Abstract
Tapasin is an integral component of the peptide-loading complex (PLC) important for efficient peptide loading onto MHC class I molecules. We investigated the function of the tapasin-related protein, TAPBPR. Like tapasin, TAPBPR is widely expressed, IFN-γ–inducible, and binds to MHC class I coupled with β2-microglobulin in the endoplasmic reticulum. In contrast to tapasin, TAPBPR does not bind ERp57 or calreticulin and is not an integral component of the PLC. β2-microglobulin is essential for the association between TAPBPR and MHC class I. However, the association between TAPBPR and MHC class I occurs in the absence of a functional PLC, suggesting peptide is not required. Expression of TAPBPR decreases the rate of MHC class I maturation through the secretory pathway and prolongs the association of MHC class I on the PLC. The TAPBPR:MHC class I complex trafficks through the Golgi apparatus, demonstrating a function of TAPBPR beyond the endoplasmic reticulum/cis-Golgi. The identification of TAPBPR as an additional component of the MHC class I antigen-presentation pathway demonstrates that mechanisms controlling MHC class I expression remain incompletely understood.
By presenting peptides at the cell surface, major histocompatibility complex class I (MHC I) molecules allow immunological monitoring of intracellular events by receptors on T, natural killer, and other cells in the immune system. Correct assembly of MHC I heterotrimers in the endoplasmic reticulum (ER) is required for stable expression of these molecules at the cell surface. The peptide loading complex (PLC), comprised of the transporter associated with antigen processing (TAP), tapasin, calreticulin, ERp57, and MHC I heavy chain (HC)/β2-microglobulin (β2m) heterodimer is central to this process (1, 2). Tapasin (or TAPBP, for TAP binding protein) is an essential component of the MHC I antigen-presentation pathway. Its proposed functions include: bridging between MHC I and the TAP transporter (3–5); increasing the levels of TAP (6, 7); and editing/optimizing peptide binding on MHC I (8–11). Although the products of MHC I alleles vary with regard to their tapasin dependence (9, 12–14), its importance is emphasized by the observations that both tapasin-deficient cell lines and tapasin knockout mice show severe reduction in cell surface MHC I expression (3, 15–17).
A human tapasin-related gene (TAPBPR) has been identified at chromosome position 12p13.3 near a MHC paralogous locus (18). Like tapasin, the encoded TAPBPR protein consists of a signal sequence, three extracellular domains comprising a unique membrane distal domain, an IgSF (immunoglobulin superfamily) V domain and an IgC1 domain, a transmembrane domain, and a cytoplasmic region (19). However, the amino acid sequence of TAPBPR is only approximately 22% identical to tapasin. A related TAPBPR gene is also found in fish and chicken, suggesting that it has a conserved function (20). We set out to examine whether, like tapasin, TAPBPR is involved in the MHC I antigen presentation pathway.
Results
Endogenous TAPBPR Is Widely Expressed and IFN-γ–Inducible.
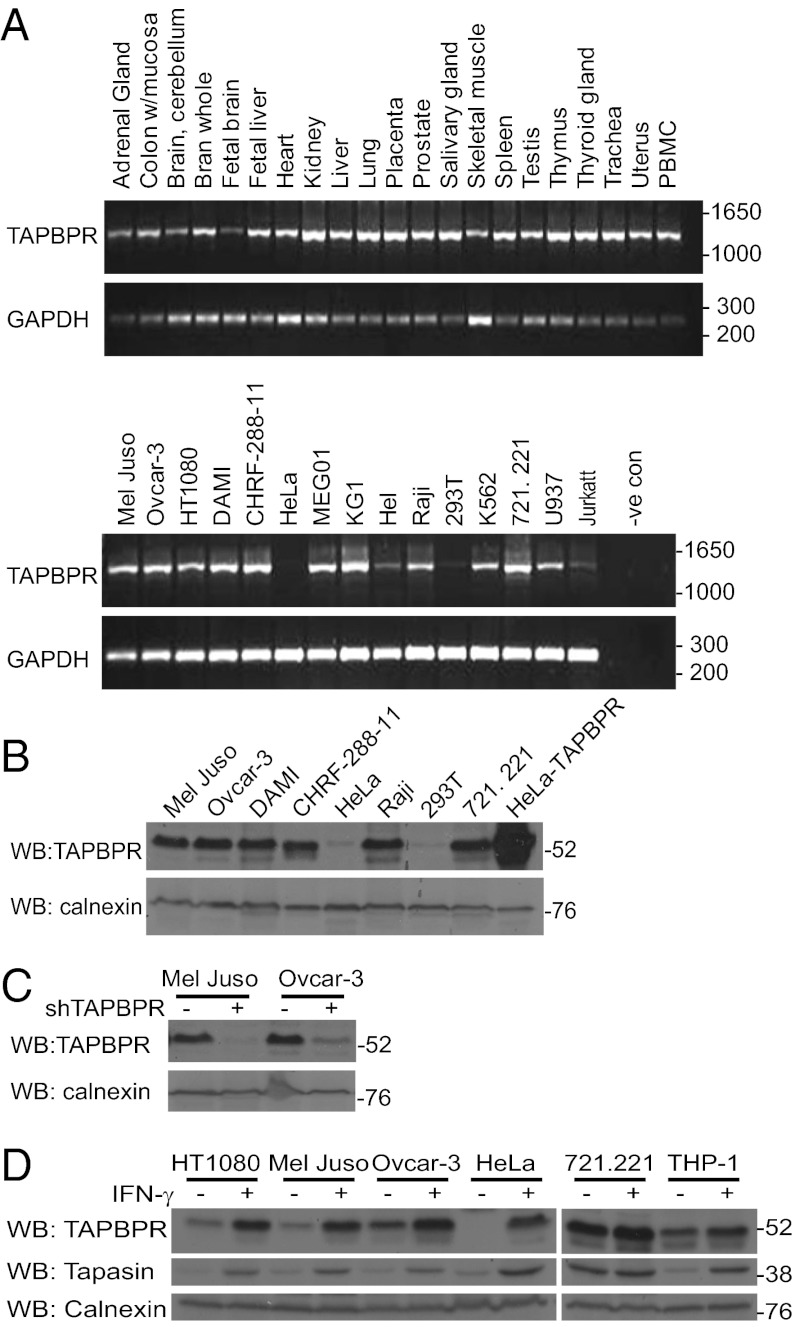
