Abstract
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the gastrointestinal tract and arises from the interstitial cells of Cajal. It is characterized by expression of the receptor tyrosine kinase CD117 (KIT). In 70–80% of GIST cases, oncogenic mutations in KIT are present, leading to constitutive activation of the receptor, which drives the proliferation of these tumors. Treatment of GIST with imatinib, a small-molecule tyrosine kinase inhibitor, inhibits KIT-mediated signaling and initially results in disease control in 70–85% of patients with KIT-positive GIST. However, the vast majority of patients eventually develop resistance to imatinib treatment, leading to disease progression and posing a significant challenge in the clinical management of these tumors. Here, we show that an anti-KIT monoclonal antibody (mAb), SR1, is able to slow the growth of three human GIST cell lines in vitro. Importantly, these reductions in cell growth were equivalent between imatinib-resistant and imatinib-sensitive GIST cell lines. Treatment of GIST cell lines with SR1 reduces cell-surface KIT expression, suggesting that mAb-induced KIT down-regulation may be a mechanism by which SR1 inhibits GIST growth. Furthermore, we also show that SR1 treatment enhances phagocytosis of GIST cells by macrophages, indicating that treatment with SR1 may enhance immune cell-mediated tumor clearance. Finally, using two xenotransplantation models of imatinib-sensitive and imatinib-resistant GIST, we demonstrate that SR1 is able to strongly inhibit tumor growth in vivo. These results suggest that treatment with mAbs targeting KIT may represent an alternative, or complementary, approach for treating GIST.
Keywords: cancer, immunotherapy, Gleevec, leiomyosarcoma
Gastrointestinal stromal tumor (GIST) is a neoplasm of mesenchymal cells with an annual incidence of 10–20 cases per million people. GIST frequently recurs locally after initial surgical resection, and advanced disease is characterized by distant metastasis to the liver, lungs, and bone (1). Approximately 20 y ago, the interstitial cells of Cajal (ICCs) were first identified as the cell-of-origin for GIST. Found in the muscle wall of the gastrointestinal tract, ICCs are a population of cells that control peristaltic contractions and are characterized by their expression of the tyrosine kinase receptor KIT (CD117), a receptor for stem cell factor (SCF) (2). SCF binding to KIT leads to receptor homodimerization and intracellular kinase activation, setting off a signaling cascade that positively regulates both the MAPK and the PI3K-AKT pathways (3). The vast majority (95%) of GISTs are positive for KIT protein expression, and 70–80% of GISTs contain activating mutations in KIT, which result in constitutive activation via auto-phosphorylation and SCF-independent signaling and cellular proliferation (4).
Imatinib is a small-molecule tyrosine kinase inhibitor (TKI) that was initially developed to inhibit the BCR-ABL fusion protein that drives the oncogenesis of chronic myelogenous leukemia. It was later discovered that imatinib could also inhibit oncogenic KIT signaling by stabilizing KIT in the inactivated form and preventing its constitutive auto-phosphorylation, leading to the rationale for its clinical use in the treatment of GIST (5–7). Before the use of imatinib, less than 5% of GIST cases responded to conventional chemotherapy, and patients with advanced disease showed a median survival of 18 mo. In contrast, treatment of patients with KIT-mutation–positive GIST with imatinib resulted in disease control in 70–85% of cases and in an increased median survival of 5 y (8). As such, GIST became one of the earliest examples of a solid tumor reacting to targeted therapy. Unfortunately, most patients eventually develop resistance to the drug, owing to mutations in KIT that render GIST cells imatinib-resistant and allow KIT signaling to remain constitutively active (1).
