Abstract
Fibroblast cell lines, designated R- and W cells, were generated, respectively, from mouse embryos homozygous for a targeted disruption of the Igf1r gene, encoding the type 1 insulin-like growth factor receptor, and from their wild-type littermates. W cells grow normally in serum-free medium supplemented with various combinations of purified growth factors, while pre- and postcrisis R- cells cannot grow, as they are arrested before entering the S phase. R- cells are able to grow in 10% serum, albeit more slowly than W cells, and with all phases of the cell cycle being elongated. An activated Ha-ras expressed from a stably transfected plasmid is unable to overcome the inability of R- cells to grow in serum-free medium supplemented with purified clones. Nevertheless, even in the presence of serum, R- cells stably transfected with Ha-ras, alone or in combination with simian virus 40 large T antigen, fail to form colonies in soft agar. Reintroduction into R- cells (or their derivatives) of a plasmid expressing the human insulin-like growth factor I receptor RNA and protein restores their ability to grow with purified growth factors or in soft agar. The signaling pathways participating in cell growth and transformation are discussed on the basis of these results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Ando K., Ajchenbaum-Cymbalista F., Griffin J. D. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993 Oct 8;75(1):73–82. [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Baserga R., Sell C., Porcu P., Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994 Feb;27(2):63–71. doi: 10.1111/j.1365-2184.1994.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Baserga R. The double life of the IGF-1 receptor. Receptor. 1992 Winter;2(4):261–266. [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Alani R., Preis L. H., Szabo E., Birrer M. J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993 Apr;8(4):877–886. [PubMed] [Google Scholar]
