Abstract
Transient receptor potential channels (TRPs) form a large family of ubiquitous non-selective cation channels that function as cellular sensors and in many cases regulate intracellular calcium. Identification of the endogenous ligands that activate these TRP receptors is still under intense investigation with the majority of these channels still remaining “orphans”. That these channels respond to a variety of external stimuli (e.g. plant-derived lipids, changes in temperature, and changes in pH) provides a framework for their abilities as cellular sensors, however, the mechanism of direct activation is still under much debate and research. In the cases where endogenous ligands (predominately lipids) have shown direct activation of a channel, multiple ligands have been shown to activate the same channel suggesting that these receptors are “promiscuous” in nature. Lipidomics of a growing class of endogenous lipids, N-acyl amides, the most famous of which is N-arachidonoyl ethanolamine (the endogenous cannabinoid, Anandamide) is providing a novel set of ligands that have been shown to activate some members of the TRP family and have the potential to deorphanize many more. Here it is argued that activation of TRPV receptors, a subset of the larger family of TRPs, by multiple endogenous lipids that are structurally analogous is a model system to drive our understanding that many TRP receptors are not promiscuous, but are more characteristically “opportunistic” in nature; exploiting the structural similarity and biosynthesis of a narrow range of analogous endogenous lipids. In addition, this manuscript will compare the activation properties of TRPC5 to the activity profile of an “orphan” lipid, N-palmitoyl glycine; further demonstrating that lipidomics aimed at expanding our knowledge of the family of N-acyl amides has the potential to provide novel avenues of research for TRP receptors.
Keywords: lipid signaling, TRP channels, N-acyl amide, Lipidomics, Cannabinoids, TRPV1, TRPV2, TRPV3, TRPV4
Introduction
Transient receptor potential channels (TRPs) form a large family of ubiquitous non-selective cation channels (Nilius and Owsianik, Vriens et al. , 2004). Numerous excellent reviews have been written about the subclasses, structure, function, and pharmacology and the reader is encouraged to read those cited here to gain a better in-depth knowledge of this exponentially growing field (Hardie, Jiang et al. , Nilius and Owsianik, Venkatachalam and Montell, 2007) In brief, TRPs are found in many species of animals ranging from standard invertebrates such as Drosophila and Caenorhabditis elegans (Harteneck et al. , 2000) to reptiles such as Xenopus laevis (Saito et al. , 2011) to mammals such as rats and humans (Neeper et al. , 2007). The TRP superfamily of channel proteins present a broad spectrum of activation mechanisms in response to a variety of stimuli including heat and cold (Clapham, 2002), pH (Yutaka and Yoshitaka, 2010), UV irradiation (Lee et al. , 2011), mechanical stimuli (Caterina and Julius, 2001), exogenous ligands such as capsaicin (the lipid in chili peppers that causes the sensations of chemical ‘heat’) (Caterina et al. , 1997) and resiniferatoxin (a lipophilic toxin found in a cactus-type plant indigenous to Morocco) (Roberts et al. , 2004) as well as many other plant, environmental and endogenous stimuli.
TRP channels, of which there are over 50 known to science, structurally consist of six transmembrane-spanning subunits and a recognized pore region formed by a short hydrophobic stretch. In mammals, TRP channels can be divided by sequence homology into six major subtypes (Venkatachalam and Montell, 2007, Vriens, Owsianik, 2004). The first three subfamilies are structurally similar to one another; these are the canonical or classical subfamily (TRPC1-7), the heat-activated vanilloid TRP channels (TRPV1-6), and the melastatins (TRPM1-8). The other three subfamilies found in mammals include the polycystin subfamily (TRPP1-3), the mucolipin subfamily (TRPML1-3), and the ankyrin reeptor (TRPA1). A seventh TRP subtype, TRPN (formerly nompC), is also recognized in Drosophila (Walker et al. , 2000). All TRP channels play roles in cell function including mobilization of calcium or other cations, temperature-sensing, osmolarity, nociception, and inflammation (Baez-Nieto et al. , 2011, Clapham, 2003, Holzer, 2011). There are a variety of endogenous TRP activators, most of which are lipophillic in chemical structure (Hardie, Jiang, Gamper, Venkatachalam and Montell, 2007)
That TRP channels respond to a variety of external stimuli (e.g. plant-derived lipids, changes in temperature, and changes in pH) provides a framework for their abilities as cellular sensors, however, the mechanism of direct activation is still under much debate and research. In the cases where endogenous ligands (predominately lipids) have shown direct activation of a TRP channel, multiple ligands have been shown to activate the same channel suggesting that these receptors are “promiscuous” in nature. Here, the discussion will focus on the activity of a novel class of lipids, N-acyl amides, and their activity at TRPV channels as well as speculate on the potential that the lipid, N-palmitoyl glycine (PalGly) may be an endogenous ligand at TRPC5. These examples illustrate how our growing understanding of the world of endogenous lipids provides novel insight into how these critical and ubiquitous TRP channels function and suggests that these interactions are more ‘opportunistic’ (i.e. evolutionarily driven) than ‘promiscuous’ (i.e. random, non-selective).
