Abstract
Symptoms of depression can be induced in humans through blockade of acetylcholinesterase (AChE) whereas antidepressant-like effects can be produced in animal models and some clinical trials by limiting activity of acetylcholine (ACh) receptors. Thus, ACh signaling could contribute to the etiology of mood regulation. To test this hypothesis, we administered the AChE inhibitor physostigmine to mice and demonstrated an increase in anxiety- and depression-like behaviors that was reversed by administration of nicotinic or muscarinic antagonists. The behavioral effects of physostigmine were also reversed by administration of the selective serotonin reuptake inhibitor fluoxetine. Administration of fluoxetine also increased AChE activity throughout the brain, with the greatest change in the hippocampus. To determine whether cholinergic signaling in the hippocampus could contribute to the systemic effects of cholinergic drugs, we infused physostigmine or virally delivered shRNAs targeting AChE into the hippocampus. Both pharmacological and molecular genetic decreases in hippocampal AChE activity increased anxiety- and depression-like behaviors and decreased resilience to repeated stress in a social defeat paradigm. The behavioral changes due to shRNA-mediated knockdown of AChE were rescued by coinfusion of an shRNA-resistant AChE transgene into the hippocampus and reversed by systemic administration of fluoxetine. These data demonstrate that ACh signaling in the hippocampus promotes behaviors related to anxiety and depression. The sensitivity of these effects to fluoxetine suggests that shRNA-mediated knockdown of hippocampal AChE represents a model for anxiety- and depression-like phenotypes. Furthermore, abnormalities in the cholinergic system may be critical for the etiology of mood disorders and could represent an endophenotype of depression.
Keywords: psychosocial stress, affective disorders
Depression affects one in six individuals worldwide, resulting in a substantial personal and economic burden. Although the precise etiology of depression is not known, a constellation of findings suggests that predisposing factors, including individual genetic makeup, interacting with stressful life events, can precipitate depressive disorders. Currently available antidepressants are effective at treating both depression and anxiety, but the therapeutic response remains variable. Only 30% of patients achieve remission during the first line of treatment with a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine, and many patients show only limited improvements. Thus, it is critical to identify the neurobiological factors that predispose individuals to stress susceptibility and depression to understand the etiology and develop therapeutics for the disorder.
A recent human imaging study has suggested that acetylcholine (ACh) levels are elevated in patients who are actively depressed, as measured by occupancy of nicotinic receptors throughout the brain, and remain high in patients who have a history of depression (1). In addition, despite the recent failure of a large clinical trial, other clinical and preclinical studies have shown that blockers of cholinergic (both muscarinic and nicotinic) receptors can induce antidepressant-like responses (2). These observations have renewed interest (3, 4) in the adrenergic-cholinergic balance hypothesis of mania and depression (5), which states that heightened cholinergic tone and decreased noradrenergic tone could lead to depressive symptoms (6). In these human studies, physostigmine, an inhibitor of acetylcholinesterase (AChE: the primary enzyme regulating ACh levels in the extracellular space), increased depressive symptoms in individuals with or without a history of depression. Similarly, hypersensitivity of the cholinergic system has been connected to the induction of depression-like behavior induced by various stressors in rodents (7). ACh levels and activity can be modulated by stress [one of the main triggers of depression (8)] in several brain regions (9). Taken together, these observations suggest that hyperactivity of brain cholinergic systems can contribute to the pathophysiology of depression; however, the mechanisms underlying this relationship are largely unknown, and it is not clear whether there is a causal relationship between ACh regulation and mood or whether these observations are solely correlative.
Previous studies have attempted to alter AChE activity genetically, but broad peripheral effects of AChE knockout result in early death and severe motor abnormalities, whereas broad overexpression results in compensatory alterations in AChE activity (10, 11). Other studies have manipulated expression of a specific, alternatively spliced isoform of AChE (AChE-R) that can be induced by stress, that results in hypersensitivity of hippocampal neurons through neurite reorganization (12), and that may protect against stress-induced neurodegeneration (13). Mice overexpressing AChE-R show heightened anxiety-like behavior (14), but it remains difficult to determine whether this is a primary consequence of altering AChE activity or whether the behavioral change is due to compensation that can occur in genetically manipulated animals during development.
In this study, we investigated the role of cholinergic signaling in anxiety- and mood-related behaviors, using pharmacological and molecular genetic techniques to alter AChE levels or activity in adulthood. We tested the hypothesis that ACh dynamics in the hippocampus are involved in anxiety- and depression-like behavior, as well as susceptibility to stress in the social defeat paradigm.
Results
Acute Treatment with Physostigmine Induces a Depression-Like Phenotype in the Tail Suspension Test.