Imatinib resistance poses a significant challenge in the clinical management of GIST. The vast majority of approaches to date have involved the use of alternative small-molecule TKIs targeting KIT or other receptor tyrosine kinases, such as PDGFRA and VEGFR (1). In the present work, we evaluate an alternative, and perhaps complementary, approach that involves targeting KIT with a monoclonal antibody (mAb), SR1 (9, 10). We show that SR1 is able to potently inhibit the growth of GIST cells in vitro and in vivo. Significantly, the SR1-mediated effects on GIST growth were seen in both imatinib-sensitive and imatinib-resistant human GIST cell lines, suggesting that treatment with SR1 may represent a fruitful approach to overcoming the significant clinical challenge of imatinib resistance in GIST.
Results
SR1 Inhibits GIST Cell-Line Growth in Vitro and Reduces KIT Cell-Surface Expression.
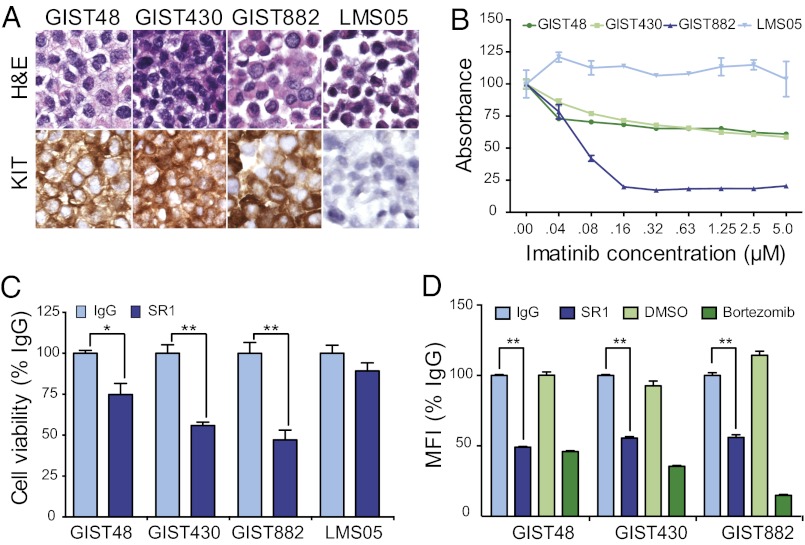
KIT protein expression was determined by immunohistochemistry (IHC) on the human GIST cell lines GIST48, GIST430, and GIST882 and as a negative control on a human leiomyosarcoma (LMS) cell line, LMS05 (Fig. 1A).
Fig. 1.
SR1 treatment slows in vitro GIST cell growth and reduces cell-surface KIT expression. KIT protein expression was analyzed by IHC on paraffin-embedded pellets of GIST48, GIST430, GIST882, and LMS05 cell lines (A). Cell viability assays were carried out to ascertain sensitivity to imatinib treatment for 72 h with GIST and LMS cell lines (B). Viable cell number, as measured by WST-1 absorbance, after 9 d in the presence of 10 μg/mL IgG control or SR1 was evaluated in GIST and LMS cells (C). Cell-surface KIT expression was evaluated in GIST cells by flow cytometry after 12 h of incubation with 10 μg/mL IgG, 10 μg/mL SR1, DMSO (1:1,000), or 100 nM bortezomib (D). All experiments were performed in triplicate. *P < 0.05 and **P < 0.01, as calculated by Student t test.
GIST48 and GIST430 were derived from clinical specimens that had developed resistance to imatinib treatment; GIST48 harbors a primary KIT exon 11 missense mutation and a secondary heterozygous KIT exon 17 missense mutation, and GIST430 harbors a primary heterozygous KIT exon 11 in-frame deletion and a secondary heterozygous KIT exon 13 missense mutation (11). In contrast, GIST882 was derived from a patient before treatment with imatinib and is sensitive to treatment with imatinib in vitro; GIST882 harbors a homozygous missense mutation in KIT exon 13 (6). Consistent with their clinical origins, GIST882 cells treated with increasing doses of imatinib strongly inhibited cell viability, whereas GIST48 and GIST430 cells were significantly less sensitive to treatment in vitro; KIT-negative LMS05 cells showed no response to increasing doses of imatinib (Fig. 1B).
