficial symbiosis (5, 6), were likely already operating when animals first appeared. Attention to this ancient repertoire of eukaryote–bacterial interactions can provide important insights into larger questions in metazoan evolution, from the origins of complex multicellularity to the drivers of morphological complexity itself.
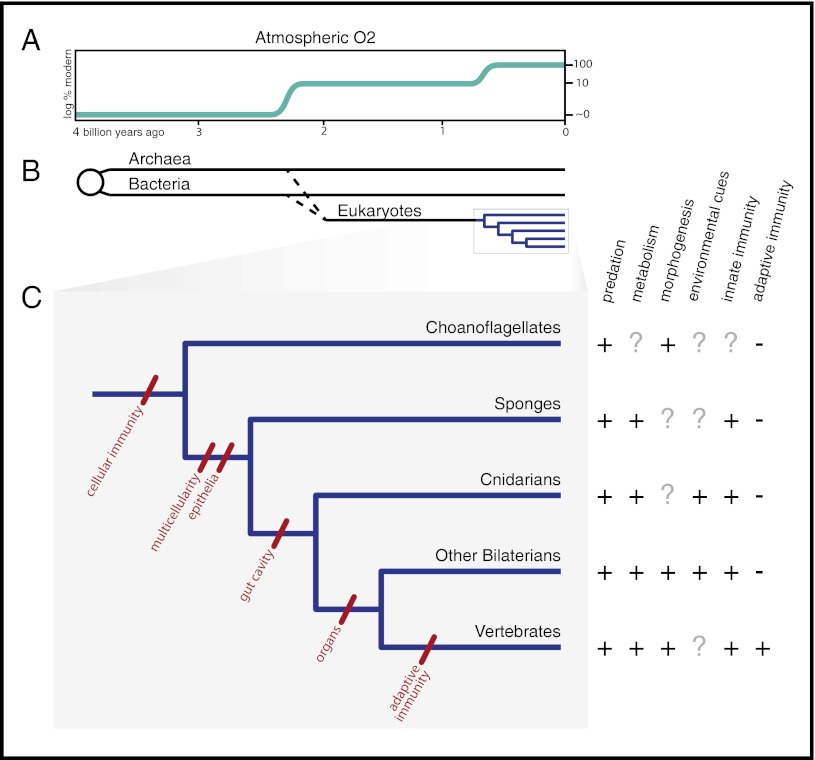
Fig. 1.
Animals through time. (A) Upper atmospheric oxygen concentration, as a percent of current levels, plotted against geological time. (B) Phylogenetic history of life on Earth, scaled to match the oxygen timeline. Note that the origin of the eukaryotes and the subsequent diversification of animals both correspond to periods of increasing atmospheric oxygen. (C) (Left) A phylogeny of choanoflagellates and selected animals, annotated to indicate the evolution of characters particularly relevant to interactions with bacteria. (Right) Interactions between bacteria and eukaryotes, corresponding to the phylogeny. Bacteria are prey, sources of metabolites, inducers of development in symbiosis (morphogenesis) and in larval settlement (environmental cues), and activators of immune systems.
Based on molecular and cellular data, animals and choanoflagellate protists are now considered sister groups, descended from a common choanoflagellate-like ancestor (Fig. 1) (7). The major underpinnings of animal–bacterial interactions—nutrition, recognition, cell adhesion, and signaling—guide two types of choanoflagellate behavior that may have been key to the origin of animals: predation (8) and colony formation (9). Extant choanoflagellates have homologs of animal signaling and adhesion proteins (e.g., cadherins and C-type lectins) that may have arisen as critical facilitators of bactivory (8). Diverse animals respond to bacterial signals as triggers for morphogenesis or behavior (e.g., larval settlement). Thus, the discovery that at least one choanoflagellate, Salpingoeca rosetta, responds to signals from specific bacteria to initiate colony formation through cell division hints at an ancient involvement of bacteria in the initiation of multicellularity (9). It will be important to learn whether intercellular cohesion in sponges, which are known to harbor hundreds of bacterial species (10–12), similarly depends on the presence of bacteria. The origin of multicellularity has been a topic of intense debate in biology, and many hypotheses have been developed about how this evolutionary milestone was achieved (13). A microbial role in animal origins does not obviate other perspectives on the evolution of complex multicellularity but adds a necessary functional and ecological dimension to these considerations.
As early animals diversified, animal–bacterial interactions continued to shape evolution in new ways (Fig. 1C). Bacteria took on a new role in animal nutrition, serving not only as prey but also as producers of digestible molecules in the animal gut. This role may have become more diverse with the evolution of a tubular gut, with one-way passage of food from mouth to anus. Bacterial influence on gut evolution certainly intensified with the subsequent origin of the coelom, a body cavity in which the organs are suspended. The advent of the coelom made gut elongation and regional specialization possible, facilitating both massive ingestion and storage for later digestion. Although the degree to which microbes have driven gut evolution is unknown, the radiation of several animal groups (e.g., ruminants) was undoubtedly enabled by alliances with their gut-associated microbiota. The evolution of form and function in other organ systems (e.g., respiratory, urogenital) may have also been influenced by interactions with bacterial partners (14). Furthermore, it is likely that the evolution of these organ system niches drove radiation of particular clades of animal-associated bacteria (15), such as the genus Helicobacter in vertebrate guts (16).
Evolution with animals, whether in symbiosis or via shared habitats, has also influenced the distribution and diversification of bacteria. For example, 90% of the bacterial species in termite guts are not found elsewhere (17). Such specialization, while increasing efficiency, comes with a cost: for every animal species that goes extinct, an unknown number of unique bacterial lineages that have evolved to depend on this animal niche disappear as well (18). On a broader scale, the evolution of animals provided novel physical environments for bacterial colonization, such as aerated deep sediments resulting from animal burrowing. Finally, human activities, which make a range of molecules not previously found in nature, such as halogenated hydrocarbons, have driven selection on bacterial catabolic pathways (19), leaving a signature of our presence in microbial metabolism.