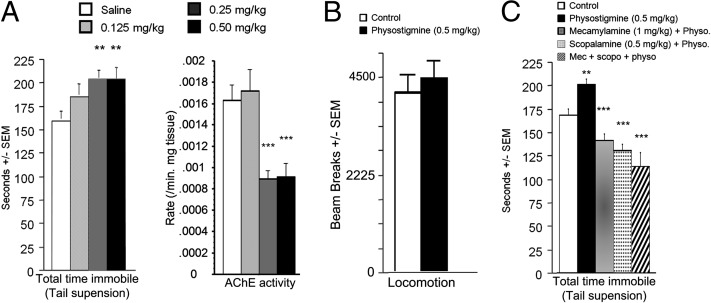
As expected, acute administration of physostigmine significantly decreased activity of AChE across brain regions (P’s < 0.05) (Fig. S1). Acute physostigmine (0–0.5 mg/kg) administration also induced an increase in immobility in the tail suspension test, suggesting that AChE blockade has a prodepressant-like effect (15) [treatment: F(3, 54) = 3.68, P = 0.017] (Fig. 1A, Left). Post hoc analyses demonstrated that physostigmine concentrations above 0.25 mg/kg were effective (0.25 mg/kg: P = 0.005; 0.5 mg/kg: P = 0.005) without affecting locomotor activity (Fig. 1B, F < 1). Physostigmine administration induced a dose-dependent decrease in brain AChE activity (Fig. 1A, Right) [treatment: F(3, 148) = 10.1, P < 0. 0001]. Post hoc analyses revealed that concentrations inducing a significant decrease in AChE activity (0.25 mg/kg, P = 0.0002; 0.5 mg/kg, P = 0.0007) were effective in the tail suspension test.
Fig. 1.
Time spent immobile in the tail suspension test after administration of the cholinesterase antagonist physostigmine 30 min before testing (A, Right). AChE activity in whole brain after administration of physostigmine (A, Left). Locomotor activity over 20 min following physostigmine injection (B). Time spent immobile in the tail suspension test after treatment with physostigmine with, or without, a nicotinic (mecamylamine) and/or muscarinic (scopolamine) antagonist (C). n = 8–12 per group. **P < 0.01; ***P < 0.001. All data are expressed as mean ± SEM.
Acute Effects of Physostigmine Are Reversed by ACh Receptor Antagonists or Treatment with the Antidepressant Fluoxetine.
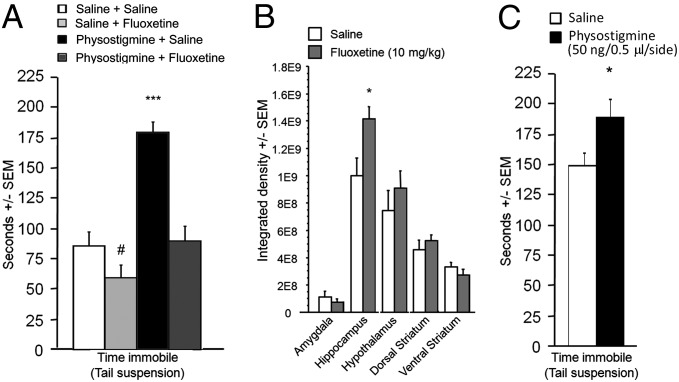
Blockade of AChE results in increased ACh signaling at its targets, both muscarinic and nicotinic acetylcholine receptors (AChRs). Acute administration of scopolamine (a broad muscarinic antagonist; 0.5 mg/kg), mecamylamine (a broad nicotinic antagonist; 1 mg/kg), or a combination of both drugs blocked the effects of physostigmine in the tail suspension test, suggesting that physostigmine’s effects are due to downstream activation of AChRs (F < 1) (Fig. 1C). In addition, administration of the antidepressant fluoxetine (10 mg/kg) also reversed the prodepressant-like effects of physostigmine observed in the tail suspension test (Fig. 2A) (saline + saline vs. physostigmine + fluoxetine: F < 1), suggesting that an antidepressant used in human depressed patients was also effective in reversing physostigmine-induced depression-like behavior.
Fig. 2.
(A) Time spent immobile in the tail suspension test after physostigmine-induced immobility and its reversal by fluoxetine; n = 9–12 per group. (B) AChE activity in microdissected brain regions following chronic treatment with fluoxetine; n = 5 per group. (C) Time spent immobile in the tail suspension test following an acute bilateral infusion of physostigmine into the hippocampus; n = 7–12 per group. #P = 0.07; *P < 0.05; ***P < 0.001. All data are expressed as mean ± SEM.
Chronic Treatment with Fluoxetine Increases Hippocampal AChE Activity.
Because fluoxetine reversed the effects of AChE blockade, we hypothesized that antidepressant administration might also alter AChE activity. We therefore treated animals with fluoxetine for 15 d, tested them in the tail suspension test to verify fluoxetine’s antidepressant-like effect, and measured AChE activity in brain slices. Fluoxetine induced a brain region-dependent increase in AChE activity [treatment × brain region: F(9, 130) = 4.16, P < 0.0001 (Fig. 2B)]. Post hoc analyses revealed that significance was reached only in the hippocampus (P = 0.04). To control for any effects of the behavioral assay on AChE activity, we repeated the evaluation of AChE activity in mice treated chronically with fluoxetine with no behavioral testing. Brain regions were microdissected and AChE activity was measured in homogenates, confirming the increase of hippocampal AChE activity following chronic fluoxetine treatment (P = 0.008) (Fig. S2). In contrast, chronic administration of physostigmine resulted in a paradoxical up-regulation of AChE activity, suggesting that there was compensation at the transcriptional level and that pharmacological antagonism cannot be used to induce a long-term increase in brain acetylcholine levels (Fig. S3).