To determine whether KIT-induced cell growth could be affected using an anti-KIT mAb, GIST48, GIST430, GIST882, and LMS05 cells were plated and allowed to adhere overnight before grown in the presence of anti-KIT mAb (clone SR1) or IgG control antibody for 9 d. SR1 was equally effective in slowing the growth rate of both imatinib-resistant cell lines (GIST48 and GIST430) and an imatinib-sensitive cell line (GIST882), whereas the KIT-negative LMS05 cells showed no significant alteration in cell viability as a result of long-term culture with SR1 (Fig. 1C). Together, these results demonstrate that anti-KIT mAb possesses the potential to inhibit KIT-mediated cell growth in GIST cells independently of their resistance or sensitivity to imatinib treatment.
We observed no obvious decreases by Western blot in levels of phosphorylated or total KIT upon treatment with SR1 (Fig. S1) and hypothesized that SR1 treatment of GIST cells could down-regulate levels of KIT expression specifically on the cell surface. To determine levels of KIT cell-surface expression, we used a second anti-KIT mAb (104D2) that binds a KIT epitope different from SR1 (12). We found no decrease in the ability of 104D2 to bind KIT in the presence of SR1 at 4 °C, a condition where receptor internalization or shedding does not occur (Fig. S2). Bortezomib, a small-molecule proteasome inhibitor that has been shown to induce down-regulation, internalization, and degradation of cell-surface KIT, was used as a positive control (13). GIST48, GIST430, GIST882, and LMS05 cells were treated with IgG, SR1, DMSO, or bortezomib for 12 h, and cell-surface KIT expression was analyzed by flow cytometry. We found that, like bortezomib, SR1 was able to induce a significant decrease in cell-surface KIT expression (Fig. 1D).
SR1 Allows for Phagocytosis of GIST Cells by Macrophages.
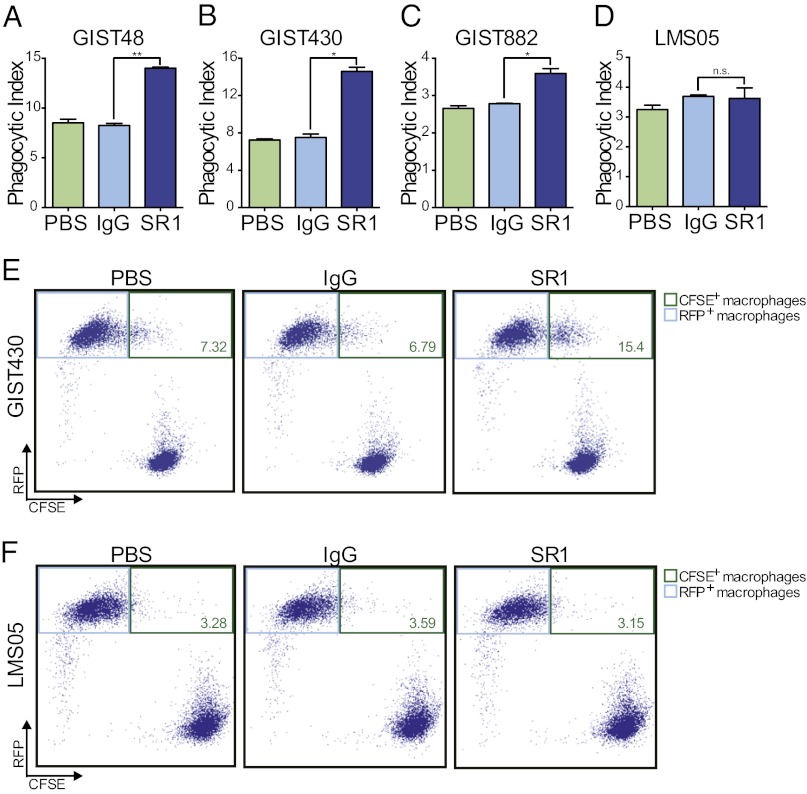
As mAbs can also mediate immune cell-mediated tumor clearance, we next performed coculture assays with macrophages to ascertain whether SR1 treatment could induce phagocytosis of GIST tumors. GIST48, GIST430, GIST882, and LMS05 cells were green fluorescently labeled and then incubated for 2 h with bone marrow-derived macrophages from red fluorescent protein (RFP)-positive mice in the presence of PBS, control IgG, or SR1. The cells were then analyzed by flow cytometry to determine the level of tumor cell phagocytosis by the macrophages. We found that in all three GIST cell lines, SR1 treatment led to a statistically significant increase in macrophage phagocytosis (Fig. 2 A–C and E and Fig. S3). In contrast, KIT-negative LMS05 cells showed no difference in their potential to be phagocytosed regardless of PBS, IgG, or SR1 treatment (Fig. 2 D and F and Fig. S3).