- Cai H., Erhardt P., Szeberényi J., Diaz-Meco M. T., Johansen T., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine is stimulated by Ras proteins during mitogenic signal transduction. Mol Cell Biol. 1992 Dec;12(12):5329–5335. doi: 10.1128/mcb.12.12.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptor tyrosine kinase substrates: src homology domains and signal transduction. FASEB J. 1992 Nov;6(14):3283–3289. doi: 10.1096/fasebj.6.14.1385243. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Phillips P. D., Sorger T., Gerhard G. Alterations in the responsiveness of senescent cells to growth factors. J Gerontol. 1989 Nov;44(6):55–62. doi: 10.1093/geronj/44.6.55. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. Inositides and the nucleus and inositides in the nucleus. Cell. 1993 Aug 13;74(3):405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Weinberg R. A. The pathway to signal achievement. Nature. 1993 Oct 28;365(6449):781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- Falco J. P., Taylor W. G., Di Fiore P. P., Weissman B. E., Aaronson S. A. Interactions of growth factors and retroviral oncogenes with mitogenic signal transduction pathways of Balb/MK keratinocytes. Oncogene. 1988 Jun;2(6):573–578. [PubMed] [Google Scholar]
- Fantl W. J., Johnson D. E., Williams L. T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Gai X. X., Rizzo M. G., Lee J., Ullrich A., Baserga R. Abrogation of the requirements for added growth factors in 3T3 cells constitutively expressing the p53 and IGF-1 genes. Oncogene Res. 1988;3(4):377–386. [PubMed] [Google Scholar]
- Geffner M. E., Bailey R. C., Bersch N., Vera J. C., Golde D. W. Insulin-like growth factor-I unresponsiveness in an Efe Pygmy. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1216–1223. doi: 10.1006/bbrc.1993.1755. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Goldring S. R. Cytokines and cell growth control. Crit Rev Eukaryot Gene Expr. 1991;1(4):301–326. [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Faria T. N., Stannard B., Roberts C. T., Jr, LeRoith D. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (alpha IR-3). J Biol Chem. 1993 Feb 5;268(4):2655–2661. [PubMed] [Google Scholar]
- Lammers R., Van Obberghen E., Ballotti R., Schlessinger J., Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990 Oct 5;265(28):16886–16890. [PubMed] [Google Scholar]