Local Infusion of Physostigmine into the Hippocampus Induces a Depression-Like Phenotype in the Tail Suspension Test.
To determine whether the change in hippocampal AChE activity could be causally related to behavior in the tail suspension test, we microinfused physostigmine into the hippocampus. Acute AChE antagonism in the hippocampus resulted in a significant increase in immobility in the tail suspension test [F(1, 22) = 4.71, P = 0.04], suggesting that increased ACh signaling in this brain structure is sufficient to induce depression-like behavior (Fig. 2C).
Knockdown of AChE in the Hippocampus Increases Anxiety- and Depression-Like Behaviors and Susceptibility to Social Stress.
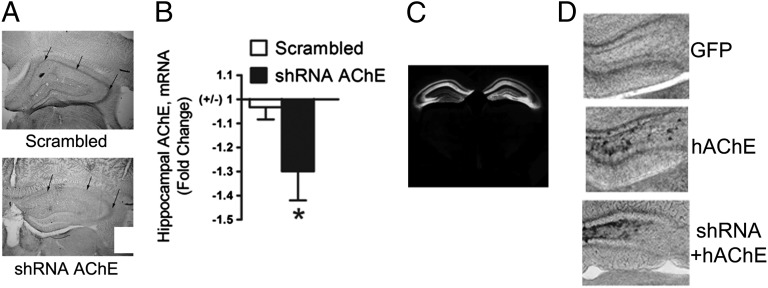
To determine whether a local, long-term change in ACh dynamics in the hippocampus could result in altered anxiety- and depression-like behavior, we generated adeno-associated virus (AAV) carrying shRNAs targeting the regions common to all isoforms of AChE (shAChE). The ability of these vectors to decrease AChE activity was validated in vitro (Fig. S4) and in vivo (Fig. 3 A and B). AAV-shAChEs were infused into the hippocampus, and accuracy of targeting was determined based on expression of GFP (Fig. 3C). Off-target effects of shRNAs are an important concern in in vivo knockdown studies. To verify that the behavioral effects of AAV-shAChE infusion were due to AChE knockdown specifically, we used the human AChE cDNA (hAChE) to design an AChE expression construct that would not be recognized by the AAV-shAChE construct. We infused AAV-hAChE into the hippocampus alone or coinfused with AAV-shAChE and verified that hAChE was expressed even when the shAChE (Fig. 3D) was present, demonstrating that hAChE was resistant to knockdown by shAChE.
Fig. 3.
AAV2-mediated delivery of AChE shRNAs (AAV-shAChE) into the hippocampus of mice. Representative photomicrograph showing hippocampus-specific decrease in AChE activity after hippocampal infusion of AAV-shRNA by stereotaxic surgery (A). Real-time PCR showed knockdown of AChE mRNA levels in shAChE-infected hippocampus compared with scrambled controls; n = 6 per group (B). GFP expression after infusion of AAV in the hippocampus demonstrating the specificity of the localization and the spread of infusion (C). Representative activity-based staining of AChE showing hippocampal AChE overexpression following infusion of the AAV-hAChE construct (D). A control group infused with AAV-GFP shows baseline AChE activity (D, Top), whereas the group infused with the human esterase construct shows increased AChE activity (D, Middle), and the overexpression is not decreased by coinfusion of the AAV-shRNA (D, Bottom). Data are expressed as mean ± SEM. *P < 0.05.
Hippocampal AChE knockdown resulted in a significant decrease in time spent in the open arms in the elevated plus maze [T(18) = 7.19, P < 0.001 (Fig. 4A, Left)] compared with hippocampal infusion of a scrambled control vector (AAV-Scr). Similarly, in the light-dark test, mice that received infusions of the AAV-shAChE into the hippocampus spent less time in the light side of the box [T(20) = 2.75, P < 0.05 (Fig. 4A, Right)] compared with mice that received AAV-Scr infusions. In both tests, no differences in the number of entries and overall activity were detected between groups, suggesting that these changes were due to increased anxiety-like behavior and not to changes in locomotor activity.
Fig. 4.
AChE knockdown in the hippocampus promotes anxiety-like behavior in mice. Infusion of shAChE into the hippocampus (n = 10 per group) decreased both time spent in open arms in the elevated plus maze test (A, Left) and time spent in the light compartment of the light–dark box (A, Right). AChE knockdown in the hippocampus increased depression-like behavior in mice. Infusion of shAChE into the hippocampus increased immobility time in the tail suspension (B, Left) and forced-swim (B, Right) tests, with no effects on locomotor activity (C). AChE knockdown in the hippocampus promotes stress susceptibility in mice. Ten days of social defeat stress decreased social interaction in WT mice (D). Infusion of shAChE into the hippocampus decreased social interaction after submaximal defeat stress compared with mice receiving scrambled shRNA infusion (E). Expression of hAChE in the hippocampus prevents AChE knockdown and rescues the behavioral effect of shAChE infusion in mice. Infusion of AAV-hAChE in the hippocampus prevented prodepressive effects of hippocampal shAChE delivery in the tail suspension and forced-swim tests, as well as in the submaximal social defeat paradigm. Fluoxetine reversed the behavioral effects of hippocampal shRNA-mediated AChE knockdown. Acute administration of fluoxetine (10 mg/kg) reversed the effects of AChE knockdown in the hippocampus in the tail suspension test (F, Left). Chronic administration of fluoxetine (10 mg/kg) reversed the effects of shAChE infusion in the social defeat paradigm (F, Right); n = 8–10 per group. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001.