To examine the expression profile of TAPBPR, we screened for endogenous human TAPBPR in human tissue and cell line cDNA panels. RT-PCR analysis showed broad expression of TAPBPR RNA (Fig. 1A) except for low levels seen in HeLa and HEK-293T cell lines. The RT-PCR data were supported by Western blot analysis for endogenous TAPBPR protein, using an antibody raised to the membrane-distal domain of TAPBPR, which detected a band of the predicted size of TAPBPR (∼52 kDa; Fig. 1B). Western blotting on cell lines stably depleted of TAPBPR mRNA using TAPBPR-specific shRNA lentiviral transduction confirmed the ∼52-kDa band as TAPBPR (Fig. 1C). The presence of an IFN-responsive element upstream of the TAPBPR gene in the rainbow trout genome suggested that its expression could be induced by IFN-γ (20). Indeed, IFN-γ treatment increased TAPBPR protein expression in all cell lines tested, with the exception of EBV-transformed B-cell lines, such as 721.221, which had high levels before treatment (Fig. 1D). As noted above, TAPBPR expression was very low in untreated HeLa cells but was induced by IFN-γ to similar levels as other cells (Fig. 1D). Therefore, like tapasin, the expression of endogenous TAPBPR is widespread and IFN-γ–inducible.
Fig. 1.
Endogenous TAPBPR is widely expressed and IFN-γ inducible. (A) RT-PCR screen for endogenous TAPBPR on a human RNA tissue panel and human cell lines. TAPBPR was amplified using primers specific for exon 1 and 7 of TAPBPR (Upper). GAPDH-specific PCR is shown as a positive control (Lower). (B) Cell lysates were blotted for endogenous TAPBPR protein using a TAPBPR mAb. A blot for calnexin is included as a loading control. (C) Depletion of TAPBPR using specific shRNA lentivirus confirms that the ∼52-kDa band detected with the TAPBPR mAb is TAPBPR. (D) Lysates from human cell lines with or without IFN-γ treatment for 24 h were blotted with TAPBPR mAb. Tapasin, detected with RsinN, is included as a positive control for IFN-γ induction and calnexin is included as a loading control.
TAPBPR Binds to a MHC I HC/β2m Heterodimer.
To identify TAPBPR cellular partners, we expressed protein A-tagged TAPBPR (ZZ-TAPBPR) in HeLa cells. Affinity chromatography with IgG-Sepharose isolated TAPBPR, and subsequent MALDI analysis revealed that TAPBPR bound the MHC HC together with β2m (Fig. 2A and Table S1). Immunoprecipitation of endogenous TAPBPR in IFN-γ–treated HeLa cells confirmed the association of endogenous TAPBPR with the MHC I HC and β2m (Fig. 2B). The association between endogenous TAPBPR and MHC I was also confirmed in other cell lines, including EBV-transformed B-cell lines (Fig. S1). To ensure that the detection of MHC I using the polyclonal TAPBPR sera was a direct consequence of TAPBPR expression, and not attributable to cross-reactivity of the antisera with other components of the MHC I pathway, we used shRNA to deplete cells of TAPBPR. The expression of TAPBPR remained suppressed even in the presence of IFN-γ (Fig. 2B). In TAPBPR-immunoprecipitated samples, MHC I HC and β2m bands were significantly reduced when TAPBPR was depleted, revealing the observed interaction between TAPBPR and the MHC I heterodimer was not attributable to antibody cross-reactivity (Fig. 2B). Total cellular levels of MHC I HC and β2m were unaffected by TAPBPR depletion (Fig. 2B). Although depletion of TAPBPR resulted in a slight decrease in the association of tapasin with the MHC I HC, TAPBPR was not critical for the association between tapasin and HC/β2m heterodimer (Fig. 2B). To determine what form of MHC I bound to TAPBPR, reciprocal immunoprecipitations of MHC I, followed by reimmunoprecipitation for TAPBPR, were performed. TAPBPR was predominantly associated with the MHC I HC/β2m heterodimer (Fig. 2C). We were unable to detect an association of TAPBPR and MHC I using W6/32, which recognizes peptide loaded heterotrimeric forms (Fig. 2C). These results suggest that TAPBPR binds to a heterodimer of MHC I in an intermediate/dynamic state of peptide loading similar to the form that tapasin binds. Alternatively, the W6/32 epitope may be masked on the MHC I HC by TAPBPR.
Fig. 2.