- Leof E. B., Lyons R. M., Cunningham M. R., O'Sullivan D. Mid-G1 arrest and epidermal growth factor independence of ras-transfected mouse cells. Cancer Res. 1989 May 1;49(9):2356–2361. [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Lu K. H., Levine R. A., Campisi J. c-ras-Ha gene expression is regulated by insulin or insulinlike growth factor and by epidermal growth factor in murine fibroblasts. Mol Cell Biol. 1989 Aug;9(8):3411–3417. doi: 10.1128/mcb.9.8.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Shibasaki F., Shibata M., Takenawa T. Ash/Grb-2, a SH2/SH3-containing protein, couples to signaling for mitogenesis and cytoskeletal reorganization by EGF and PDGF. EMBO J. 1993 Sep;12(9):3467–3473. doi: 10.1002/j.1460-2075.1993.tb06021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Medema R. H., Bos J. L. The role of p21ras in receptor tyrosine kinase signaling. Crit Rev Oncog. 1993;4(6):615–661. [PubMed] [Google Scholar]
- Medema R. H., Wubbolts R., Bos J. L. Two dominant inhibitory mutants of p21ras interfere with insulin-induced gene expression. Mol Cell Biol. 1991 Dec;11(12):5963–5967. doi: 10.1128/mcb.11.12.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol. 1993 Sep;3(9):296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
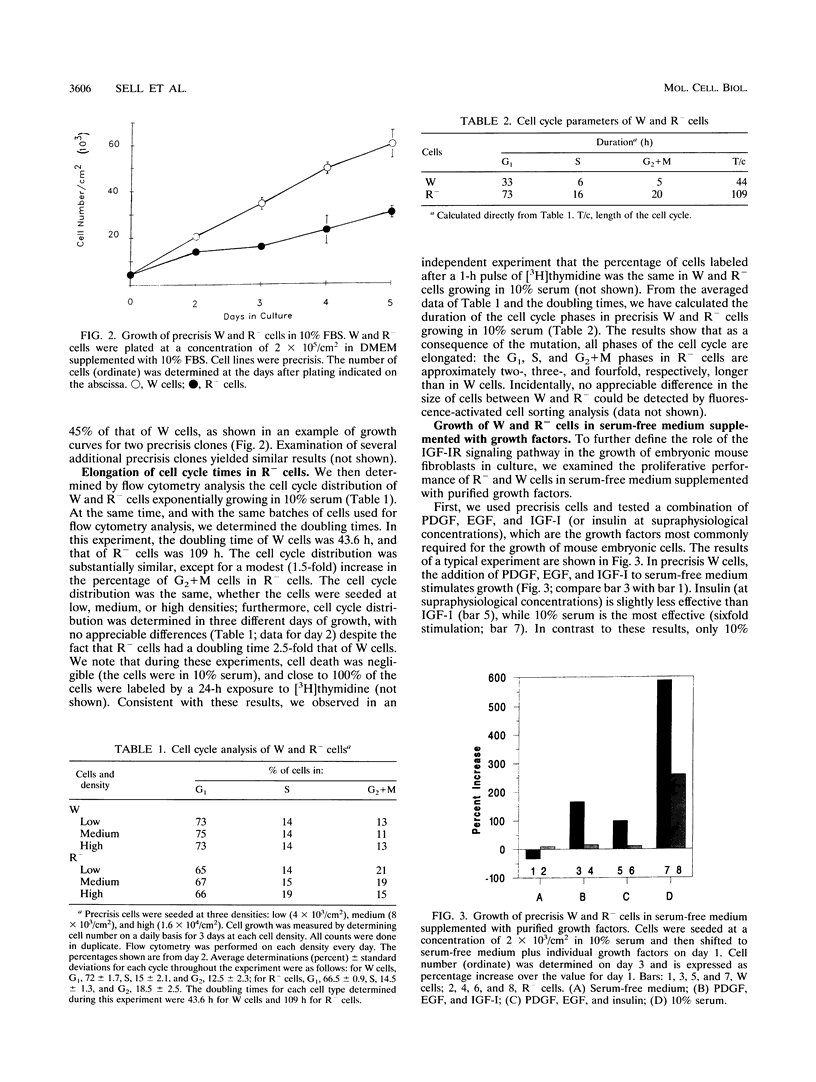
- Panayotou G., Waterfield M. D. The assembly of signalling complexes by receptor tyrosine kinases. Bioessays. 1993 Mar;15(3):171–177. doi: 10.1002/bies.950150305. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Pietrzkowski Z., Mulholland G., Gomella L., Jameson B. A., Wernicke D., Baserga R. Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res. 1993 Mar 1;53(5):1102–1106. [PubMed] [Google Scholar]
- Pietrzkowski Z., Sell C., Lammers R., Ullrich A., Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992 Sep;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Wernicke D., Porcu P., Jameson B. A., Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992 Dec 1;52(23):6447–6451. [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
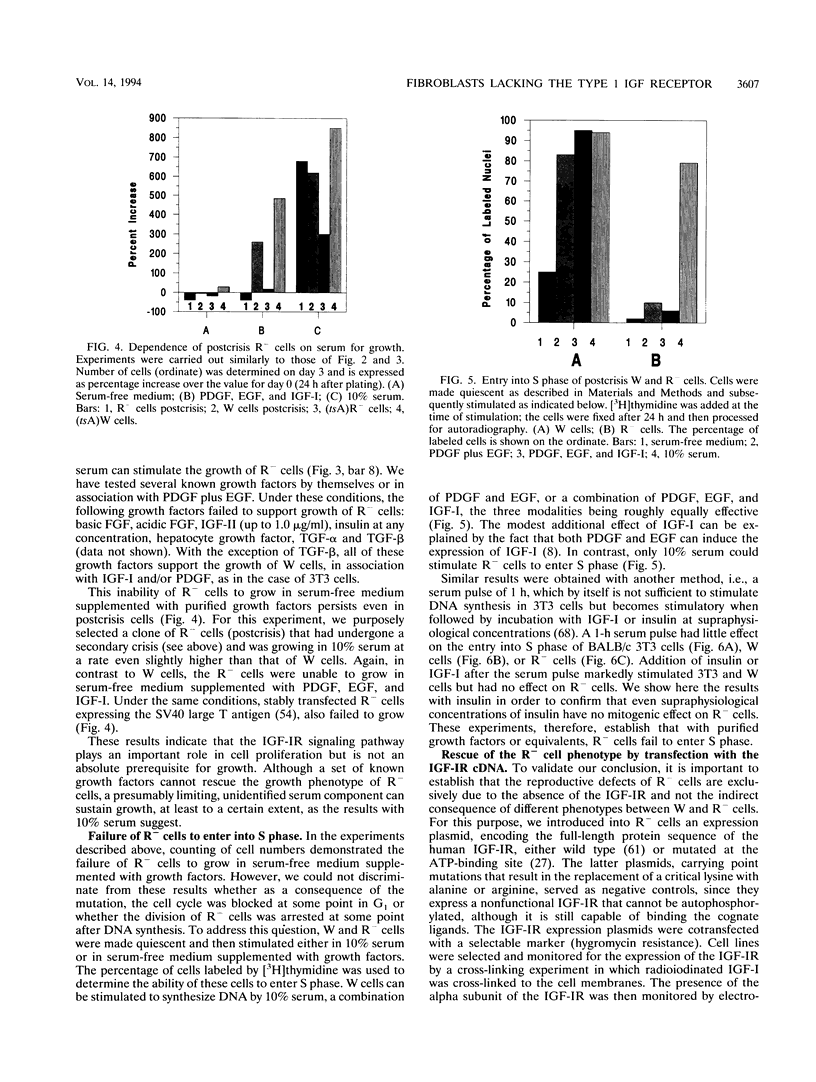