In both the tail suspension and the forced-swim tests, AAV-shAChE infusion into the hippocampus resulted in increased immobility time [tail suspension: T(20) = 3.19, P < 0.01; forced swim: T(19) = 2.35, P < 0.05 (Fig. 4B)] compared with AAV-Scr controls. No difference was observed in locomotor activity between these groups (Fig. 4C), suggesting that knockdown of hipopocampal AChE increased depression-like behaviors. Conversely, coinfusion of AAV-hAChE prevented the behavioral effects of AAV-shAChE infusion in the tail suspension test and the forced-swim test.
Although these latter tests have been useful as tests of antidepressant efficacy, human depression generally results from emotional stress rather than physical stress. To determine whether ACh dynamics in the hippocampus alters the response to socially induced stressors, we evaluated behavior in the social defeat model. As a first step, we confirmed that mice that experience repeated aggression (social defeat) exhibit social avoidance (16). Mice were subjected to 10 d of social defeat stress, followed 24 h later by the social interaction test. As expected, mice exposed to chronic social defeat stress were significantly more likely to avoid a target mouse during social interaction testing compared with nonstressed controls [T(16) = 3.79, P < 0.01 (Fig. 4D)].
We then tested whether hippocampal AChE knockdown altered stress susceptibility using a submaximal version of social defeat, which does not induce social avoidance in wild-type mice. AAV-shAChE or AAV-Scr vectors were infused into the hippocampus, mice were allowed to recover for 4 wk, and animals were then subjected to three social defeat episodes in 1 d. Twenty-four hours later, social avoidance was measured in the social interaction test. As expected, control animals that were subjected to submaximal defeat stress displayed levels of social interaction that were comparable to nondefeated mice (Fig. 4E). In contrast, mice that received AAV-shAChE infusions into the hippocampus displayed significant social avoidance (P < 0.05) after submaximal defeat stress. Importantly, AAV-shAChE infusion did not influence social behavior in the absence of stress, indicating that hippocampal AChE knockdown increased the susceptibility to social defeat stress rather than altering behavior on its own. ANOVA for interaction scores detected a significant main effect of AChE knockdown [F(1,31) = 4.17, P < 0.05] with no significant main effect of stress [F(1,31) = 0.59, P = 0.45], as well as a significant knockdown X stress interaction [F(1,31) = 5.13, P < 0.05]. Bonferroni post hoc comparison further revealed significant social avoidance in AChE knockdown mice compared with controls that was present only after social defeat stress (P < 0.05), with no significant effect of knockdown in naive nonstressed mice. Coinfusion of AAV-hAChE prevented the behavioral effects of AAV-shAChE infusion in the submaximal social defeat paradigm (Fig. 4E), whereas locomotor activity was unaffected. Although infusion of AAV-hAChE into the hippocampus increased AChE activity, the overexpression construct had no effect on any of the behavioral paradigms tested (Fig. S5).
Behavioral Effects of AChE Knockdown Can Be Reversed with Fluoxetine Treatment.
To determine whether the prodepressant-like effects of hippocampal AChE knockdown were sensitive to antidepressant administration, mice infused with shAChE into the hippocampus were treated with fluoxetine (10 mg/kg) and tested in the tail suspension test or social defeat models. Acute administration of fluoxetine (10 mg/kg) decreased immobility in mice with hippocampal shAChE infusions to a level similar to control animals [treatment: F(2, 26) = 8.17, P = 0.0018) (Fig. 4F)].
Unlike the tail suspension test, social defeat behavior is sensitive to chronic, but not acute, treatment with antidepressants that are effective in human depressed subjects (17, 18). Chronic treatment with fluoxetine (10 mg/kg for 15 d) prevented the effects of hippocampal AAV-shAChE infusion on submaximal social defeat behavior [saline vs. fluoxetine: F(1, 18) = 67.1, P < 0.001) (Fig. 4F)].