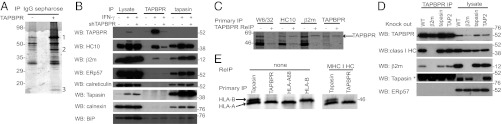
TAPBPR binds to a MHC I HC/β2m heterodimer. (A) Coomassie-stained gel from IgG-Sepharose pull-downs on HeLa (-) and HeLa transiently transfected with ZZ-TAPBPR (+). The proteins in all bands were identified by mass spectrometry. Proteins labeled 1, 2, and 3 were unique to the ZZ-TAPBPR lane and were identified as ZZ-TAPBPR, MHC I HC, and β2m, respectively (see Table S1 for peptide mass fingerprinting and peptide fragmentation data). (B) Total cellular levels of TAPBPR and tapasin were isolated from HeLa-S and HeLa-S stably transduced with TAPBPR-specific shRNA (shTAPBPR) with or without IFN-γ treatment for 66 h, followed by solubilization in 1% digitonin. Western blot analysis on lysates, and on TAPBPR and tapasin immunoprecipitates, was performed as indicated. (C) IFN-γ–treated HeLa-S were metabolically labeled for 4 h before solubilization in 1% digitonin. After immunoprecipitation of MHC I HC (W6/32 and HC10), β2m and TAPBPR (using rabbit anti-TAPBPR) proteins were eluted, denatured, and reimmunoprecipitated with the TAPBPR mAb. (D) TAPBPR was isolated by immunoprecipitation from WT, β2m knockout, tapasin knockout, or TAP2 knockout IFN-γ–treated KBM-7 cells lysed in 1% digitonin. Western blot analysis was performed as indicated. *Secondary antibody cross-reacting with antibody HC. (E) MHC I HCs were isolated from IFN-γ–treated HeLa-S following a 20 min metabolic label by immunoprecipitating tapasin, TAPBPR, HLA-A68, or HLA-B molecules (using 4E mAb). Reimmunoprecipitation using HC10 on tapasin and TAPBPR primary immunoprecipitates confirmed that both bands were MHC I HC. All experiments were independently repeated at least three times.
TAPBPR Does Not Bind to Tapasin, ERp57, Calreticulin, or Calnexin.
Unlike tapasin, no association was detected between TAPBPR and ERp57, calreticulin, or calnexin (Fig. 2B), nor did tapasin and TAPBPR associate (Fig. 2B). No specific association was observed between TAPBPR and BiP (Fig. 2B). In addition, binding of tapasin to components of the MHC I presentation pathway was unaffected by TAPBPR depletion.
β2m Is Essential for the Association Between TAPBPR and MHC I, but Neither Tapasin Nor TAP Is Required.
To evaluate the MHC I components required for TAPBPR association with MHC I, we used KBM-7 cells in which the genes for β2m, tapasin, or TAP2 were disrupted by transposon mutagenesis (21). Immunoprecipitation revealed a critical requirement for β2m in the association of TAPBPR with the HC (Fig. 2D). This was confirmed in IFN-γ–treated HeLa, in which depletion of β2m by shRNA similarly resulted in the loss of association between TAPBPR and the MHC I HC (Fig. S2A). TAPBPR appeared to be less abundant in the absence of β2m, suggesting TAPBPR may be unstable if it fails to associate with MHC I (Fig. 2D). This phenomenon may be cell type-specific or influenced by IFN-γ because TAPBPR expression was stable in Daudi (β2m-deficient cells) and was not enhanced by reconstitution with β2m (Fig. S2B). The interaction between TAPBPR and the MHC I HC occurred in the absence of tapasin or a functional TAP heterodimer, demonstrating that tapasin and TAP are not required (Fig. 2D).
TAPBPR Preferentially Associates with HLA-A in HeLa Cells.
When immunoprecipitating tapasin in IFN-γ–treated HeLa, two MHC I HC bands were detected (Fig. 2E). The upper HC band migrated at the same molecular weight as the HLA-B HC (HLA-B15), whereas the lower HC band migrated at the same position as the HLA-A HC in HeLa, HLA-A68. Only the lower HC band coimmunoprecipitated with TAPBPR, suggesting TAPBPR preferentially associates with the product of the HLA-A locus in IFN-γ–treated HeLa (Fig. 2E). The two bands at ∼45 kDa associated with tapasin and TAPBPR were confirmed as MHC I HC by reimmunoprecipitating with HC10 (Fig. 2E). Although HC10 shows broad reactivity to HLA-B and -C molecules, it reacts with a number of HLA-A molecules that contain a PxxWDR motif at amino acids 57–62 in the α1 domain, including HLA-A68 found in HeLa (22, 23).
Association Between TAPBPR and MHC I Occurs in the ER.
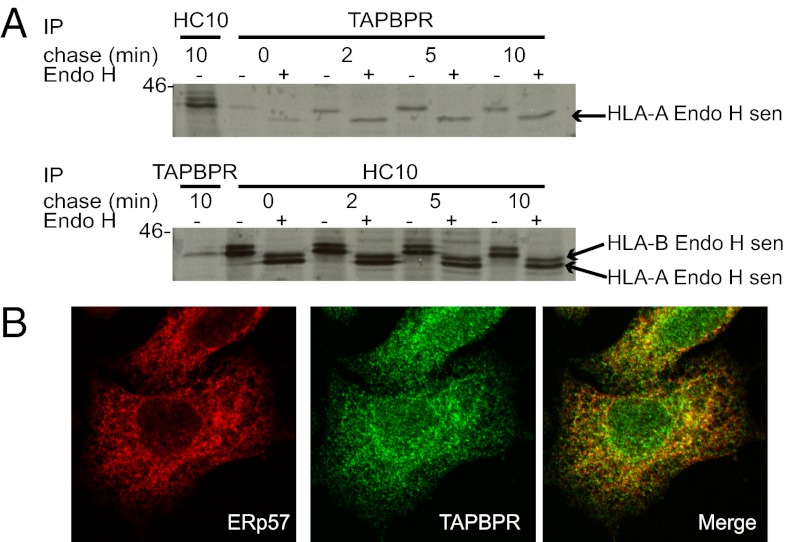
To understand more about the role of TAPBPR in MHC I assembly, we performed pulse-chase analysis and confocal microscopy. The association between endogenous TAPBPR and MHC I HC occurred rapidly following MHC I synthesis. Association was observed 2 min after synthesis of the HC (Fig. 3A), consistent with an interaction in the ER. In support of this, TAPBPR-associated HCs were Endoglycosidase H (Endo H)-sensitive (Fig. 3A). Furthermore, a significant proportion of TAPBPR colocalized with the ER resident protein ERp57 (Fig. 3B).