Fig. 2.
SR1 treatment enables GIST cell phagocytosis by macrophages. Macrophage phagocytosis of GIST and LMS cells was evaluated in the presence of PBS, 10 μg/mL IgG, or 10 μg/mL SR1 by flow cytometry. Macrophages that had successfully phagocytosed tumor cells were defined as RFP- and CFSE-double–positive cells (phagocytic index). SR1 treatment led to a significant increase in macrophage phagocytosis of GIST48 (A), GIST430 (B), and GIST882 (C) cells compared with IgG and PBS controls. SR1 treatment had no effect on macrophage phagocytosis of LMS05 cells (D). Representative dot plots are shown for GIST430 (E) and LMS05 (F). Upper right gates (green outline) were drawn to identify double-positive populations. The percentages of double-positive cells are labeled in green. All experiments were performed in triplicate. *P < 0.05, **P < 0.01, and n.s.P > 0.05, as calculated by Student t test.
SR1 Inhibits Growth of Both Imatinib-Sensitive and Imatinib-Resistant GIST Xenografts in Mice.
We next evaluated whether anti-KIT mAbs could inhibit the growth of xenotransplanted GIST tumors in mice. Imatinib-resistant GIST48 and GIST430 cells, and imatinib-sensitive GIST882 cells, were first transduced in vitro with a lentivirus designed to express GFP and luciferase, enabling the use of bioluminescent imaging to monitor tumor engraftment and growth in vivo. For all cell lines, 100,000 cells were injected into the peritoneal cavity of 4- to 8-wk-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) immunodeficient mice, which lack functional T-cells and B-cells but retain macrophages capable of phagocytosis (14). Two weeks after transplantation, engraftment was evaluated using bioluminescent imaging; GIST48 tumors failed to engraft, but tumor cell engraftment was confirmed in GIST430 and GIST882 mice. The animals were then randomized according to baseline tumor luminescence and treatment commenced (full treatment protocols are available in Table S1).
In imatinib-resistant GIST430 xenografts, treatment with SR1 potently inhibited tumor growth, as evidenced by an ∼10-fold decrease in average bioluminescent signal compared with the IgG-treated controls after 8 wk of treatment (Fig. 3 A and B and Table S2). Mice were killed after 8 wk of treatment and autopsies were performed under fluorescent light to aid in identification of tumor masses (Fig. 3C). All mice treated with control IgG showed gross tumor masses whereas only one SR1-treated animal showed a gross tumor. The identity of the tumor masses found in control- and SR1-treated mice was confirmed by histologic examination and IHC. GIST430 xenografts showed a proliferation of a monotonous spindle-cell neoplasm in various locations in the peritoneal cavity. No preference of these tumor masses was found for a specific location although one lesion grew in the wall of the intestine, reminiscent of the site of origin for GIST in human disease. Most tumor masses were large and contained no residual indication of lymph node architecture. The identity of the tumor cells was confirmed by IHC for KIT and DOG1, a marker for GIST independent of the KIT pathway (15, 16). All tumor masses showed consistent expression of both KIT and DOG1 (Fig. 3D). The single tumor that was grossly identified in an SR1-treated GIST430-bearing mouse expressed KIT at a level that did not appear different from the control treated group, but the immunoperoxidase technology is likely not sensitive enough to determine the decrease in surface expression of KIT seen on flow cytometry studies, especially because intracellular KIT may still react by immunodetection in formalin-fixed, paraffin-embedded (FFPE) sections.