Intertwining Genomes
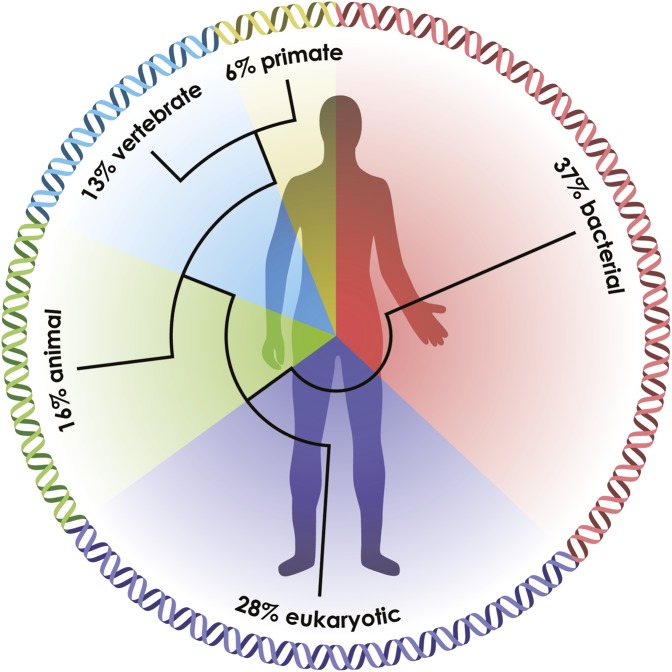
The long history of shared ancestry and alliances between animals and microbes is reflected in their genomes. Analysis of the large number of full genome sequences presently available reveals that most life forms share approximately one third of their genes, including those encoding central metabolic pathways (20). Not surprisingly, many animal genes are homologs of bacterial genes, mostly derived by descent, but occasionally by gene transfer from bacteria (21). For example, 37% of the ∼23,000 human genes have homologs in the Bacteria and Archaea, and another 28% originated in unicellular eukaryotes (20) (Fig. 2). Among these homologous genes are some whose products provide the foundation for signaling between extant animals and bacteria (22).
Fig. 2.
The ancestry of humans reflected in the genomic signature. A phylogenetic analysis of the human genes reveals the relative percentage of the genome that arose at a series of stages in biological evolution (20).
The intertwining of animal and bacterial genomes is not just historical: by coopting the vastly more diverse genetic repertoire present in its bacterial partners (23), a host can rapidly expand its metabolic potential, thereby extending both its ecological versatility and responsiveness to environmental change. For instance, many invertebrates have intracellular bacterial symbionts whose genes encode metabolic capabilities lacking in animals, such as the synthesis of essential amino acids (24), photosynthesis (25), or chemosynthesis (26). Certain marine invertebrates that feed on algae maintain algal plastids as photosynthetically active symbionts, a behavior that allows the host to use photosynthate as a food source for extended periods (27). These metabolic add-ons allow the animal to thrive by adapting to otherwise noncompetitive lifestyles (e.g., feeding on nutrient-poor diets such as plant sap) (28) or environments (e.g., oligotrophic habitats) (26). Further, such phenomena fit the definition of epigenetic features. Recent studies have revealed that bacterial pathogens (29) and other environmental factors (30) can alter the activities of epigenetic machinery. It is to be anticipated that such influences will extend to all types of animal–bacterial interactions, including those described above.
Microbial communities in the vertebrate gut respond to the host diet over both daily and evolutionary time scales, endowing animals with the flexibility to digest a wide variety of biomolecules and cope with and even flourish under conditions of diet change (15, 31). For example, the gut microbiome of most people in the United States is adapted to digest a high-fat, high-protein diet, whereas populations in rural Malawi and the Amazonas of Venezuela have distinct microbial consortia and functional gene repertoires optimized for breaking down complex carbohydrates (32). The gut microbiome adapts to changing diets and conditions not only by shifting community membership but also by changing gene content via horizontal gene transfer. For instance, the gut bacterium Bacteroides plebeius, found in some Japanese people, bears a gene transferred horizontally from the marine bacterium Zobellia galactanivorans, giving the gut symbiont the capacity to degrade seaweed polysaccharides (33). More generally, human-associated bacteria have a 25-fold higher rate of gene transfer than do bacteria in other environments, highlighting the important role of gene transfer in host-associated bacterial communities (34).
Bioinformatic analyses have revealed that interactions with animals also influence the size and content of the genomes of their bacterial partners. Although not all genome-size reduction occurs in symbiosis, a long history of intimate association with insects has resulted in highly reduced genomes in their intracellular symbionts; for example, the endosymbiont Candidatus Hodgkinia cicadicola of the Arizona cicada has a genome size <144 kb, smaller than that of some organelles (35). Recent studies have shown that genome reduction also occurs in segmented filamentous bacteria (Candidatus Savagella), members of the mammalian microbiota that are critical for the maturation of the immune system (36). Conversely, in Bacteroides thetaiotaomicron, another member of the mammalian intestinal microbiota, adaptation to a gut habitat rich in complex carbohydrates has driven the expansion of at least two gene families: glycan-utilization genes, which constitute 18% of this species’ genome (37), and diverse sulfatases that allow B. thetaiotaomicron to digest host mucin (38). The genomic basis for other microbial adaptations among gut microbes is less clear. One possible selection pressure is host temperature. In aquatic environments such as the deep sea, host fishes and invertebrates conform to the temperature of the environment, so temperature-driven coevolution would be unlikely in these habitats. In contrast, terrestrial environments often have broad, short-term (daily) and long-term (seasonal) fluctuations in temperatures. It is in these habitats that endothermy (maintaining a constant body temperature by metabolic means) evolved as a shared character in birds and mammals. Most enteric bacteria of birds and mammals have growth optima at ∼40 °C, suggesting the unexplored possibility that this trait resulted from coevolution of these bacteria with their endothermic hosts. The reciprocal may also be true, i.e., an animal’s microbial partners may have played a role in selecting for the trait of endothermy. Constant high temperature speeds up bacterial fermentation, providing rapid and sustained energy input for the host. These benefits are apparent when comparing conventional to germ-free mammals, which require one-third more food to maintain the same body mass (39). Keeping their microbes working at optimum efficiency likely offered a strongly positive selection pressure for the evolution of genes associated with the trait of endothermy in birds and mammals.