N-acyl amides and TRPV channels: opportunities abound
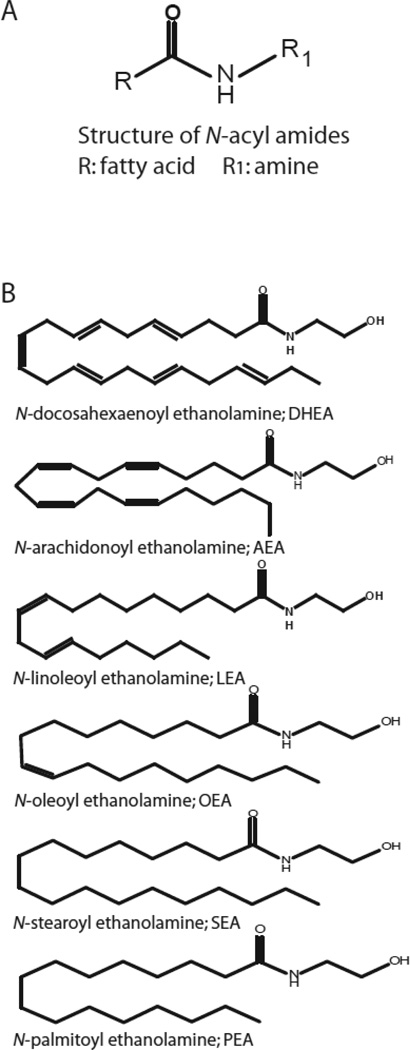
Traditionally in the world of biochemistry and cell-cell communication lipids were assumed to serve as simple structural compartments of cellular membranes without any role in biological functions. However, progress facilitated by research techniques such as mass spectrometry have broadened the understanding of lipids beyond their structural roles, and is helping to describe the specific molecular mechanisms by which they exert their signaling actions. The importance of N-acyl amides as signaling molecules emerged in the 1950s, when Kuehl and colleagues identified N-palmitoyl ethanolamine (PEA; Fig. 1B), in soybeans, peanuts and egg yolk and demonstrated it to have anti-inflammatory effects (Kuehl, 1957). During the last decade over 50 additional members of this family of lipids, including the endocannabinoid (eCB), N-arachidonoyl ethanolamine (Anandamide; AEA), form the class of so-called “N-acyl amino acids” (Bradshaw et al. , 2009, Bradshaw and Walker, 2005, Milman et al. , 2006, Saghatelian et al. , 2006). Structurally, these molecules consist of fatty acids conjugated to an amine by an amide bond (Fig. 1A). The most is known species in this class of lipids are the N-acyl ethanolamines, including the aforementioned AEA and PEA, as well as 4 additional endogenous structural analogs illustrated in Fig. 1B.
Figure 1. Molecular structure of N-acyl amides.
A) Basic molecular structure of all N-acyl amides. B) Molecular structure of 6 N-acyl ethanolamines.