Fig. 3.
TAPBPR is expressed in the ER. (A) IFN-γ–treated HeLa cells were pulse-labeled for 2 min and chased for the indicated times. MHC I HCs associated with TAPBPR (by immunoprecipitation with rabbit anti-TAPBPR) or the total free HC10 reactive HC population were immunoprecipitated from Triton X-100 lysates. Samples were treated with or without Endo H. (B) Confocal micrograph of endogenous TAPBPR in IFN-γ–treated HeLa. Fixed and permeabilized cells were stained with mouse anti-ERp57 and rabbit anti-TAPBPR. All experiments were independently repeated at least three times.
TAPBPR Slows Anterograde Transport of MHC I.
To determine how TAPBPR affects MHC I export, we compared the folding and transport of MHC I in HeLa in the presence or absence of GFP-TAPBPR. Transport of all forms of MHC I were delayed in the presence of TAPBPR (Fig. 4A). Only ∼50% of the W6/32-reactive MHC I HC were resistant to Endo H digestion after 180 min in GFP-TAPBPR–expressing cells compared with 100% after 45 min in the parental HeLa (WT). Furthermore, less W6/32-reactive and more HC10-reactive MHC I HCs were detected in the presence of TAPBPR. These data show that TAPBPR affects MHC I folding and transport and suggest TAPBPR increases the presence of an open conformation of MHC I. In keeping with the data on endogenous TAPBPR, GFP-TAPBPR immunoprecipitated with HC10- and β2m-specific antibodies but not with the W6/32 mAb (Fig. 4A). To determine whether endogenous TAPBPR also slowed MHC I transport, we examined MHC I export following TAPBPR depletion in IFN-γ–treated HeLa. Depletion of TAPBPR increased the rate of MHC I transport, as measured by the gain of Endo H resistance over a 30-min chase period (Fig. 4B). Similar results were observed using a number of antibodies including W6/32, β2m, and an HLA-A68–specific reagent (Fig. 4B). In keeping with the binding preference of TAPBPR for HLA-A in HeLa, increased MHC I export was only seen for the HLA-A protein product, represented by the lower band, and not the upper HLA-B band (Fig. 4B). At the 20-min chase time point, a difference of ∼20% was observed for Endo H-resistant HLA-A HC immunoprecipitated with W6/32 (Endo H resistance: WT, 33.6%; shTAPBPR, 53.2%) or the HLA-A68–specific mAb (WT, 39.9%; shTAPBPR, 60.1%) following TAPBPR depletion. In agreement with more rapid folding of HLA in the absence of TAPBPR, the HLA-A HC lost HC10 reactivity faster in the absence of TAPBPR (Fig. 4B). Supporting the hypothesis that expression of TAPBPR affected folding and forward transport of HLA molecules, cell surface expression of conformational MHC I was significantly reduced, and increased expression of free MHC I HC was observed in HeLa transduced with TAPBPR (Fig. 4C). However, stable depletion of TAPBPR in IFN-γ–treated cells did not reveal an obvious cell surface phenotype on steady-state MHC I expression (Fig. 4D).
Fig. 4.
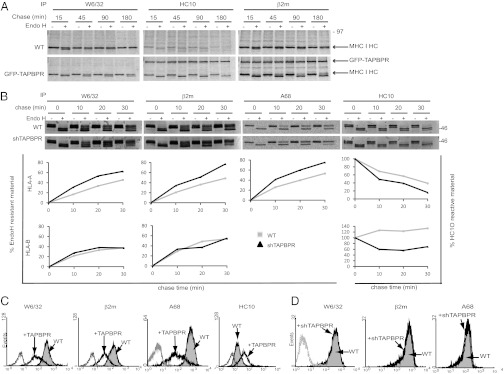
TAPBPR slows forward transport of MHC I. (A and B) HeLa (WT) and HeLa expressing GFP-TAPBPR (A) or IFN-γ–treated HeLa-S (WT) and IFN-γ–treated HeLa-S cells stably depleted of TAPBPR by shRNA transduction (shTAPBPR) (B) were radiolabeled for 10 min and chased for the indicated times. After lysis in 1% Triton X-100, immunoprecipitation was performed with W6/32, anti-β2m, HC10, and anti-HLA-A68, followed by treatment with or without Endo H. Densitometry on individual HLA HC bands was performed to calculate changes in export and folding rate for B. (C and D) Cytofluorometric analysis of MHC I expression (W6/32, β2m, HLA-A68, HC10) on WT HeLa-M (gray filled histogram) and HeLa-M cells stably transduced with an untagged TAPBPR (black line histogram) (C) or IFN-γ–treated HeLa-S (gray filled histogram) and IFN-γ–treated HeLa-S stably depleted of TAPBPR with shTAPBPR (black line histogram) (D). Staining with an isotype control is included (gray line). All experiments were independently repeated at least three times.