Partners in Animal Development
Animal development has traditionally been viewed as an autonomous process directed by the genome. Because it both originated and evolved in a microbe-rich environment, animal development deserves a reexamination, at least in part, as an orchestration of animal-encoded ontogeny and interdomain communication (40, 41). Although relatively few studies have been reported until recently, these early data lead us to anticipate that microbes play a role in providing signals for multiple developmental steps.
From their earliest stages of development, animals use sophisticated mechanisms to manage their microbial environment. Physical barriers, such as capsules, chorions, and mucus, protect eggs by excluding microbes, and chemical barriers, including antimicrobial peptides (AMPs), shape the composition of the associated microbiota (42). Conversely, several animals recruit specific bacteria to their embryonic surfaces to provide protection against potential pathogens (43). For example, the shrimp Palaemon macrodactylus is protected from the fungus Lagenidium callinectes by 2,3-indolinedione that is produced by an Alteromonas sp. on the embryo’s surface (44). Although many animals, including a wide variety of insects, have transovarial (i.e., via the egg to the embryo) transmission of bacterial partners (28, 45), we have no persuasive evidence to date that these microbes or their metabolites influence embryogenesis. Whereas developmentally important symbioses have been documented throughout the postembryonic (larval and juvenile) stages of vertebrate and arthropod life cycles, the roles of symbiotic microbes during normal embryonic development are just beginning to be studied. Unlike vertebrates whose embryos develop inside enclosures that physically block bacterial associations, many invertebrates acquire their symbionts through the female germ line. Here, we may expect to find regulatory signals being generated by microbes and interactions between host and symbiont development (46). It is apparent that evolution has selected for anatomical, cellular, and molecular determinants that act during this period to prepare newborn animals for interactions with the microbial world.
Ample evidence shows that microbes act directly as agents of postembryonic development. For example, fucosyltransferases decorate the surface of the embryonic mammalian intestine with fucose residues that provide a nutrient source for gut microbes, including B. thetaiotaomicron, as they colonize the newborn (47). In the squid-vibrio system, a complex organ forms during embryogenesis that facilitates subsequent colonization by the symbiotic bacterium Vibrio fischeri (48). The products of horizontally acquired microbes can be essential for a range of developmental functions, including influences on larval growth rate and body size in invertebrates (49), postembryonic maturation and renewal of epithelia in invertebrates and vertebrates (50–53), development and specification of the gut-associated lymphoid tissues in vertebrates (54), activation of the immune system in tsetse flies (55), and normal brain development in mammals (56, 57). Intriguingly, the host regulatory pathways that control immune responses to microbes appear also to have central roles in animal development, underscoring the intimate relationships between development and host–microbe interactions (58, 59).
Perhaps the most pervasive example of microbial signaling in animal development is the induction of settlement and metamorphosis of many marine invertebrate larvae (60). This transition is an absolute requirement for completion of the animal’s life cycle and is contingent on induction by exogenous morphogenetic cues, many of which are produced by bacteria associated with a particular environmental surface (60). Marine invertebrate metamorphoses offer valuable models for exploring the basis of bacterial signaling in animal development in a setting where the very persistence of marine ecosystems depends on it.
Coming full circle, the influence of microbes on animal reproduction can be observed with particular clarity in invertebrates (61). Most insect orders carry vertically transmitted parasites that can affect the processes of sexual determination, maturation, and reproductive success. For example, various Wolbachia strains feminize crustacean genetic males, kill males, or induce clonal production of females in some insects (62). However, in one case, the association with a Wolbachia strain has become essential for reproduction; the wasp Asobara tabida requires this microbe for egg maturation (63). Recent studies have shown that, in both invertebrates and vertebrates, the microbiota can even influence reproductive behavior (64). Changes in cuticular-hydrocarbon profiles linked to specific bacterial symbionts in the gut of Drosophila melanogaster correlate with mate choice (65), and several lines of evidence suggest that olfactory cues associated with mate choice in vertebrates are produced by their resident microbiota (66).
Interdomain Communication
Although animals and bacteria have different forms and lifestyles, they recognize one another and communicate in part because, as described above, their genomic “dictionaries” share a common and deep evolutionary ancestry. One modality of interdomain communication, that occurring during bacterial pathogenesis, has been extensively explored for over a century. However, how might bacterial signaling structure the biology of the healthy host?
Biologists now know that bacteria have social behaviors, communicating with each other through chemical signaling, such as quorum sensing (67, 68); more recently, interdomain quorum signaling between bacteria and their eukaryotic partners has become evident (22, 69–71). In addition to quorum signals, bacteria use cell surface–derived molecules to communicate with their hosts, affecting host processes both at the cellular level [e.g., apoptosis, Toll-like receptor (TLR) signaling (52, 72)], as well as at the organ-system level (Fig. 3). Conversely, host-derived signal molecules like nitric oxide (NO) can be sensed directly by microbes (73). It is intriguing to consider that these kinds of communication evolved to maintain an association’s balance with its hundreds of beneficial species and that pathogens have “hijacked” these conversations to enhance their fitness through disease. For example, Salmonella typhimurium has adapted the quorum-sensing regulator QseC to act as a receptor for the host hormone norepinephrine and thereby tie the regulation of virulence genes to the hormone’s presence in the tissue (74). Some hosts, such as the marine macroalga Delisea pulchra, respond to quorum-signaling pathogens by producing halogenated furanones that act as signal mimics, blocking the microbes’ communication (75).