Fig. 3.
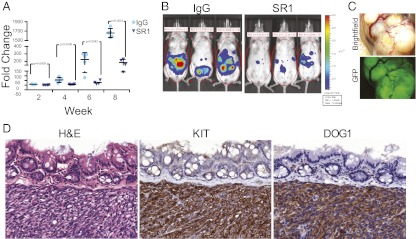
SR1 treatment inhibits tumor growth in a xenotransplantation model of imatinib-resistant GIST. Eight weeks of treatment with SR1 significantly decreased GIST430 xenograft growth compared with IgG control treatment (A). Representative images of SR1 and IgG control-treated mice are shown (B). Fluorescent microscopy was used to evaluate the presence of GFP-positive growths after animal sacrifice (C). The identity of GIST430 xenotransplanted tumors was confirmed by H&E staining and by IHC for GIST markers KIT and DOG1 (D).
In contrast to the mice injected with GIST430, the mice that carried GIST882 xenografts showed significantly smaller lesions. Despite this inability of GIST882 cells to generate large tumor masses, we saw a strong inhibition of in vivo tumor growth in mice treated with SR1 compared with those treated with control IgG, with the SR1-treated mice showing a fivefold decrease in average bioluminescent signal compared with IgG-treated controls after 6 wk of treatment (Fig. 4 A and B and Table S3). GIST882-bearing animals were euthanized, and no grossly apparent tumor masses were evident. However, under fluorescent light, small tumor foci were seen in many areas in control mice (Fig. 4C, Left) whereas only rare and smaller foci were seen in mice treated with SR1 (Fig. 4C, Right).
Fig. 4.
SR1 treatment inhibits tumor growth in a xenotransplantation model of imatinib-sensitive GIST. Six weeks of treatment with SR1 significantly decreased GIST882 xenograft growth compared with IgG control treatment (A). Representative images of SR1 and IgG control-treated mice are shown (B). Fluorescent microscopy was used to evaluate the presence of GFP-positive growths after animal sacrifice. White arrows denote tumor nodules (C).
Discussion
The development of imatinib, and the discovery of its efficacy for inhibiting oncogenic KIT mutations in GIST, represented a significant milestone in the progress of targeted anticancer therapeutics. Unfortunately, secondary KIT mutations arise in GISTs during imatinib treatment, leading to resistance to imatinib and subsequent recurrence of tumors. Most approaches to date have focused on second-generation small-molecule TKIs targeting other active sites in KIT or other proteins implicated in GIST tumor growth, such as PDGFRA, VEGFR, and HSP90 (1). One such molecule is sunitinib, a TKI approved for second-line treatment of GIST that shows only a modest and short-lived effect on disease progression in a subset of patients and that carries with it a risk of adverse events that sometimes necessitate dose reduction or cessation (17). The limited efficacy of sunitinib and other TKIs for imatinib-resistant GIST highlights the need for alternative approaches to treat these tumors.
In parallel with efforts focusing on improving small-molecule drug candidates for oncology indications, the development of mAbs as therapeutic molecules for the treatment of cancer has offered a large number of treatment options across a broad range of tumor types. mAbs are capable of exerting multiple, and often simultaneous, effects on cancer cells via their interaction with their targets. When successful, these mAb-induced interactions can ultimately combine to produce antitumor effects in vitro and in vivo through various modes of action, including inhibition of the target’s ability to activate downstream signaling targets, internalization and degradation of a cell-surface target, phagocytosis, and/or antibody-dependent cell-mediated cytotoxicity (ADCC), a process through which target cells are lysed by cytotoxic granules released by natural killer (NK) cells, granulocytes, and other leukocytes (18).