Discussion
The ability of ACh signaling to alter mood in human subjects was first discovered several decades ago (5, 6). However, the brain regions involved in cholinergic control of anxiety- and depression-like symptoms have remained unknown, mainly due to the complex nature of mood regulation and the neuromodulatory role of ACh (see ref. 19 for review). The effects of ACh are dependent on the site, pattern, and timescale of release. ACh release can be induced by environmental stressors in many brain areas, including the prefrontal cortex and the hippocampus, two regions known to be involved in depression and mood regulation (20). ACh signaling can coordinate the response of neuronal networks involving these stress-sensitive brain areas, all of which could be relevant for behaviors related to anxiety and depression. AChE is the key enzyme in ACh breakdown and is extremely efficient at modulating extracellular ACh levels (21). AChE is also the target of drugs used as treatments for Alzheimer’s disease and is also targeted by nerve agents, and insecticides. Therefore, it is of great interest to determine whether alterations of AChE activity and cholinergic tone may mediate stress-induced changes leading to anxiety- and depression-related behaviors.
In the current set of experiments, C57BL/6J male mice injected with physostigmine showed increased immobility in the tail suspension test, replicating human studies showing that a pharmacological increase in cholinergic tone can precipitate symptoms of depression (5, 6). Recently, human imaging using a nicotinic tracer that can be displaced by endogenous ACh showed that there is decreased nicotinic receptor availability in depressed subjects, with no change in receptor number as measured postmortem (1), further suggesting that heightened cholinergic tone could be associated with symptoms of depression. In the current study, we determined that depression-like consequences of AChE antagonism were due to signaling through its downstream receptors because both muscarinic and nicotinic AChR antagonists reversed the effects of physostigmine in the tail suspension test. Both clinical and preclinical studies have shown that AChR blockers can have antidepressant-like effects (22, 23), suggesting that reversal of a hypercholinergic state could result in an antidepressant response. Finally, the SSRI fluoxetine also abolished physostigmine-induced increases in immobility in the tail suspension test, validating the interpretation that these effects of physostigmine are depression-like.
Although acute administration of physostigmine is a useful way to test the hypothesis that increased ACh signaling can lead to depression-like symptoms, it has limitations as a mouse model of depression and has limited utility for mechanistic studies of ACh effects on circuits related to depression-like behavior. Whereas acute administration of physostigmine results in increased ACh signaling, chronic administration of the drug results in increased transcription and translation of the AChE gene that can compensate for pharmacological blockade (24). shAChE infusions therefore represent a useful model of chronic increased ACh signaling in the hippocampus. The current set of experiments demonstrates that shAChE infusion results in long-term decreases in AChE activity and in increases in anxiety- and depression-like behavior. Thus, hippocampal AChE knockdown is a useful model of cholinergic dysfunction leading to anxiety- and depression-like behavior.
The pharmacological and molecular genetic studies described here show that increased ACh signaling in the hippocampus is sufficient to recapitulate the consequences of peripheral physostigmine administration and to identify a brain region involved in ACh-mediated regulation of anxiety- and depression-like behaviors. The hippocampus, amygdala, and prefrontal cortex receive a high level of cholinergic input from the basal forebrain complex, and in particular, from the medial septum and nucleus basalis (25). Several studies have shown that stress increases ACh release in a brain region-specific manner (20). Importantly, although increasing cholinergic tone globally or in the hippocampus induces depression-like symptoms, a recent study also showed that decreasing cholinergic tone in the striatum can lead to depression-like symptoms, likely through interneuron-dependent disinhibition of striatal neurons (26). This highlights the fact that cholinergic signaling is not homogenous throughout the brain and that the hippocampus may be a critical node mediating cholinergic effects on stress-related behaviors. In the current study, we found that chronic administration with fluoxetine up-regulated AChE specifically in the hippocampus. Previous studies have demonstrated changes in AChE activity, a switch in expression of particular AChE isoforms, and increased ACh release in the hippocampus following exposure to stress (27, 28). ACh signals through muscarinic and nicotinic receptors to modulate the dynamic properties of the hippocampus, generating a range of stable oscillatory network states (29) that may mediate the stress response.
Despite the clear effects of stress on hippocampal activity, stress-induced activity may also lead to a compensatory increase in hippocampal AChE activity, reducing extracellular acetylcholine levels and suppressing cholinergic neurotransmission (27). Psychological stress (27) and various environmental stimuli such as AChE inhibitors (30) and head injury (31) all increase AChE transcription. These findings clearly demonstrate that stress can alter cholinergic signaling at different levels; however, it was not known whether changes in hippocampal cholinergic transmission could alter the behavioral response to stress (32). In the current study, we showed that facilitating cholinergic signaling during social defeat stress by knockdown of AChE activity in the hippocampus precipitated significant social avoidance in mice. AChE knockdown in the absence of exposure to defeat stress did not change social behavior at baseline. Our results further suggest that maintaining high ACh levels in the hippocampus during stress may intensify the stress response and alter coping skills in adverse situations. Supporting this conclusion, pharmacological inhibition of AChE during elevated stress was recently shown to increase anxiety-like behavior in mice without similar effects in nonstressed controls (33). Similarly, rats with increased cholinergic sensitivity are more susceptible to the immobility-inducing effects of mild stressors (34). Consistent with the results shown here, acute pharmacological AChE inhibition by physostigmine has also been shown to increase immobility in the forced-swim test in rats (35).