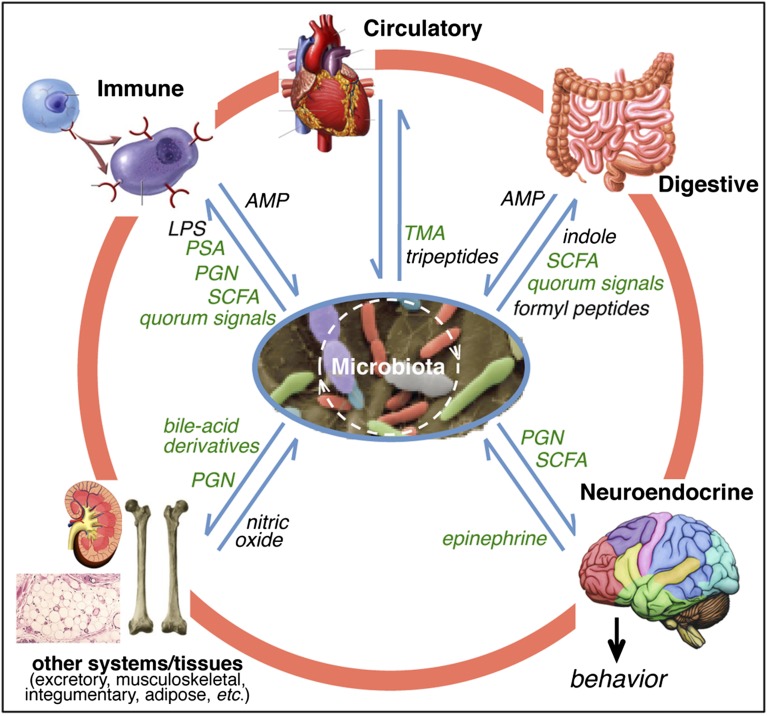
Fig. 3.
Signaling within and between the animal and its microbiota. Members of the microbiota, such as those in and on the gut, oral cavity, and skin, communicate among themselves and exchange signals with the animal’s organ systems, participating in the body’s homeostasis. Some of the signals promoting this balance are mentioned in the text (green), whereas other representatives are not (black; Tables S1 and S2). The microbiota also influences animal behavior, creating a direct interface with other organisms. AMP, antimicrobial peptides; LPS, lipopolysaccharide; PGN, peptidoglycan; PSA, polysaccharide A; SCFA, short-chain fatty acids; TMA, trimethylamine oxide.
The gut is likely the site of the most dynamic and consequential bacteria signaling that benefits animal hosts, because of the sheer numbers and diversity of its microbes and the inherent permeability and sensitivity of the gut epithelium. For example, acetate, a short-chain fatty acid (SCFA) produced by the gut bacterium Acetobacter, stimulates insulin signaling in D. melanogaster, thereby promoting host growth rates and reducing sugar and lipid levels (49). In mammals, SCFAs affect fat deposition, appetite-related hormone titers, and food consumption, which in turn can modulate the composition of the microbiota and have major consequences for health and behavior (76, 77). Not surprisingly, the composition of the gut microbiota and its SCFA production are influenced by diet. The resultant interplay among diet, the microbiota, and their metabolites is, in turn, implicated in the development of major metabolic disorders including obesity and diabetes (78). As much as a third of an animal’s metabolome—i.e., the diversity of molecules carried in its blood—has a microbial origin; thus, the circulatory system extends the chemical impact of the microbiota throughout the human body (79), transporting metabolites that influence the physiology and metabolism of distant organs and perhaps other bacterial communities (80, 81). Some dietary constituents can be modified by gut microbiota into deleterious compounds; for example, the conversion of dietary phosphatidylcholine into the proatherosclerotic metabolite, trimethylamine, can jeopardize cardiovascular health (82). Furthermore, recent studies link the gut microbiota to brain physiology and animal behavior (83). For instance, germ-free mice have defects in brain regions that control anxiety (57), and feeding probiotic bacteria to normal mice reduces depression-like behaviors (84, 85). The finding that TLRs, which transduce bacterial signals to host cells, are present on enteric neurons reveals one mechanism by which microbiota can communicate with the central nervous system through the brain–gut axis (72). Thus, maintaining homeostasis with the normal microbiota is essential to a healthy nervous system.
As the guardian of an animal’s internal environment, its immune system coordinates cellular and biochemical responses to alterations in the molecular landscape (86, 87), creating a robust equilibrium between the healthy host and its normal microbiota. The complexity of components that comprise this system reflects the great chemical diversity present in the microbial world. Pattern-recognition receptors (PRRs) of the innate immune system can have enormous repertoires, particularly in the invertebrates. PRRs recognize microbe-associated molecular patterns (MAMPs), such as bacteria-specific cell surface molecules (88). For example, peptidoglycan (PGN), a cell wall constituent of bacteria, interacts with PRRs to induce developmental processes in vertebrates and invertebrates (52, 54). The gut-associated lymphoid tissues of mammals mature with the presentation of peptidoglycan monomer by the gut microbiota during their early establishment, and the same molecule induces the regression of a juvenile-specific epithelium that facilitates colonization by the symbiont in the squid–vibrio system. Similarly, a polysaccharide produced and exported by Bacteroides fragilis, a constituent of the normal microbiota, signals the PRRs of immune cells to suppress gut inflammation (89). Disturbance of equilibria maintained by MAMP–PRR interactions can lead to a wide variety of pathologic states, including inflammatory bowel disease and diabetes (90, 91). Further, SCFAs produced by gut bacteria help the host defend against enteric infections (92), revealing molecular symbiosis between the microbiota and the immune system. Finally, immunologists are beginning to examine the possibility that, in addition to a role in pathogenesis, a principal selection pressure acting on the form and function of the adaptive immune system is the need to maintain balance among the complex, coevolved consortia that form persistent symbioses with the mucosal surfaces of several organ systems in the vertebrate host (86, 93–95).
Nested Ecosystems
Since the dawn of metazoan evolution, the ecology of animals has depended on bacterial communities. The fossil record provides evidence that some animal forms in the Ediacaran grazed on dense assemblages of bacteria on hard substrates (96) and that burrowing animals originated in association with microbial mats (97). Biologists increasingly recognize that, in extant animals, developmental and physiological signaling are processes whose understanding benefits from an ecological perspective (98).
Viewing animals as host–microbe ecosystems has given us new insights into the maintenance of human health. The application of ecological approaches, including successional assembly and diversity analysis, has proven valuable in understanding how animal–microbial alliances function (99–101). For example, human infants born vaginally have a very different succession during the early phases of gut colonization and possibly long-term composition of their microbiota than those delivered by Caesarean section (102). The effects of this difference in infant delivery on adult health remain to be discovered. We know that imbalances in the mature human microbiome have been correlated with a spectrum of diseases, including obesity and diabetes (77). A recent metacommunity analysis of the gut microbiota of obese and lean twins revealed that obesity is associated with a significantly less stable and more variable microbial community (103). Although most research on consortia is currently focused on humans and vertebrate model systems, such as mice and zebrafish, similarly complex interactions occur in all animal species. Viewing bacterial colonization of animals as an ecological phenomenon adds clarity to an understanding of the mechanisms and routes by which phylogenetically rich and functionally diverse microbial communities become established and evolve on and within animal hosts.