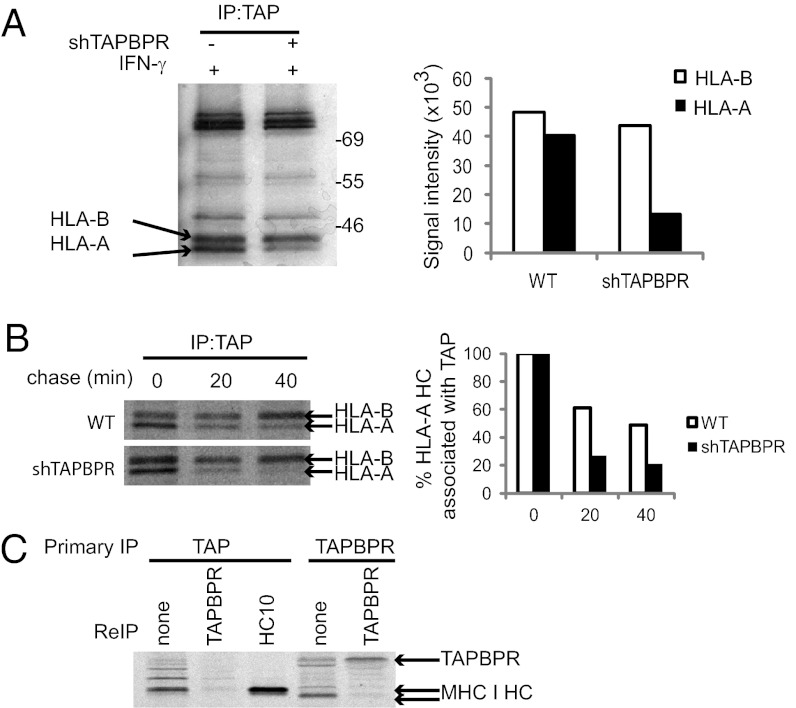
TAPBPR Prolongs Association of MHC I with the PLC.
Because TAPBPR influenced MHC I export, we tested how it affected the composition of the PLC. Isolation of the PLC by TAP1 immunoprecipitation revealed a major effect of TAPBPR. Following depletion of TAPBPR, less HLA-A was associated with the PLC (a decrease of ∼67%), with no difference in the HLA-B association (Fig. 5A). The reduced association of HLA-A could be explained by either a lack of recruitment or a faster off rate from the PLC. In the absence of TAPBPR, recruitment of HLA-A onto the PLC appeared normal, but the HCs failed to remain associated with TAP (Fig. 5B). Therefore, TAPBPR appears to be required for prolonged association of the HLA-A HC with the PLC.
Fig. 5.
TAPBPR prolongs association of MHC I with the PLC but is not an integral component of the PLC. (A and B) IFN-γ–treated HeLa-S and HeLa-S shTAPBPR were metabolically labeled for 2 h with no chase (A) or for 20 min followed by the chase times indicated (B). Samples were lysed in 1% digitonin. The PLC was isolated by immunoprecipitation using 148.3. Densitometry of the HLA bands was performed on phospho-screen images. Both experiments were independently repeated three times. (C) IFN-γ–treated HeLa-S were metabolically labeled for 4 h, followed by lysis in 1% digitonin. Primary immunoprecipitation was performed using Ring4C for the PLC or TAPBPR, followed by elution in 1% SDS. Reimmunoprecipitation was performed for TAPBPR or with HC10 as a positive control. The input for reimmunoprecipitation compared with primary TAP immunoprecipitation lane is 30× and 10× for TAPBPR and HC10, respectively. This experiment was repeated six times using either Ring4C or 148.3 for the primary TAP immunoprecipitation. In no case was endogenous TAPBPR detected on the PLC.
TAPBPR Is Not an Integral Component of the PLC.
Given the strong influence of TAPBPR on the association of MHC I HC with TAP, we wanted to determine whether TAPBPR, like tapasin, was an integral component of the PLC. In immunoprecipitation experiments, we could not observe an association between TAP and endogenous TAPBPR in IFN-γ–treated HeLa, as determined by lysis in digitonin (Fig. 5C). However, after overexpression of GFP-TAPBPR in HeLa we did detect some limited association between TAPBPR and TAP, suggesting that a transient association may occur between TAPBPR and the PLC (Fig. S3).
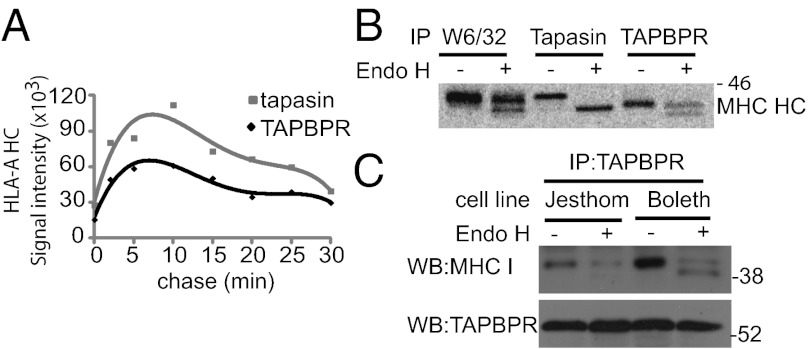
Newly Synthesized MHC I Bind to Tapasin and TAPBPR with Similar Kinetics.
Because TAPBPR is apparently not a component of the PLC, where does it fit in the MHC I antigen presentation pathway? To approach this, we determined whether TAPBPR associated with MHC I before or after the PLC. Newly synthesized MHC I molecules were labeled in IFN-γ–treated HeLa cells by a short pulse (2 min) and followed through the cell during the chase period. In these experiments, HLA-A molecules exhibited similar binding kinetics to TAPBPR or tapasin (Fig. 6A). The peak signal of MHC I binding to tapasin and TAPBPR occurred at ∼10 min for both proteins (Fig. 6A).
Fig. 6.
TAPBPR associates with the HLA-A HC with similar kinetics as tapasin, but TAPBPR transports through the Golgi. (A) Tapasin- and TAPBPR-reactive MHC I HC were isolated from IFN-γ–treated HeLa-S cells labeled for 2 min, followed by the chase time indicated. Densitometry was performed on the band corresponding to the HLA-A HC. (B) W6/32-, tapasin-, and TAPBPR-reactive MHC I HC were immunoprecipitated from IFN-γ–treated HeLa-S cells labeled for 4 h followed by treated with Endo H. (C) Total cellular levels of TAPBPR were isolated by immunoprecipitation from Jesthom and Boleth EBV transformed B cell lines. Samples were treated with Endo H, and MHC I associated with TAPBPR was detected by Western blotting with HC10 and HCA2. Blotting with anti-TAPBPR was included to demonstrate the amount of TAPBPR isolated in the immunoprecipitation.