Studies of the cholinergic influence on anxiety have yielded more mixed results than studies of depression-like behavior. For example, acute injection of high doses of nicotine into the hippocampus has anxiogenic effects that may be mediated by activation of α7 nicotinic acetylcholine receptors (nAChRs) (36), whereas lower doses of nicotine tend to produce anxiolytic effects (37). Interestingly, the anxiogenic effects of nicotine in the hippocampus were induced only under experimental conditions involving an unfamiliar arena or high light (38), which indicates that nAChR stimulation can produce distinct behavioral outcomes depending on the level of stress generated by the test itself. It follows that, under experimental conditions that generate low levels of anxiety, infusions of either a nicotinic or a muscarinic cholinergic receptor antagonist into the hippocampus can have anxiogenic effects (32, 38). Together with our findings, these studies indicate that the effect of cholinergic tone on anxiety-like behavior may depend on levels of stress.
In summary, the results presented here identify a cholinergic mechanism in the hippocampus that regulates behavioral susceptibility to stress and to anxiety- and depression-like behaviors in mice. In particular, this study shows that whereas other brain nuclei are also essential for regulating mood and anxiety, maintaining hippocampal ACh at homeostatic levels is critical for regulation of emotional behaviors. Furthermore, increasing hippocampal ACh signaling is sufficient to induce behaviors related to anxiety and depression. The current dataset also suggests that the ability of physostigmine to induce symptoms of depression in humans may be due to increases in hippocampal ACh levels. In addition, knockdown of AChE in the hippocampus appears to be an animal model of depression-like behavior with relevant predictive validity for human antidepressant response due to its reversibility by chronic fluoxetine administration and good construct validity based on data suggesting that individuals who are actively depressed may have increased brain ACh levels (1). The current study builds on previous studies of the Flinders sensitive line of rats in which alterations in cholinergic signaling contribute to depression-like phenotypes (34). The experiments presented here also identify the hippocampus as one area critical for the effects of ACh signaling on anxiety and depression, providing a manipulation in adulthood that bypasses potential developmental alterations.
Methods
Animals.
C57BL/6J male mice (10–12 wk of age) were obtained from Jackson Laboratory and housed under standard laboratory conditions (21 ± 2 °C, 12 h light–dark cycle with food and water available ad libitum). Upon arrival, mice were split into groups of five mice and randomly assigned to experimental groups. Mice were allowed to acclimate to the laboratory for 1–2 wk before experiments. Male CD1 mice (20–40 wk of age) obtained from Charles River were used as aggressors in the social defeat paradigm and housed under the same conditions, unless specified otherwise.
Drugs.
Chemicals were obtained from Sigma-Aldrich, and injectable solutions were diluted in phosphate buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4). Thirty minutes before behavioral testing, solutions were injected i.p. unless stated otherwise (local infusions). For experiments that used two drugs (Fig. 2A), mice were injected with saline or physostigmine followed by saline or fluoxetine 15 min later, and tail suspension testing was performed 30 min after the last injection.
Measurement of AChE Activity.
In brain slices, AChE activity was measured as previously described (39, 40) and based on colorimetric reaction (see SI Methods for details). In microdissected brain nuclei, enzymatic activity was determined using a similar method modified for microassays using DACE-100-QuantiChrom Acetylcholinesterase Assay kit following the manufacturer’s recommendations. Each measurement was performed in triplicate.
AChE Knockdown by AAV-shRNAs.
Three shRNAs directed against AChE were constructed using published methods (41) by selecting unique 24 base sequences and tested in vitro in N2A cells (see Fig. S4 for details). To achieve long-term in vivo knockdown, efficacious shRNA-AChEs (shAChE) or a scrambled construct (Scr) were incorporated into AAV-2. AAV-shAChE or AAV-Scr (a scrambled sequence with no known target) was infused bilaterally by stereotaxic surgery into the hippocampus. Mice were left to recover for 21 d to allow for knockdown, and efficacy of AAV infusions was further evaluated both by measuring AChE activity in sections and by quantifying mRNA levels in microdissected tissue (see SI Methods for details). At the end of behavioral experiments, animals were perfused and brain sections were examined with fluorescence microscopy to validate the infusion and infection sites by visualization of GFP.
Behavioral Testing.
The sequence of behavioral tests following AAV infusions started with the elevated plus maze, followed by the light–dark test, tail suspension test, and the forced-swim test. Tests were performed 48–72 h apart to limit any effects of one test on the next.
At least 3 wk after the last behavioral test in the battery, mice were exposed to either chronic or submaximal social defeat episodes, followed by the social interaction test. To validate that submaximal social defeat measures were unaffected by preceding behavioral testing, we also tested mice naive to any prior behavioral testing. Naive mice displayed similar levels of social avoidance after AChE knockdown compared with mice that had been tested in the behavioral battery. For all procedures, mice were habituated to testing rooms for at least 30 min before behavioral measures were taken, and testing took place between 1000 and 1800 hours. All procedures were approved by the Yale University Animal Care and Use Committee and conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Elevated Plus Maze.