An ecological perspective influences not only our understanding of animal–microbiome interactions but also their greater role in biology. The ecosystem that is an individual animal and its many microbial communities [i.e., the holobiont (104)] does not occur in isolation but is nested within communities of other organisms that, in turn, coexist in and influence successively larger neighborhoods comprising ever more complex assemblages of microbes, fungi, plants, and animals (Fig. 4). Hydrothermal vent communities illustrate the role of animal–microbe associations in such nested ecosystems. At vents and other reducing habitats, chemoautotrophic symbionts provide organic nutrients for animal hosts in at least seven different phyla. The activities of these individual symbioses contribute to larger communities that include nonsymbiotic animal and microbial species that are able to exist through the symbiotic primary production that is not driven by solar energy but rather by sulfide, hydrogen, methane, and other reduced energy sources (26, 105). Similarly, nested within broader terrestrial ecosystems, bacterial communities in floral nectar can influence the way animals such as pollinators interact with plants. In these instances, the bacteria change the chemical properties of the nectar, making it more or less attractive to the pollinator, which changes the pollinator–plant dynamic (106).
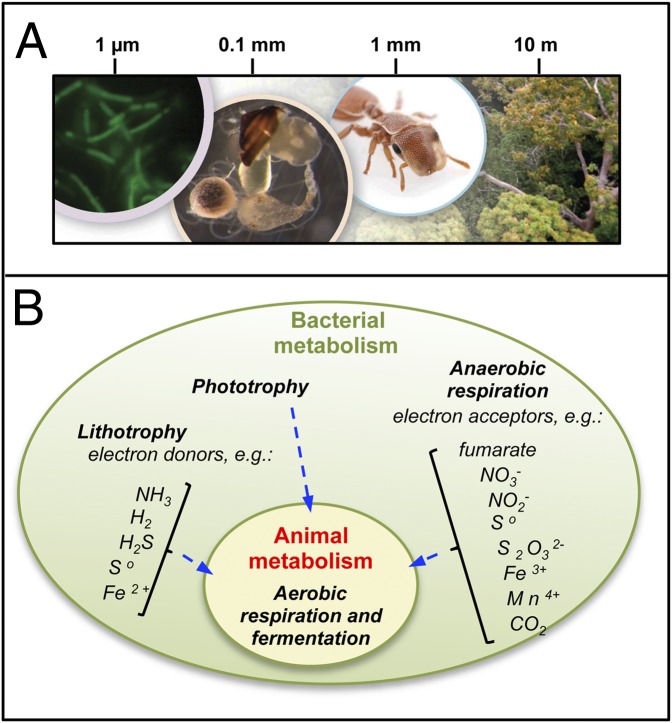
Fig. 4.
Nested ecological interactions of animals and bacteria and their underlying metabolic bases. (A) A forest canopy insect illustrates the cascading effects of animal-bacterial interactions across multiple spatial scales. Bacterial symbionts (Left), residing in the gut (Center Left), are essential to nutritional success of insect species (Center Right) in tropical forest canopies (Right), where they often make up a majority of animal biomass. (B) Diversity of energy metabolism in bacteria and animals. Animals can ferment and aerobically respire but are unable to perform the vast diversity of other, ecologically vital, energy-harvesting processes. Beyond phototrophy, which they share with plants, bacteria can also contribute to primary production by using inorganic energy sources (lithotrophy) to fix CO2. Animals are directly or indirectly dependent on bacteria for extracting energy and cycling biomolecules, whereas animals actively contribute to bacterial productivity through bioturbation, nutrient provisioning, and as habitats for colonization and shelter.
Bacteria are critical determinants of animal population and community structures, even in ecosystems where intimate symbioses are not the driving force. Recent studies demonstrate that the larvae of many benthic marine invertebrates require specific microbial cues for their recruitment from the plankton, and these larval responses to bacteria influence the structuring of many marine benthic communities (60, 107). For example, certain strains of the biofilm-forming bacterium Pseudoalteromonas luteoviolacea produce chemical cues that stimulate settlement and metamorphosis by Hydroides elegans, a polychaete worm that fouls docks and the hulls of ships worldwide (60, 108), as well as a sea urchin (109) and a coral (107). Surface biofilms on many marine animals serve important functions in determining the very nature of the animals’ ecological interactions with other organisms (110). Similarly, the acquisition of an appropriate microbiome at critical life history stages of many animals affects their subsequent behavioral patterns and thus the stability of their ecological roles in their communities (64). Bacteria feeding on dead animals in the sea, and likely on land, repel animal scavengers by producing noxious metabolites; these products allow the bacteria to effectively outcompete organisms 10,000 times their size (111).
Conversely, invasive animals can alter the activities of indigenous bacteria, with significant effects on their shared habitat. For example, rats introduced onto small Pacific islands decimated seabird populations, resulting in decreased sea-to-land transport of nutrients (guano) and altered decomposition and nutrient cycling by soil microbes (112). In another study, European earthworm species introduced to North American hardwood forests led to significant changes in soil microbial biomass and the metabolic quotient of the soil ecosystem (113). In each of these situations, an introduction led to a substantial reduction in ecosystem productivity. Applying metacommunity and network analyses (114) to such animal–bacterial interactions will be essential for the design of effective strategies for managing ecosystems in the face of the environmental perturbations, such as pollution, invasive species, and global climate change, that challenge the biosphere.
Challenges
For much of her professional career, Lynn Margulis (1938–2011), a controversial visionary in biology, predicted that we would come to recognize the impact of the microbial world on the form and function of the entire biosphere, from its molecular structure to its ecosystems. The weight of evidence supporting this view has finally reached a tipping point. The examples come from animal–bacterial interactions, as described here, and also from relationships between and among viruses, Archaea, protists, plants, and fungi. These new data are demanding a reexamination of the very concepts of what constitutes a genome, a population, an environment, and an organism. Similarly, features once considered exceptional, such as symbiosis, are now recognized as likely the rule, and novel models for research are emerging across biology. As a consequence, the New Synthesis of the 1930s and beyond must be reconsidered in terms of three areas in which it has proven weakest: symbiosis, development, and microbiology (115). One of these areas, microbiology, presents particular challenges both to the species concept, as formulated by Ernst Mayr in 1942, and to the concept that vertical transmission of genetic information is the only motor of selectable evolutionary change.