In the present work, we show that treatment of GIST cell lines with the anti-KIT mAb SR1 resulted in a significant decrease in cell growth and in cell-surface KIT expression, suggesting that the SR1-mediated growth inhibition in GIST cells may be occurring due to internalization and degradation of KIT. We also show that SR1 treatment increased phagocytosis of GIST cells by macrophages. Further study is needed, however, to fully elucidate additional consequences of SR1 treatment on GIST cells. The impact of SR1 treatment on GIST cell interactions with additional immune effector cells, such as T-cells, B-cells, and NK cells, which are not present in the mice used for our xenotransplantation studies, needs to be investigated. In the future, SR1, or other KIT-specific mAbs, could be modified to enhance affinity to KIT and/or to potentiate one or more mAb-mediated antitumor cell functions, such as receptor internalization, receptor homodimerization inhibition, macrophage phagocytosis, or ADCC. Also, although this report focused on treatment of GIST cells, SR1 or other anti-KIT mAb treatment may prove to be useful in other KIT-positive tumors, such as pancreatic adenocarcinoma, testicular seminoma, melanoma, neuroblastoma, and breast cancer (19–23).
In conclusion, we show that treatment of human GIST cell lines with the anti-KIT mAb SR1 can inhibit tumor growth in vitro and in vivo and that decreasing cell-surface KIT expression and immune cell-mediated tumor clearance are two mechanisms that likely contribute to SR1’s efficacy. Importantly, SR1-mediated inhibition in tumor growth was independent of imatinib sensitivity or resistance in human GIST cell lines, suggesting that anti-KIT mAb therapy may effectively address the significant clinical problem of imatinib resistance in GIST and thereby form the rationale for evaluating the clinical efficacy of mAb therapy in GIST patients.
Materials and Methods
Cell Culture.
GIST cell lines were developed in the Fletcher laboratory at Brigham and Women's Hospital, Boston, using a protocol approved by the Brigham and Women's Hospital institutional research board (6, 11). Their derivations and culture conditions have been described previously (6, 11, 24). SR-1 (mouse IgG2A) binds to human c-kit and was purified by Protein G from supernatant obtained from hybridoma cells cultured under standard conditions.
Cell Viability Assays.
For imatinib sensitivity assays, cells were seeded at a density of 4,000 cells per well in 96-well tissue culture plates and allowed to adhere overnight. Imatinib mesylate (Santa Cruz Biotechnology) was dissolved in DMSO to a stock concentration of 10 mM, and cells were then treated with a range of concentrations from 0 to 5 μM; total DMSO was held constant. After 72 h, cell numbers were assessed using WST-1 reagent (Roche); viable cell numbers are directly related to WST-1 absorbance. For SR1 sensitivity assays, cells were seeded at a density of 4,000 cells per well in 96-well plates and allowed to adhere overnight. Cells were then cultured in the presence of SR1 or mouse IgG control antibody at a concentration of 10 μg/mL. The antibody-containing media was refreshed every 3 d, and cell viability was assessed using WST-1 reagent (Roche) after 9 d of antibody treatment. Purified SR1 mAb was provided by the laboratory of Judith Shizuru (Stanford University School of Medicine). Control mouse IgG antibody was purchased from Innovative Research.
Cell-Surface Protein Expression Assays.
A total of 500,000 tumor cells were plated in each well of a six-well tissue culture plate (BD Biosciences), allowed to adhere overnight, and treated in triplicate with 10 μg/mL SR1, 10 μg/mL IgG control, DMSO (1:1,000), or 100 nM bortezomib (Selleck Chemicals) for 12 h. The cells were then dissociated using TrypLE (Life Technologies), washed with PBS, and stained with anti-KIT mAb (clone 104D2) directly conjugated to phycoerythrin (STEMCELL Technologies), which can bind to KIT in the presence of SR1 (Fig. S2). The cells were then washed twice, stained with DAPI, and analyzed on an LSRFortessa cell analyzer (BD Biosciences).