TAPBPR Transports MHC I Through the Golgi.
In contrast to tapasin, TAPBPR does not contain any obvious ER retention motif in its cytoplasmic tail, giving rise to the possibility of a function of TAPBPR beyond the ER. Human TAPBPR does not contain any N-linked glycosylation sites, making it difficult to examine its transmission through the Golgi directly. We, therefore, examined Endo H sensitivity of the MHC I associated with TAPBPR as a surrogate, to determine whether TAPBPR had moved through the Golgi. Immunoprecipitation of TAPBPR in radiolabeled cells, followed by Endo H digestion, revealed that >50% of the TAPBPR:MHC I complex had trafficked through the medial Golgi (Fig. 6B). This is in contrast to the tapasin:MHC I complex, which clearly remained Endo H-sensitive and, therefore, premedial Golgi (Fig. 6B). The association of TAPBPR with Endo H-resistant forms of MHC I was also observed in other cell types, such as EBV-transformed lymphoblastoid cell lines (Fig. 6C). Where does TAPBPR traffic to after the medial Golgi? We failed to observe significant cell surface expression of TAPBPR on IFN-γ–treated HeLa, suggesting that TAPBPR does not accumulate at the cell surface (Fig. S4A). However, in HeLa transduced with TAPBPR, a significant portion of TAPBPR was expressed at the cell surface (Fig. S4A). This surface expressed TAPBPR was quickly internalized into the endosomal system where it colocalized with Early Endosome Antigen 1 (Fig. S4C) and MHC I (Fig. S4D).
Discussion
The current model of the MHC I antigen presentation pathway involves a number of proteins, namely calnexin, calreticulin, ERp57, tapasin, TAP1 and TAP2, and endoplasmic reticulum aminopeptidase1/2, which, together, assist in the folding and peptide loading of the MHC I heterodimer in the ER. Here, we have identified TAPBPR as an additional component of the pathway.
TAPBPR shares many properties with tapasin. Expression of both proteins is inducible with IFN-γ, as is the case for other components of the MHC I presentation pathway (24–26). Tapasin and TAPBPR both appear to bind to a similar conformation of MHC I: a HC/β2m heterodimer (3). TAPBPR has a half-life of ∼24 h (Fig. S5), similar to the half-life of tapasin and MHC I (27). Therefore, like tapasin, TAPBPR is an MHC I dedicated binding protein. However, TAPBPR is clearly different to tapasin in several ways. First, TAPBPR does not bind ERp57 or calreticulin. Second, TAPBPR is not essential for efficient peptide loading onto MHC I in the ER. In fact, in cells transduced with TAPBPR, export of MHC I was delayed and surface expression of MHC I was reduced, suggesting an opposing function to tapasin (16, 17, 28, 29). Third, TAPBPR is not an integral component of the PLC. In keeping with this, TAPBPR lacks a charged residue in its transmembrane domain, a property of tapasin that is proposed to facilitate its interaction with TAP (15, 30). Fourth, TAPBPR expression is not restricted to the ER.
Where does TAPBPR fit into the MHC I pathway? Given the essential requirement for β2m in the interaction between TAPBPR and MHC I, TAPBPR must bind after β2m has associated with the HC. However, TAP and tapasin are not required for TAPBPR to associate with MHC I. This implies that peptide may not be crucial for the association of TAPBPR with the HC/β2m complex and that TAPBPR may be capable of binding to empty MHC I. Consistent with this, MHC I molecules are more thermolabile and peptide receptive in cells overexpressing TAPBPR, and the association of TAPBPR with the HC can be outcompeted with high-affinity peptides (Fig. S6). The precise position of the initial TAPBPR:MHC I association in the ER in relation to the PLC is unclear. Given the influence of TAPBPR expression on the off rate of MHC I from the PLC, TAPBPR could function after the PLC. However, the ability of TAPBPR to bind to MHC I in the absence of a functional PLC places TAPBPR either before the PLC or suggests that TAPBPR and tapasin compete for MHC I at a similar point in the folding pathway, with TAPBPR possibly representing an alternative folding pathway for MHC I. In support of the latter or a function for TAPBPR after the PLC, TAPBPR does not bind to a number of components of the known MHC I folding pathway in the ER including calnexin, calreticulin, ERp57, tapasin, and TAP. In addition, TAPBPR is not required for MHC I to bind the PLC. Furthermore, tapasin and TAPBPR bind to newly synthesized MHC I HC with similar kinetics.
The function of TAPBPR in the MHC I presentation pathway remains enigmatic. The strong influence of TAPBPR on the PLC is intriguing given that we are unable to demonstrate an association of endogenous TAPBPR with TAP. If TAPBPR does represent an alternative folding pathway for MHC I, this pathway is unlikely to be totally separate from the PLC-mediated pathway given the clear phenotypic effect of TAPBPR depletion on the association of HLA-A with the PLC. Despite this, we were unable to observe a significant effect of endogenous TAPBPR depletion on surface expression of MHC I, suggesting a subtle effect of TAPBPR on MHC I surface expression, possibly involving peptide selection. If tapasin and TAPBPR do compete for MHC I association, in the absence of TAPBPR, MHC I could bind to tapasin instead, which would result in no obvious effect of TAPBPR depletion on surface levels of MHC I.