Members of the TRPV family that consists of TPRV1-6, of which TRPV1-4 (also known as the thermoTRPs for their responses to heat) are the most targeted TRP receptors for drug discovery as they are major players in mechanisms of pain and inflammation (Di Marzo et al. , 2002). The discovery of TRPV1 (formerly VR1) receptors in dorsal root ganglia (DRG) in 1997 provided a breakthrough in the field of nociception because the receptor was identified as a molecular integrator of different types of noxious stimuli (Caterina, Schumacher, 1997). TRPV1 receptors are mainly expressed in nervous tissue such as Aδ and C fibers as well as DRG, trigeminal and nodose ganglia, and sensory neurons of the jugular ganglia as well as many brain areas (Caterina and Julius, 2001, Nagy et al. , 2004, Tominaga et al. , 1998) (Starowicz K, 2008). Additionally, they are expressed in non-neuronal tissues such as keratinocytes (Denda, 2010) (Huang, 2010), pancreas (Schwartz et al. , 2011), and smooth muscle (Benko et al. , 2012). Two commonly known exogenous chemical activators of TRPV1 are capsaicin (Caterina, Schumacher, 1997) and resiniferatoxin (RTX) (Starowicz K, 2008), both are highly lipophilic. The activity of these exogenous ligands is also sensitive to changes in the physical and chemical environment. Thermal environments above 42°C also activate TRPV1 receptors as well as environments with a low (acidic) pH (Tominaga and Caterina, 2004). TRPV1 receptor activity by exogenous ligands can be modulated by endogenous molecules as well, including a number of factors known to participate in inflammation such as nerve growth factors (Di Marzo, Blumberg, 2002), prostaglandins E2 and I2 (Moriyama et al. , 2005), and ATP (Moriyama et al. , 2003). Other identified endogenous activators for TRPV1 are lipoxygenase products of arachidonic acid (e.g. 12-(s)-HPETE, 9-HODE) (Hwang et al. , 2000, Patwardhan et al. , 2009), LTB4 (McHugh et al. , 2006) and N-(4-hydroxyphenyl) arachidonoylethanolamide (AM404), which is a metabolite of paracetamol (De Petrocellis et al. , 2000). Each of these lipids, likewise are small molecular weight lipids that share a general structural homology in being derived directly from a single fatty acid chain or fatty acid amide that is oxygenated.
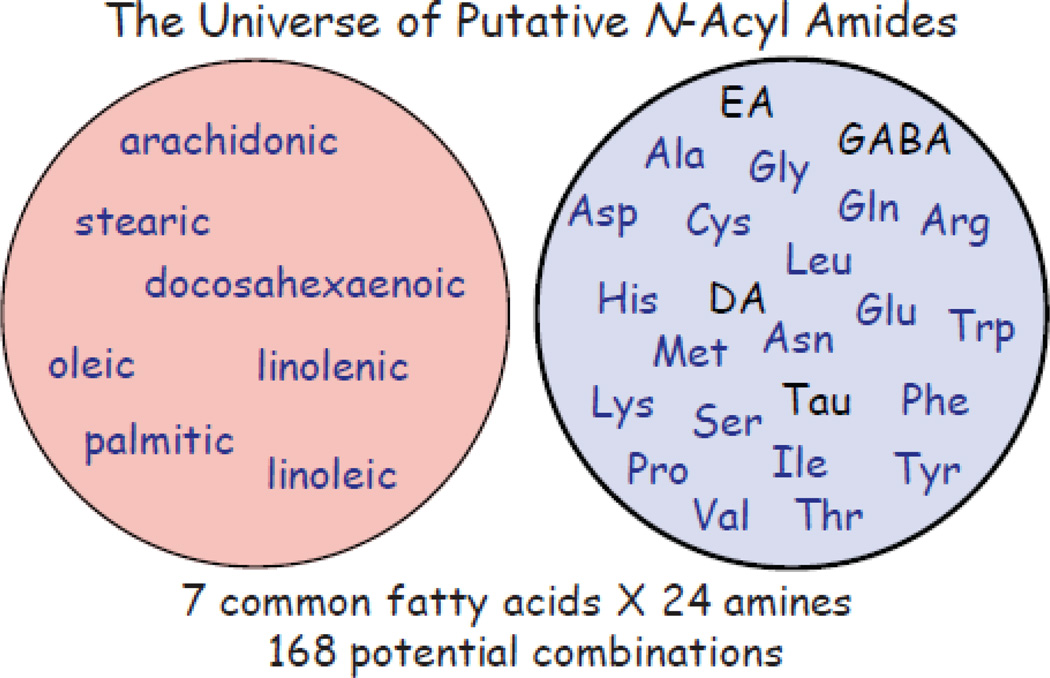
Multiple members of the N-acyl amide class of lipids have been shown to activate a member of the TRPV family, TRPV1 (Di Marzo, Blumberg, 2002). N-arachidonoyl ethanolamide (AEA), isolated in 1992 and identified as an endogenous ligand for the cannabinoid receptors, CB1 and CB2 (Devane WA, 1992), was the first N-acyl amide shown to activate TRPV1 channels (Zygmunt et al. , 1999). In 2002, Huang and colleagues demonstrated that N-arachidonoyl dopamine (NADA), structurally similar to the exogenous TRPV1 agonist capsaicin as well as an AEA structural analog with activity at CB1, is a more potent endogenous ligand for TRPV1 receptors (Huang et al. , 2002). Subsequently, another endogenous structural analog to both AEA and NADA was discovered, N-oleoyl dopamine (OLDA), that was, likewise, shown to activate TRPV1 receptors at low nanomolar concentrations and cause changes in pain behavior (Chu et al. , 2003). Around the same time, Ben Cravatt and colleagues showed that 20uM of N-arachidonoyl taurine actives both TRPV1 and TRPV4 receptors, demonstrating that non-eCB type N-acyl amides could also activate TRPs (Saghatelian, McKinney, 2006). All N-acyl amides discussed in this review are, likewise, structurally analogous to AEA, NADA, and OLDA with the difference being the species of fatty acid and the amine forming the amide bond. Combinatorial predictions of 7 of the most common fatty acids conjugated to 24 ubiquitous amines gives a potential 168 N-acyl amide combinations (Figure 2). To date, lipidomics techniques have focused on identifying and characterizing those N-acyl amides with this simple structure, however, there is the theoretical potential for the amine to instead be a peptide (2 or more amines conjugated with amide bonds and then conjugated to the fatty acid). Combinatorial predictions of this class of lipids are an exponential function and currently only present in theory.