It is imperative that human societies recognize the centrality of the relationships between microbes and other organisms for the health of both individuals and the environments in which they live. The current focus on studies of humans and their microbiota has provided compelling evidence that the composition and activity of resident microbes play crucial roles in shaping the metabolic and regulatory networks that define good health, as well as a spectrum of disease states. Nonetheless, the underlying ecological mechanisms are still poorly defined, and the development of tools to translate this understanding into novel therapies presents an ongoing challenge.
In broader-scale ecosystems, evidence is mounting that seemingly minor environmental perturbations have major long-term impacts. A full understanding of the consequences will require us to expand our investigations of the associated changes in microbial communities in soil, freshwater, and marine habitats. How are such microbial assemblages affected by the introduction of nonnative species of plants and animals, the increases in temperature due to global climate change, and the acidification of the oceans? Although a few studies (116, 117) have revealed its importance, the impact of acidification has thus far focused largely on eukaryotic calcification processes (118). This emphasis leaves us still ignorant of how marine ecosystems may be changed if small shifts in seawater pH or temperature alter the compositions of bacterial communities that are crucial for recruitment of the next generations of plants and animals into their native habitats. The maintenance and restoration of ecosystems that support sustainable agriculture and carbon-neutral energy production depend on recognition of the interactions between microorganisms and animals, plants, and fungi, and the robustness of these relationships in response to anthropogenic and other perturbations. Whether an ecosystem is defined as a single animal or the planet’s biosphere, the goal must be to apply an understanding of the relationships between microbes and other organisms to predict and manipulate microbial community structure and activity so as to promote ecosystem health.
These challenges present a vast and exciting frontier for the field of biology and call on life scientists to alter significantly their view of the fundamental nature of the biosphere. Ambitious large-scale interdisciplinary research efforts, such as the Human Microbiome Project and the Earth Microbiome Project, aim to provide a basic understanding of microbial variation across a wide range of body and environmental habitats in both the normal and perturbed states. Effective project design and the resulting large data sets are driving advances in quantitative methods, such as the creation and refinement of techniques to improve approximation algorithms, dimensionality reduction, and visualization of the results (119). These efforts have highlighted the need for genomic standards, open-source integrated analysis pipelines, and increased low-cost computational power. A compelling goal for the future is to apply these technologies, the resultant data, and the emerging intellectual framework to a wide array of biological questions. Such a synthesis promises to generate a more accurate vision of life on earth.
Successful development of research on our microbial world will result only with the breakdown of existing intellectual barriers, not only between the subdisciplines of biology, but also across the natural sciences, mathematics, computer science, and engineering. Such integration will be fostered by the active promotion of cross-disciplinary units at universities, collaboration among professional societies, and novel approaches by the funding agencies to support the development of this new frontier (120). The progress of change across the field will also require reformulation of educational goals, including development of ways of teaching biology that are as revolutionary as those that occurred in the 1950s in the wake of both the New Synthesis and the launch of Sputnik. Because of advances described here, we foresee a day when microbiology will be a centerpiece not only of biological research, but also of high school, undergraduate, and graduate biology education.
Supplementary Material
Acknowledgments
We thank N. Glasser for assistance with graphics and D. Haraway and E. A. C. Heath-Heckman for helpful discussion and comments on the manuscript. The work of this group was supported by National Science Foundation Grant EF-0905606 to the National Evolutionary Synthesis Center (NESCent). This effort was also supported by fellowships to M.M.-N. from the John Simon Guggenheim Foundation and the Gordon and Betty Moore Foundation Visiting Scholar Program at the California Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218525110/-/DCSupplemental.
References
- 1.Pace NR, Sapp J, Goldenfeld N. Phylogeny and beyond: Scientific, historical, and conceptual significance of the first tree of life. Proc Natl Acad Sci USA. 2012;109(4):1011–1018. doi: 10.1073/pnas.1109716109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu D, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature. 2009;462(7276):1056–1060. doi: 10.1038/nature08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert SF, Sapp J, Tauber AI. A symbiotic view of life: We have never been individuals. Q Rev Biol. 2012;87(4):335–341. doi: 10.1086/668166. [DOI] [PubMed] [Google Scholar]
- 4.Knoll AH. Life on a Young Planet. Princeton, NJ: Princeton Univ Press; 2003. [Google Scholar]
- 5.Dopheide A, Lear G, Stott R, Lewis G. Preferential feeding by the ciliates Chilodonella and Tetrahymena spp. and effects of these protozoa on bacterial biofilm structure and composition. Appl Environ Microbiol. 2011;77(13):4564–4572. doi: 10.1128/AEM.02421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowack EC, Melkonian M. Endosymbiotic associations within protists. Philos Trans R Soc Lond B Biol Sci. 2010;365(1541):699–712. doi: 10.1098/rstb.2009.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr M, Leadbeater BS, Hassan R, Nelson M, Baldauf SL. Molecular phylogeny of choanoflagellates, the sister group to Metazoa. Proc Natl Acad Sci USA. 2008;105(43):16641–16646. doi: 10.1073/pnas.0801667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols SA, Dayel MJ, King N. Genomic, phylogenetic, and cell biological insights into metazoan origins. In: Telford MJ, Littlewood DTJ, editors. Evolution: Genes, Genomes, Fossils and Trees. Oxford, UK: Oxford Univ Press; 2009. [Google Scholar]
- 9.Alegado RA, et al. 2012. A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals, eLife 1:e00013.