The mechanisms controlling MHC I expression beyond the ER are enigmatic. Post-PLC, MHC I molecules are exported from the ER via a selective process, involving trafficking motifs in the HC cytoplasmic tails and Bap31, which facilitates recruitment into COP-II coated vesicles (31–36). A bottleneck in the MHC I pathway appears to occur before the medial Golgi with the transport of suboptimally loaded MHC I molecules and PLC components blocked at this step (4, 37–40). Our most intriguing finding is the ability of TAPBPR to stay associated with MHC I beyond the ER/cis-Golgi, a function that clearly sets TAPBPR apart from all other known components of the MHC I presentation pathway. The identification of a “TAPBPR-chaperoned” export pathway for MHC I, together with the apparent preference of TAPBPR for HLA-A, hints that alternative trafficking pathways exist for the products of various MHC I loci. The identification of TAPBPR as an additional antigen presentation pathway component clearly demonstrates that our knowledge of the mechanisms controlling MHC I expression are still incomplete and that the control exerted by MHC I presentation pathway extends beyond the ER.
Materials and Methods
Details of constructs, screening for TAPBPR RNA expression, affinity chromatography using ZZ-TAPBPR, flow cytometry, and immunofluorescence are provided in SI Materials and Methods.
Antibodies.
The following antibodies were used: the pan-MHC I mAb W6/32 (recognizes a conformation epitope on HC dependent on β2m and peptide) (41), HC10 (recognizes HLA-A, -B, and -C containing a PxxWDR motif at amino acids 57–62 in the α1 domain) (22, 23), HCA2 [recognizes HLA containing a xLxTLRGx motif at amino acids 77–84 on α1 domain; a kind gift from Hidde Ploegh (Harvard University, Cambridge, MA)] (42, 43), mAb specific for conformational A2 and A68 (One Lambda), the HLA-B–reactive mAb 4E, rabbit anti-human β2m (Dako), rabbit anti-ERp57 (ab10287; Abcam), mouse anti-ERp57 (ab13506; Abcam), rabbit anti-BiP (ab21685; Abcam), rabbit anti-calnexin (Enzo Life Sciences), rabbit anti-calreticulin (Calbiochem), rabbit anti-GFP (ab290; Abcam), mouse anti-GFP (Roche), RsinN (rabbit anti-sera to the N terminus of tapasin), the tapasin-specific mAb Pasta1, the TAP1-specific mAbs 148.3 and Ring4C [all kind gifts from Peter Cresswell (Yale University School of Medicine)], mAb raised against amino acids 23–122 of full-length human TAPBPR (ab57411; Abcam). IgG1 and IgG2a isotype control antibodies were also used (Dako). Species-specific fluorescent secondary antibodies were from Molecular Probes.
Production of Anti-Human TAPBPR Monoclonal Antibodies and Polyclonal Sera.
cDNA encoding the extracellular portion of human TAPBPR (amino acids 22–406) was cloned into the mammalian expression vector SigpIg Plus consisting of the CD33 signal sequence and a human IgG1 fragment crystallizable region (Fc) tag. HeLa cells stably transfected with TAPBPR-SigpIg plus were maintained in RPMI 1640 with 1% FCS. Secreted TAPBPR-Fc fusion protein was purified from culture supernatant on protein A-Sepharose column (GE Healthcare). After dialysis into PBS, the TAPBPR-Fc fusion protein was used to immunize two rabbits (Covalab). Serum was depleted of Fc-reactive species using human IgG-Sepharose (GE Healthcare). The IgG fraction was purified using protein A-Sepharose. mAbs were raised against recombinant expressed TAPBPR using standard methods as described in Skjoedt et al. (44).
Radiolabeling, Immunoprecipitation, and Gel Electrophoresis.
Starved cells were labeled with [35S]methionine/cysteine Promix (Amersham Pharmacia) at 37 °C for the indicated time. For pulse chase analysis labeled cells were chased for the indicated times at 37 °C in medium containing excess cold methionine/cysteine, then washed in iced-cold PBS. Cells were lysed in either 1% Triton X-100 (Sigma) or 1% digitonin (Calbiochem) in Tris-buffered saline (TBS) (20 mM Tris-HCl, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA) containing 10 mM N-Ethylmaleimide (Sigma), 1 mM PMSF and protease inhibitors (Roche). After centrifugation, supernatants were precleared on IgG- and protein A-Sepharose beads (GE Healthcare). Immunoprecipitations were performed on lysates using the indicated antibody. Beads were washed thoroughly in 0.1% detergent in TBS. For reimmunoprecipitations, proteins were eluted in 1% SDS-TBS (plus 10 mM DTT if reducing conditions required), followed by a 10-fold dilution in 1% Triton X-100 in TBS (plus 20 mM iodoacetamide if DTT was used), and then immunoprecipitated using the indicated antibodies. Samples were heated at 80 °C for 10 min in reducing sample buffer. For Endo H treatment, proteins were digested with 1,000 units of Endo Hf (New England Biolabs) for 1 h at 37 °C. Samples were separated by gel electrophoresis. For metabolically labeled proteins, gels were fixed and dried, and then images were obtained using a phospho-screen (Perkin-Elmer) or on film. PhosphorImager analysis was performed using Typhoon Trio variable mode imager (GE Healthcare) together with ImageQuantTL software. To calculate the percentage of Endo H-resistant material, HC10-reactive material, or TAP-associated HC, the following calculation was used for the individual bands corresponding to the HLA-A and -B HC: signal intensity at specific time point/signal intensity at the 0 time point × 100. For immunoblotting, proteins were transferred onto an Immobolin transfer membrane (Millipore) and blotting with the indicated antibodies.
Supplementary Material
Acknowledgments
This work was funded by a Wellcome Trust Career Development award (Grant 085038 to L.H.B.). J.T. is supported by the Wellcome Trust and the Medical Research Council with partial support by the National Institute for Health Research Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222342110/-/DCSupplemental.