Figure 2. Combinatorial construct for putative N-acyl amides.
The circle on the left contains the names of 7 abundant fatty acids found in mammalian systems, whereas, the circle on the right contains the standard abbreviations for the 20 common amino acids and 4 common amines (EA=ethanolamine; DA=dopamine; GABA= gamma-aminobutyric acid; Tau=taurine). These substrates are depicted in a “randomized” scenario to suggest that there is an equal likelihood that conjugations are possible for each fatty acid with each amine. To date, we have generated or purchased 81 of these combinations, therefore, studies are still ongoing to determine the full complement of endogenous N-acyl amides.
A critism of the data that AEA and other N-acyl amides are ‘real’ endogenous ligands for the TRPV receptors is that their production levels, potency, and distribution are potentially not a direct match for the expression and activational EC50 values of each of these receptors. For example, NADA and OLDA, which have low nanomolar potency at TRPV1 receptors (a theoretical ‘physiological’ range of activity), are mainly produced in striatum and to a much lesser extent in areas of the brain including hippocampus and cerebellum and is virtually absecent in dorsal root ganglia (DRG) (Huang, Bisogno, 2002), where TRPV1 receptors are highly expressed (Caterina and Julius, 2001). Alternatively, AEA and N-arachidonoyl taurine, which are more ubiquitous activate TRPV1 and TRPV4 receptors at µM concentrations, yet conventional means to determine endogenous levels would suggest that those EC50 values do not match what is being produced endogenously (Saghatelian, McKinney, 2006, Zygmunt, Petersson, 1999). Our group recently challenged TRPV1 receptors with over 80 N-acyl amides and discovered 8 novel N-acyl amides that act as agonists for TRPV1: 3 N-acyl GABAs, 2 novel N-acyl ethanolamides, and 3 N-docosahexaenoyl amides, each of these N-acyl amides, likewise, showed potency at low µM concentrations (data published in abstract form, ICRS 2012). Unpublished data from our lab demonstrated that when these 8 N-acyl amides are added together in a single assay, the EC50 was in the nanomolar range, suggesting that there is a potential additive effect of multiple endogenous ligands acting at the same time. This is similar to the so-called ‘Entourage' effects seen at TRPV1 observed for other N-acyl ethanaolamides (Ho et al. , 2008) wherein other endogenous structural analogs that are not agonists change the kinetics of TRPV1 to an agonist; however, the difference here is that each of these lipids is also an agonist at TRPV1, whereas, the typical entourage are not. Therefore, all together, there are currently at least 12 endogenous lipid activators of TRPV1 and the possibility that they may amplify the signaling effects at TRPV1 may be more aptly described as an ‘Ensemble’ effect. Until the full complement of potential N-acyl amides are available as standards it is not possible to know if additional N-acyl amides exist that will additionally activate TRPV1 (and other TRPs). That a single TRP channel is activated or modulated by multiple endogenous and exogenous lipids appears to be a normal mode of operation for many TRPs (Beech). Perhaps the rapid activation and in some cases sensitization of these receptors has evolved with the production of multiple structurally analogous lipids that share biosynthetic and metabolic pathways. These data suggest that TRPV1 has evolved as an opportunistic receptor that is activated by a wide range of structurally similar endogenous lipid ligands.