- 10.Hentschel U, Piel J, Degnan SM, Taylor MW. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol. 2012;10(9):641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt S, et al. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012;6(3):564–576. doi: 10.1038/ismej.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas T, et al. Functional genomic signatures of sponge bacteria reveal unique and shared features of symbiosis. ISME J. 2010;4(12):1557–1567. doi: 10.1038/ismej.2010.74. [DOI] [PubMed] [Google Scholar]
- 13.Grosberg RK, Strathmann RR. The evolution of multicellularity: A minor major transition. Annu Rev Ecol Evol Syst. 2007;38(1):621–654. [Google Scholar]
- 14.Herbst T, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6(10):776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J Clin Invest. 2009;119(9):2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hongoh Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci Biotechnol Biochem. 2010;74(6):1145–1151. doi: 10.1271/bbb.100094. [DOI] [PubMed] [Google Scholar]
- 18.Staley JT. Biodiversity: Are microbial species threatened? Curr Opin Biotechnol. 1997;8(3):340–345. doi: 10.1016/s0958-1669(97)80014-6. [DOI] [PubMed] [Google Scholar]
- 19.Janssen DB, Dinkla IJ, Poelarends GJ, Terpstra P. Bacterial degradation of xenobiotic compounds: Evolution and distribution of novel enzyme activities. Environ Microbiol. 2005;7(12):1868–1882. doi: 10.1111/j.1462-2920.2005.00966.x. [DOI] [PubMed] [Google Scholar]
- 20.Domazet-Loso T, Tautz D. An ancient evolutionary origin of genes associated with human genetic diseases. Mol Biol Evol. 2008;25(12):2699–2707. doi: 10.1093/molbev/msn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet. 2008;9(8):605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- 22.Hughes DT, Sperandio V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6(2):111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapierre P, Gogarten JP. Estimating the size of the bacterial pan-genome. Trends Genet. 2009;25(3):107–110. doi: 10.1016/j.tig.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 25.Venn AA, Loram JE, Douglas AE. Photosynthetic symbioses in animals. J Exp Bot. 2008;59(5):1069–1080. doi: 10.1093/jxb/erm328. [DOI] [PubMed] [Google Scholar]
- 26.Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat Rev Microbiol. 2008;6(10):725–740. doi: 10.1038/nrmicro1992. [DOI] [PubMed] [Google Scholar]
- 27.Rumpho ME, Pelletreau KN, Moustafa A, Bhattacharya D. The making of a photosynthetic animal. J Exp Biol. 2011;214(Pt 2):303–311. doi: 10.1242/jeb.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas AE. The Symbiotic Habit. Princeton, NJ: Princeton Univ Press; 2010. [Google Scholar]
- 29.Bierne H, Hamon M, Cossart P. 2012. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med 2(12):a010272.
- 30.Feil R, Fraga MF. Epigenetics and the environment: Emerging patterns and implications. Nat Rev Genet. 2011;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 31.Muegge BD, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hehemann JH, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464(7290):908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 34.Smillie CS, et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature. 2011;480(7376):241–244. doi: 10.1038/nature10571. [DOI] [PubMed] [Google Scholar]
- 35.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10(1):13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 36.Kuwahara T, et al. The lifestyle of the segmented filamentous bacterium: A non-culturable gut-associated immunostimulating microbe inferred by whole-genome sequencing. DNA Res. 2011;18(4):291–303. doi: 10.1093/dnares/dsr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4(5):447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjdia A, Martens EC, Gordon JI, Berteau O. Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 2011;286(29):25973–25982. doi: 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bäckhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert SF, Epel D, Tauber AI. Ecological Developmental Biology. Sunderland, MA: Sinauer; 2008. [Google Scholar]
- 41.Pradeu T. A mixed self: The role of symbiosis in development. Biol Theory. 2011;6(1):80–88. [Google Scholar]
- 42.Fraune S, et al. In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides. Proc Natl Acad Sci USA. 2010;107(42):18067–18072. doi: 10.1073/pnas.1008573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamdoun A, Epel D. Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA. 2007;104(6):1745–1750. doi: 10.1073/pnas.0610108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Turnes MS, Hay ME, Fenical W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science. 1989;246(4926):116–118. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- 45.Thacker RW, Freeman CJ. Sponge-microbe symbioses: Recent advances and new directions. Adv Mar Biol. 2012;62:57–111. doi: 10.1016/B978-0-12-394283-8.00002-3. [DOI] [PubMed] [Google Scholar]
- 46.Serbus LR, et al. A feedback loop between Wolbachia and the Drosophila gurken mRNP complex influences Wolbachia titer. J Cell Sci. 2011;124(Pt 24):4299–4308. doi: 10.1242/jcs.092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273(5280):1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery MK, McFall-Ngai M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120(7):1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 49.Shin SC, et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334(6056):670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 50.Becker T, et al. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463(7279):369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 51.Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4570–4577. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFall-Ngai MJ, Heath-Heckman EAC, Gillette AA, Peyer SM, Harvie EA. The secret languages of coevolved symbioses: Insights from the Euprymna scolopes-Vibrio fischeri symbiosis. Semin Immunol. 2012;24(1):3–8. doi: 10.1016/j.smim.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osman D, et al. Autocrine and paracrine unpaired signalling regulate intestinal stem cell maintenance and division. J Cell Sci. 2012 doi: 10.1242/jcs.113100. in press. [DOI] [PubMed] [Google Scholar]
- 54.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 55.Weiss BL, Maltz M, Aksoy S. Obligate symbionts activate immune system development in the tsetse fly. J Immunol. 2012;188(7):3395–3403. doi: 10.4049/jimmunol.1103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 57.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boehm AM, et al. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proc Natl Acad Sci USA. 2012;109(48):19697–19702. doi: 10.1073/pnas.1209714109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryu JH, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 60.Hadfield MG. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Annu Rev Mar Sci. 2011;3:453–470. doi: 10.1146/annurev-marine-120709-142753. [DOI] [PubMed] [Google Scholar]
- 61.Engelstadter J, Hurst GDD. The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst. 2009;40:127–149. [Google Scholar]
- 62.Rigaud T, Pennings PS, Juchault P. Wolbachia bacteria effects after experimental interspecific transfers in terrestrial isopods. J Invertebr Pathol. 2001;77(4):251–257. doi: 10.1006/jipa.2001.5026. [DOI] [PubMed] [Google Scholar]