The elevated plus maze was made of black plexiglas, had four 30- × 5-cm arms, and was elevated 50 cm above the floor. Two arms were enclosed by 15-cm walls; the other two arms had a 3-mm edge to prevent slipping, and all arms were illuminated equally. A 5- × 5-cm platform at the center was considered a neutral area. One hour before the experiment, animals were placed in the testing room. At the beginning of the test, mice were placed in the center of the maze facing an open arm and were allowed to explore the maze for 5 min. The percentage of time spent in the open arms [100 * (time in open arms/(total time – time in the center)] was used as the primary measure of anxiety-like behavior. Number of entries into each arm was also recorded to detect any unforeseen change in global activity.
Light–Dark Test.
The light–dark test was performed as described previously (23). The apparatus consisted of two opaque Plexiglas compartments of the same size connected by a central opening (18- × 10- × 13-cm dimensions: light compartment illuminated by a 60-W desk lamp through a transparent Plexiglas cover). Mice were placed into the dark compartment facing away from the opening and observed for 5 min after the first cross was made. Number of entries into the dark side and time spent in the dark compartment were measured.
Tail Suspension Test.
The tail suspension test is used routinely for antidepressant screening and to identify depression-like behavior in mice (15). Mice were gently suspended by the tail, and videotapes were scored for time spent immobile over 6 min. Immobility was defined as no movement except for respiration. Subjects were returned to their home cage at the end of the test.
Forced-Swim Test.
Mice were placed in clear glass beakers (18 cm in diameter) filled with 15 cm water (∼25 °C). Videotapes were scored for time spent immobile over a 15-min period as described previously (42). Immobility was defined as the minimal amount of movement made by the mouse to stay afloat. Care was taken not to put the nose of the mouse below the water level when initially placed in the water, and mice that appeared to be in distress were removed immediately. Subjects were returned to their home cage at the end of the test.
Social Defeat Stress.
Suprathreshold defeat paradigm.
Mice were subjected to a social defeat stress protocol adapted from ref. 43 and based on previous work showing that chronic social defeat can induce depression-like endophenotypes (44). During each defeat episode, a C57BL/6J test mouse was placed in the home cage of an unfamiliar, aggressive CD1 mouse for 10 min, during which time the C57BL/6J mouse displayed subordinate posturing. For the chronic social defeat paradigm, defeated mice were housed for 24 h with the aggressive CD1 mouse separated only by a metal grid, and this process of social defeat and cohousing was repeated daily for 10 consecutive days.
Submaximal defeat paradigm.
To test whether manipulations of AChE could potentiate an animal’s susceptibility to psychosocial stress induced by repeated defeat, we also used the submaximal defeat paradigm as reported previously (43). For the submaximal social defeat paradigm, C57BL/6J mice were subjected to three social defeat episodes in 1 d, and each defeat episode was separated by 15 min of rest.
Before defeat episodes, CD1 mice (6–12 mo of age) were screened for aggressive behavior, and only CD1s with attack latency shorter than 1 min were used in defeat episodes (45). Immediately after the 10th defeat of the chronic social defeat paradigm, or the third defeat of the submaximal social defeat paradigm, C57BL/6J mice were singly housed over night, and the next day a social interaction test was performed in a different room between 1000 and 1400 hours, unless stated otherwise.
Social interaction.
At the end of either the 10-d or the submaximal social defeat paradigm, approach–avoidance behavior toward an unfamiliar social target was measured as described previously (16). Experimental mice were placed within a novel white plastic open field (39 × 39 cm) in a dark environment with dimmed red light for two consecutive sessions of 2.5 min. In the first session (no target), the open field contained an empty metal grid cage (10 × 6 cm) at one end of the arena. In the second session (target present), conditions were similar, but the metal grid cage contained an unfamiliar CD1 mouse. In between the two sessions, the experimental mouse was returned to its home cage for 1–2 min. Videotapes were scored for time spent by the experimental mouse in the interaction zone (an 8-cm wide corridor around the metal grid cage) and for time spent in the open-field corners opposite the cage. The interaction score was calculated as 100 * (interaction time, target present)/(interaction time, no target).
Locomotor Activity.
Mice were placed in a clean Plexiglas cage (48 × 22 × 18 cm) for 20 min, and locomotor activity was recorded using the OptiMax system (Columbus Instruments). Subjects were returned to their home cage at the end of the test.
Statistical Analysis.
For comparisons of two groups, we used two-tailed Student t test, and ANOVA (with “drug treatment” or “virus” and/or “stress” as between-subject factors) and the post hoc t test with Bonferroni corrections were used when relevant. For analysis of AChE mRNA levels after shAChE infusions, we used a one-tailed Student t test. Values of P < 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants MH077681 and DA033945.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219731110/-/DCSupplemental.