Western Blotting.
Protein lysates were prepared from GIST430 and GIST882 cell monolayers using RIPA buffer (Thermo Scientific) supplemented with protease and phosphatase inhibitors (Roche) and PMSF (Sigma-Aldrich). Protein concentrations were determined with the Bio-Rad Protein Assay (Bio-Rad Laboratories). Electrophoresis and immunoblotting for KIT, phospho-KIT Y703, phospho-KIT Y719, and β-actin were carried out as previously described (25). Protein expression and phosphorylation changes were visualized by chemiluminescence, captured using a GelDoc system (Bio-Rad), and processed using GIMP and Inkscape software.
Macrophage Phagocytosis Assays.
C57BL/Ka Rosa26-mRFP1 transgenic mice were used for red-fluorescent macrophage derivation (26), carried out as described previously (27). Tumor cells were green fluorescently labeled with 1 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) for 10 m at 37 °C, washed twice with HBSS (Invitrogen), and incubated for 30 m in Iscove’s modified Dulbecco’s media (IMDM) (Invitrogen) with mouse IgG or SR-1. A total of 50,000 macrophages were then incubated with 100,000 tumor cells for 2 h in Ultra-low Cluster 96-well plates (BD Biosciences), washed twice, stained with DAPI, and analyzed on an LSRFortessa cell analyzer (BD Biosciences). The phagocytic index was defined as the percentage of macrophages that had successfully phagocytosed tumor cells (i.e., RFP-positive and CFSE-high cells) (Fig. 2 E and F and Fig. S3), and differences in phagocytosis between treatment groups were evaluated using Student t tests.
Xenotransplantation Studies.
All animal procedures were approved by the Administrative Panel on Laboratory Animal Care at Stanford University. Lentiviral production of a pCDH-CMV-EF1-puro construct (Systems Bioscience) containing a ubiquitin promoter driving the expression of a fusion protein containing the Luc2 (pgl4) luciferase gene (Promega) and the eGFP gene (Becton Dickinson) was carried out using standard protocols. NSG mice were used for xenotransplantation studies (14). GIST48, GIST430, and GIST882 cells were transduced with lentivirus, and 100,000 GFP+ cells were injected intraperitoneally into 4- to 8-wk-old NSG mice as described previously (28). Bioluminescent activity was visualized in vivo after D-luciferin injection (Biosynth) on an IVIS Spectrum (Caliper Life Sciences) instrument and quantified using Image 4.0 software, as described previously (28). Total flux (photons/second) values were obtained from mice. Mice were matched based on total flux 2 wk after cell engraftment and subsequently treated weekly via i.p. injections with SR1 (500 μg) or mouse IgG. Mice were imaged every 2 wk, and differences in tumor growth were assessed using Student t test. At autopsy, tumor masses were visualized using a fluorescent dissecting microscope (Leica). Each symbol in Figs. 3A and 4A (GIST430 and GIST882, respectively) represent an individual mouse. All treatment cohorts consist of five mice, with the exception of the SR1 cohort in the GIST430 treatment (n = 4) due to the death of one animal while performing bioluminescent imaging before initiation of treatment.
Histology and Immunohistochemistry.
KIT and DOG1 protein expression was evaluated on FFPE cell pellets or on full cross-sections of FFPE xenograft tumors, which were dissected after animal sacrifice, as described previously (24). Samples were then stained with primary antibodies against KIT (Dako; 1:200) and DOG1 (Leica; clone K9, 1:100) on a Benchmark autostainer (Ventana Medical Systems). The IHC reactions were visualized using mouse versions of the EnVision + system (Dako) with diaminobenzidine.