- 63.Dedeine F, et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA. 2001;98(11):6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Microbiology. Animal behavior and the microbiome. Science. 2012;338(6104):198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- 65.Sharon G, et al. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA. 2010;107(46):20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archie EA, Theis KR. Animal behavior meets microbial ecology. Anim Behav. 2011;82(2011):425–436. [Google Scholar]
- 67.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsek MR, Greenberg EP. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005;13(1):27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 69.González JF, Venturi V. A novel widespread interkingdom signaling circuit. Trends Plant Sci. 2012;42(11):2834–2839. doi: 10.1016/j.tplants.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Karlsson T, Turkina MV, Yakymenko O, Magnusson KE, Vikström E. The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration. PLoS Pathog. 2012;8(10):e1002953. doi: 10.1371/journal.ppat.1002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevens AM, Schuster M, Rumbaugh KP. Working together for the common good: Cell-cell communication in bacteria. J Bacteriol. 2012;194(9):2131–2141. doi: 10.1128/JB.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143(4):1006–1016, e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, et al. H-NOX-mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fischeri. Proc Natl Acad Sci USA. 2010;107(18):8375–8380. doi: 10.1073/pnas.1003571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreira CG, Sperandio V. Interplay between the QseC and QseE bacterial adrenergic sensor kinases in Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun. 2012;80(12):4344–4353. doi: 10.1128/IAI.00803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hentzer M, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22(15):3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 77.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 78.Burcelin R. Regulation of metabolism: A cross talk between gut microbiota and its human host. Physiology (Bethesda) 2012;27(5):300–307. doi: 10.1152/physiol.00023.2012. [DOI] [PubMed] [Google Scholar]
- 79.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 80.Swann JR, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wikoff WR, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 84.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayer EA. Gut feelings: The emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eberl G. A new vision of immunity: Homeostasis of the superorganism. Mucosal Immunol. 2010;3(5):450–460. doi: 10.1038/mi.2010.20. [DOI] [PubMed] [Google Scholar]
- 87.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: Can we draw a line? Trends Immunol. 2004;25(12):640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Round JL, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 93.Chung H, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fedonkin MA, Simonetta A, Ivantsov AY (2007) New data on Kimberella, the Vendian mollusc-like organism (White Sea region, Russia): Paleoecological and evolutionary implications, Geol Soc London Spec Publ 286(1):157–179.
- 97.Seilacher A. Biomat-related lifestyles in the Precambrian. Palaios. 1999;14(1):86–93. [Google Scholar]
- 98.Camp JG, Kanther M, Semova I, Rawls JF. Patterns and scales in gastrointestinal microbial ecology. Gastroenterology. 2009;136(6):1989–2002. doi: 10.1053/j.gastro.2009.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fierer N, et al. From animalcules to an ecosystem: Application of ecological concepts to the human microbiome. Annu Rev Ecol Evol Syst. 2012;43:137–155. [Google Scholar]
- 101.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dominguez-Bello MG, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holmes I, Harris K, Quince C. Dirichlet multinomial mixtures: Generative models for microbial metagenomics. PLoS ONE. 2012;7(2):e30126. doi: 10.1371/journal.pone.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bourne DG, et al. Microbial disease and the coral holobiont. Trends Microbiol. 2009;17(12):554–562. doi: 10.1016/j.tim.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Petersen JM, et al. Hydrogen is an energy source for hydrothermal vent symbioses. Nature. 2011;476(7359):176–180. doi: 10.1038/nature10325. [DOI] [PubMed] [Google Scholar]
- 106.Vannette RL, Gauthier M-P, Fukami T. Nectar bacteria, but not yeast, weaken a plant-pollinator mutualism. Proc Biol Sci. 2013;280(1752):20122601. doi: 10.1098/rspb.2012.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tran C, Hadfield MG. Larvae of Pocillopora damicornis (Anthozoa) settle and metamorphose in response to surface-biofilm bacteria. Mar Ecol Prog Ser. 2011;433:85–96. [Google Scholar]
- 108.Huang Y, Callahan S, Hadfield MG. Recruitment in the sea: Bacterial genes required for inducing larval settlement in a polychaete worm. Sci Rep. 2012;2:228. doi: 10.1038/srep00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huggett MJ, Williamson JE, de Nys R, Kjelleberg S, Steinberg PD. Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia. 2006;149(4):604–619. doi: 10.1007/s00442-006-0470-8. [DOI] [PubMed] [Google Scholar]
- 110.Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F. The second skin: Ecological role of epibiotic biofilms on marine organisms. Front Microbiol. 2012;3:292. doi: 10.3389/fmicb.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burkepile DE, et al. Chemically mediated competition between microbes and animals: Microbes as consumers in food webs. Ecology. 2006;87(11):2821–2831. doi: 10.1890/0012-9658(2006)87[2821:cmcbma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 112.Fukami T, et al. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol Lett. 2006;9(12):1299–1307. doi: 10.1111/j.1461-0248.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 113.Eisenhauer N, Schlaghamersky J, Reich PB, Frelich LE. The wave towards a new steady state: Effects of earthworm invasion on soil microbial functions. Biological Invasions. 2011;13(10):2191–2196. [Google Scholar]
- 114.Massol F, et al. Linking community and ecosystem dynamics through spatial ecology. Ecol Lett. 2011;14(3):313–323. doi: 10.1111/j.1461-0248.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- 115.Koonin EV. The Origin at 150: Is a new evolutionary synthesis in sight? Trends Genet. 2009;25(11):473–475. doi: 10.1016/j.tig.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Weinbauer MG, Maier C, Dai MH, Gattuso J-P. Effect of ocean acidification on microbial diversity and on microbial driven biochemistry and ecosystem functioning. Aquat Microb Ecol. 2010 [Google Scholar]
- 117.Witt V, Wild C, Anthony KR, Diaz-Pulido G, Uthicke S. Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environ Microbiol. 2011;13(11):2976–2989. doi: 10.1111/j.1462-2920.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- 118.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez A, Knight R. Advancing analytical algorithms and pipelines for billions of microbial sequences. Curr Opin Biotechnol. 2012;23(1):64–71. doi: 10.1016/j.copbio.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Committee on a New Biology for the 21st Century (2009) A New Biology for the 21st Century: Ensuring the United States Leads the Coming Biology Revolution Board on Life Sciences, National Research Council, National Academies, The National Academies Press, (Washington, DC) [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.