References
- 1.Saricicek A, et al. Persistent β2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(8):851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: A randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janowsky DS. Serendipity strikes again: Scopolamine as an antidepressant agent in bipolar depressed patients. Curr Psychiatry Rep. 2011;13(6):443–445. doi: 10.1007/s11920-011-0239-6. [DOI] [PubMed] [Google Scholar]
- 4.Mineur YS, Picciotto MR. Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31(12):580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 6.Risch SC, et al. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res. 1981;4(1):89–94. doi: 10.1016/0165-1781(81)90012-3. [DOI] [PubMed] [Google Scholar]
- 7.Dilsaver SC, Peck JA, Overstreet DH. The Flinders Sensitive Line exhibits enhanced thermic responsiveness to nicotine relative to the Sprague-Dawley rat. Pharmacol Biochem Behav. 1992;41(1):23–27. doi: 10.1016/0091-3057(92)90053-i. [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Meerson A, et al. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40(1–2):47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrabovska A, Duysen EG, Sanders JD, Murrin LC, Lockridge O. Delivery of human acetylcholinesterase by adeno-associated virus to the acetylcholinesterase knockout mouse. Chem Biol Interact. 2005;157–158:71–78. doi: 10.1016/j.cbi.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Camp S, et al. Contributions of selective knockout studies to understanding cholinesterase disposition and function. Chem Biol Interact. 2010;187(1–3):72–77. doi: 10.1016/j.cbi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meshorer E, et al. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science. 2002;295(5554):508–512. doi: 10.1126/science.1066752. [DOI] [PubMed] [Google Scholar]
- 13.Sternfeld M, et al. Excess “read-through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc Natl Acad Sci USA. 2000;97(15):8647–8652. doi: 10.1073/pnas.140004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas R, et al. Nicotine relieves anxiogenic-like behavior in mice that overexpress the read-through variant of acetylcholinesterase but not in wild-type mice. Mol Pharmacol. 2008;74(6):1641–1648. doi: 10.1124/mol.108.048454. [DOI] [PubMed] [Google Scholar]
- 15.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 17.Vialou V, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13(6):745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry. 2010;167(11):1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark GP, Rada PV, Shors TJ. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience. 1996;74(3):767–774. doi: 10.1016/0306-4522(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 21.Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat Rev Neurosci. 2009;10(5):383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: A preliminary study. J Clin Psychopharmacol. 2008;28(3):340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 23.Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52(5):1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufer D, Friedman A, Seidman S, Soreq H. Anticholinesterases induce multigenic transcriptional feedback response suppressing cholinergic neurotransmission. Chem Biol Interact. 1999;119–120:349–360. doi: 10.1016/s0009-2797(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 25.Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann N Y Acad Sci. 1995;757:169–179. doi: 10.1111/j.1749-6632.1995.tb17472.x. [DOI] [PubMed] [Google Scholar]
- 26.Warner-Schmidt JL, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA. 2012;109(28):11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393(6683):373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 28.Gilad GM. The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology. 1987;12(3):167–184. doi: 10.1016/0306-4530(87)90002-3. [DOI] [PubMed] [Google Scholar]
- 29.Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol. 2005;562(Pt 1):81–88. doi: 10.1113/jphysiol.2004.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soreq H, Seidman S. Acetylcholinesterase: New roles for an old actor. Nat Rev Neurosci. 2001;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 31.Shohami E, et al. Antisense prevention of neuronal damages following head injury in mice. J Mol Med (Berl) 2000;78(4):228–236. doi: 10.1007/s001090000104. [DOI] [PubMed] [Google Scholar]
- 32.Smythe JW, Bhatnagar S, Murphy D, Timothy C, Costall B. The effects of intrahippocampal scopolamine infusions on anxiety in rats as measured by the black-white box test. Brain Res Bull. 1998;45(1):89–93. doi: 10.1016/s0361-9230(97)00311-0. [DOI] [PubMed] [Google Scholar]
- 33.Martinowich K, et al. Roles of p75(NTR), long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biol Psychiatry. 2012;71(1):75–83. doi: 10.1016/j.biopsych.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overstreet DH. The Flinders sensitive line rats: A genetic animal model of depression. Neurosci Biobehav Rev. 1993;17(1):51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 35.Hasey G, Hanin I. The cholinergic-adrenergic hypothesis of depression reexamined using clonidine, metoprolol, and physostigmine in an animal model. Biol Psychiatry. 1991;29(2):127–138. doi: 10.1016/0006-3223(91)90041-j. [DOI] [PubMed] [Google Scholar]
- 36.File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66(1):65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 37.Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167(3):251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- 38.File SE, Gonzalez LE, Andrews N. Endogenous acetylcholine in the dorsal hippocampus reduces anxiety through actions on nicotinic and muscarinic1 receptors. Behav Neurosci. 1998;112(2):352–359. doi: 10.1037//0735-7044.112.2.352. [DOI] [PubMed] [Google Scholar]
- 39.Andrä J, Lojda Z. A histochemical method for the demonstration of acetylcholinesterase activity using semipermeable membranes. Histochemistry. 1986;84(4–6):575–579. doi: 10.1007/BF00482994. [DOI] [PubMed] [Google Scholar]
- 40.Karnovsky MJ, Roots L. A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 41.Mineur YS, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332(6035):1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mineur YS, et al. α4β2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav Pharmacol. 2011;22(4):291–299. doi: 10.1097/FBP.0b013e328347546d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38(2):315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson MB, et al. A novel role of the WNT-dishevelled-GSK3β signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31(25):9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.