Supplementary Material
Acknowledgments
We thank A. Logan, A. M. Ring, G. Krampitz, J. Oak, R. Li, X. Guo, S. K. Gupta, E. Gilbert, and members of the Stanford Immunodiagnosis Laboratory for technical assistance and helpful discussions. Grant support came from National Institutes of Health Grants CA 112270 and CA 139490, the National Cancer Institute (F30 CA168059 to K.W.), Deutsche Forschungsgemeinschaft (Grant VO 1976/1 to A.K.V.), the Life Raft Group, the GIST Cancer Research Fund, the Lacob Program of Excellence in Gynecologic–Ovarian Cancer Research and Treatment, and the Ludwig Institute for Cancer Research. B.E. is a recipient of the National Science Foundation Graduate Research Fellowship. Dedicated to the memory of our colleague Angela Lee Riepel, who lost her life to GIST.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222893110/-/DCSupplemental.
References
- 1.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 2.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 3.Yuzawa S, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130(2):323–334. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96(3):925–932. [PubMed] [Google Scholar]
- 6.Tuveson DA, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene. 2001;20(36):5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 7.Mol CD, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279(30):31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- 8.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum Pathol. 2002;33(5):466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 9.Broudy VC, et al. Isolation and characterization of a monoclonal antibody that recognizes the human c-kit receptor. Blood. 1992;79(2):338–346. [PubMed] [Google Scholar]
- 10.Ashman LK, Bühring HJ, Aylett GW, Broudy VC, Müller C. Epitope mapping and functional studies with three monoclonal antibodies to the c-kit receptor tyrosine kinase, YB5.B8, 17F11, and SR-1. J Cell Physiol. 1994;158(3):545–554. doi: 10.1002/jcp.1041580321. [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66(18):9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 12.Bühring HJ, et al. Modulation of p145c-kit function in cells of patients with acute myeloblastic leukemia. Cancer Res. 1993;53(18):4424–4431. [PubMed] [Google Scholar]
- 13.Fang HT, et al. Bortezomib interferes with C-KIT processing and transforms the t(8;21)-generated fusion proteins into tumor-suppressing fragments in leukemia cells. Proc Natl Acad Sci USA. 2012;109(7):2521–2526. doi: 10.1073/pnas.1121341109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 15.West RB, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1):107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa I, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32(2):210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, et al. Clinical efficacy and safety of sunitinib after imatinib failure in Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol. 2011;41(1):57–62. doi: 10.1093/jjco/hyq164. [DOI] [PubMed] [Google Scholar]
- 18.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito I, et al. The stem cell factor-c-kit system and mast cells in human pancreatic cancer. Lab Invest. 2002;82(11):1481–1492. doi: 10.1097/01.lab.0000036875.21209.f9. [DOI] [PubMed] [Google Scholar]
- 20.Kemmer K, et al. KIT mutations are common in testicular seminomas. Am J Pathol. 2004;164(1):305–313. doi: 10.1016/S0002-9440(10)63120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodman SE, Davies MA. Targeting KIT in melanoma: A paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010;80(5):568–574. doi: 10.1016/j.bcp.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen PS, Chan JP, Lipkunskaya M, Biedler JL, Seeger RC. The Children’s Cancer Group Expression of stem cell factor and c-kit in human neuroblastoma. Blood. 1994;84(10):3465–3472. [PubMed] [Google Scholar]
- 23.Johansson I, et al. Increased gene copy number of KIT and VEGFR2 at 4q12 in primary breast cancer is related to an aggressive phenotype and impaired prognosis. Genes Chromosomes Cancer. 2012;51(4):375–383. doi: 10.1002/gcc.21922. [DOI] [PubMed] [Google Scholar]
- 24.Edris B, et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol. 2012;227(2):223–233. doi: 10.1002/path.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühlenberg T, et al. Inhibitors of deacetylases suppress oncogenic KIT signaling, acetylate HSP90, and induce apoptosis in gastrointestinal stromal tumors. Cancer Res. 2009;69(17):6941–6950. doi: 10.1158/0008-5472.CAN-08-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11(4):519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Edris B, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. 2012;109(17):6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.