References
- 1.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 2.Van Hateren A, et al. The cell biology of major histocompatibility complex class I assembly: Towards a molecular understanding. Tissue Antigens. 2010;76(4):259–275. doi: 10.1111/j.1399-0039.2010.01550.x. [DOI] [PubMed] [Google Scholar]
- 3.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Sjögren HO, Hellman U, Pettersson RF, Wang P. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proc Natl Acad Sci USA. 1997;94(16):8708–8713. doi: 10.1073/pnas.94.16.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan P, et al. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J Immunol. 2002;168(4):1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- 6.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line. 220. Immunity. 1998;8(2):221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 7.Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur J Immunol. 1999;29(6):1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Purcell AW, et al. Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J Immunol. 2001;166(2):1016–1027. doi: 10.4049/jimmunol.166.2.1016. [DOI] [PubMed] [Google Scholar]
- 9.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16(4):509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 10.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8(8):873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 2007;26(6):1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peh CA, et al. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8(5):531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 13.Park B, Lee S, Kim E, Ahn K. A single polymorphic residue within the peptide-binding cleft of MHC class I molecules determines spectrum of tapasin dependence. J Immunol. 2003;170(2):961–968. doi: 10.4049/jimmunol.170.2.961. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JW, Sewell A, Price D, Elliott T. HLA-A*0201 presents TAP-dependent peptide epitopes to cytotoxic T lymphocytes in the absence of tapasin. Eur J Immunol. 1998;28(10):3214–3220. doi: 10.1002/(SICI)1521-4141(199810)28:10<3214::AID-IMMU3214>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Ortmann B, et al. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277(5330):1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 16.Garbi N, et al. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nat Immunol. 2000;1(3):234–238. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- 17.Grandea AG, 3rd, et al. Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice. Immunity. 2000;13(2):213–222. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 18.Du Pasquier L. The phylogenetic origin of antigen-specific receptors. Curr Top Microbiol Immunol. 2000;248:160–185. [PubMed] [Google Scholar]
- 19.Teng MS, et al. A human TAPBP (TAPASIN)-related gene, TAPBP-R. Eur J Immunol. 2002;32(4):1059–1068. doi: 10.1002/1521-4141(200204)32:4<1059::AID-IMMU1059>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Landis ED, et al. Identification and regulatory analysis of rainbow trout tapasin and tapasin-related genes. Immunogenetics. 2006;58(1):56–69. doi: 10.1007/s00251-005-0070-5. [DOI] [PubMed] [Google Scholar]
- 21.Duncan LM, et al. Fluorescence-based phenotypic selection allows forward genetic screens in haploid human cells. PLoS ONE. 2012;7(6):e39651. doi: 10.1371/journal.pone.0039651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137(7):2299–2306. [PubMed] [Google Scholar]
- 23.Perosa F, et al. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171(4):1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 24.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24(20):3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trowsdale J, et al. Sequences encoded in the class II region of the MHC related to the ‘ABC’ superfamily of transporters. Nature. 1990;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- 26.Saric T, et al. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3(12):1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 27.Bangia N, Cresswell P. Stoichiometric tapasin interactions in the catalysis of major histocompatibility complex class I molecule assembly. Immunology. 2005;114(3):346–353. doi: 10.1111/j.1365-2567.2005.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood R, Shimizu Y, Sekhon GS, DeMars R. Novel allele-specific, post-translational reduction in HLA class I surface expression in a mutant human B cell line. J Immunol. 1994;153(12):5525–5536. [PubMed] [Google Scholar]
- 29.Grandea AG, 3rd, Androlewicz MJ, Athwal RS, Geraghty DE, Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995;270(5233):105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- 30.Petersen JL, et al. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. J Immunol. 2005;174(2):962–969. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- 31.Boyle LH, Gillingham AK, Munro S, Trowsdale J. Selective export of HLA-F by its cytoplasmic tail. J Immunol. 2006;176(11):6464–6472. doi: 10.4049/jimmunol.176.11.6464. [DOI] [PubMed] [Google Scholar]
- 32.Cho S, Ryoo J, Jun Y, Ahn K. Receptor-mediated ER export of human MHC class I molecules is regulated by the C-terminal single amino acid. Traffic. 2011;12(1):42–55. doi: 10.1111/j.1600-0854.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 33.Abe F, Van Prooyen N, Ladasky JJ, Edidin M. Interaction of Bap31 and MHC class I molecules and their traffic out of the endoplasmic reticulum. J Immunol. 2009;182(8):4776–4783. doi: 10.4049/jimmunol.0800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladasky JJ, et al. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol. 2006;177(9):6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquet ME, Cohen-Doyle M, Shore GC, Williams DB. Bap29/31 influences the intracellular traffic of MHC class I molecules. J Immunol. 2004;172(12):7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- 36.Spiliotis ET, Manley H, Osorio M, Zúñiga MC, Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13(6):841–851. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 37.Garstka M, et al. Peptide-receptive major histocompatibility complex class I molecules cycle between endoplasmic reticulum and cis-Golgi in wild-type lymphocytes. J Biol Chem. 2007;282(42):30680–30690. doi: 10.1074/jbc.M701721200. [DOI] [PubMed] [Google Scholar]
- 38.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan S, Xu Y, Brenner MB. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994;263(5145):387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- 40.Paulsson KM, Jevon M, Wang JW, Li S, Wang P. The double lysine motif of tapasin is a retrieval signal for retention of unstable MHC class I molecules in the endoplasmic reticulum. J Immunol. 2006;176(12):7482–7488. doi: 10.4049/jimmunol.176.12.7482. [DOI] [PubMed] [Google Scholar]
- 41.Barnstable CJ, et al. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 42.Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol. 1990;2(2):113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 43.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: Epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35(3):177–188. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 44.Skjoedt MO, et al. Two mannose-binding lectin homologues and an MBL-associated serine protease are expressed in the gut epithelia of the urochordate species Ciona intestinalis. Dev Comp Immunol. 2010;34(1):59–68. doi: 10.1016/j.dci.2009.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.