- Reiss K., Porcu P., Sell C., Pietrzkowski Z., Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992 Nov;7(11):2243–2248. [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Van Wyk J. J., Pledger W. J. Inhibition of the mitogenic effects of plasma by a monoclonal antibody to somatomedin C. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2389–2392. doi: 10.1073/pnas.81.8.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990 Jul;70(3):591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sell C., Rubini M., Rubin R., Liu J. P., Efstratiadis A., Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
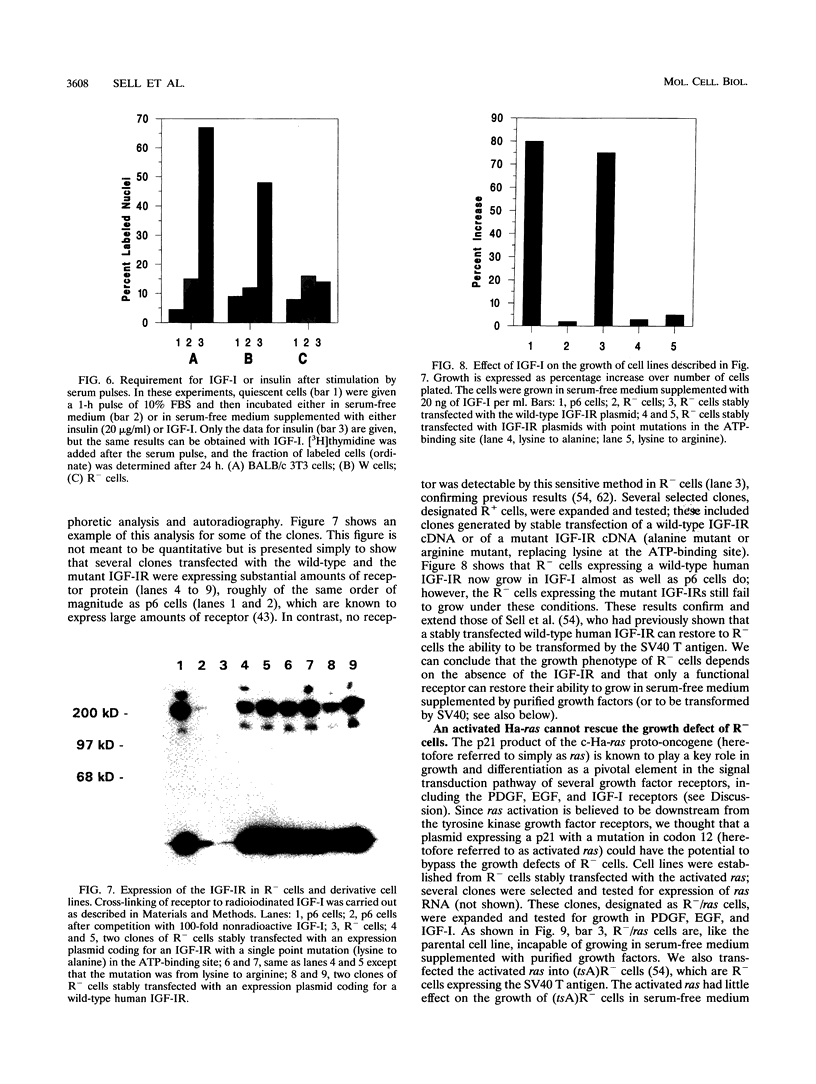
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993 Jun 25;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz E., Nugent P., Pietrzkowski Z., Baserga R. The role of the IGF1 receptor in the regulation of cdc2 mRNA levels in fibroblasts. Exp Cell Res. 1992 Apr;199(2):275–278. doi: 10.1016/0014-4827(92)90435-b. [DOI] [PubMed] [Google Scholar]
- Surmacz E., Reiss K., Sell C., Baserga R. Cyclin D1 messenger RNA is inducible by platelet-derived growth factor in cultured fibroblasts. Cancer Res. 1992 Aug 15;52(16):4522–4525. [PubMed] [Google Scholar]
- Tobe K., Matuoka K., Tamemoto H., Ueki K., Kaburagi Y., Asai S., Noguchi T., Matsuda M., Tanaka S., Hattori S. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993 May 25;268(15):11167–11171. [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara J. A., Portela A., Ortín J., Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. B., Yamauchi K., Pessin J. E. Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. J Biol Chem. 1993 Oct 25;268(30):22231–22234. [PubMed] [Google Scholar]
- Yamada H., Omata-Yamada T., Wakabayashi-Ito N., Carter S. G., Lengyel P. Isolation of recessive (mediator-) revertants from NIH 3T3 cells transformed with a c-H-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1822–1827. doi: 10.1128/mcb.10.4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinouchi M., Baserga R. The role of the IGF-1 receptor in the stimulation of cells by short pulses of growth factors. Cell Prolif. 1993 Mar;26(2):139–146. doi: 10.1111/j.1365-2184.1993.tb00014.x. [DOI] [PubMed] [Google Scholar]