Comparing activation profiles of N-palmitoyl glycine and TRPC5: they may be perfect for each other
Members of the TRPC family are store-operated channels in species ranging from Drosophila to humans (Wu et al. , 2000). The Drosophila TRP channel has a sequence that is 40% identical to human TRPC1 (Wes et al. , 1995). TRPC channels can be modulated by calcium depletion and the receptors tend to be regulated by phospholipases C and D (Harteneck and Gollasch, 2011, Hiu-Yee et al. , 2009, Inoue et al. , 2009), which are integral to the biosynthesis and metabolism of the lipids diacylglycerol and phosphatidylinositol-4,5-bisphosphate (PIP2) (Loïc et al. , 2008, William et al. , 2009). TRPC3 activation is mediated by inositol 1,4,5-triphosphate receptors (InsP(3)R) and TRPC channel activation in species ranging from Drosophila to humans is inhibited by the antagonist molecule 2-aminoethoxydiphenyl borate (2-AB) (Ma et al. , 2001). A recent review by David Beech’s group elegantly outlined the activation profile (though mostly modulation) of TRPC5 by a range of endogenous factors (Jiang, Gamper). In this review a case was clearly made that 1) TRPC5 is activated or modulated by multiple lipids, though none with obvious specificity, 2) TPRC5 has a functional role in neuronal communication, 3) TRPC5’s role as a cellular sensor is integrally tied to signaling through a GPCR, and 4) TRPC5 activity is blocked by SK&F96365. Functionally, the Beech group review listed roles in neuronal growth cone axonal guidance, smooth muscle cell migration, and activation of specific amygdala circuitry during the fear response for TRPC5. Recently, others have shown that TRPC5 plays a role in transduction of noxious cold (Zimmermann et al.) and that TRPC5 is down regulated in a model of neuropathic pain (Staaf et al. , 2009). Finally, TRPC5 has been shown to be activated by nitric oxide (Yoshida et al. , 2006), which is important for the development of neuropathic pain (Wang et al. , 2008).
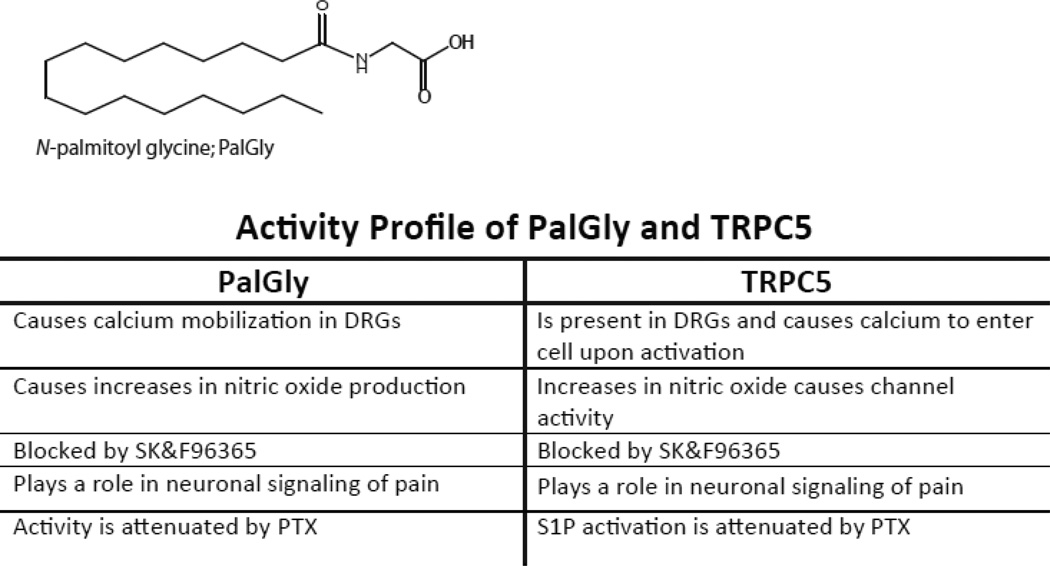
Rimmerman and colleagues recently published a report of a novel endogenous N-acyl amide, N-palmitoyl glycine (PalGly; Fig. 3A), which appears to activate a TRP-like channel in DRGs (Rimmerman et al. , 2008). The similarities to TRPC5 activation and the activity of PalGly in DRGs are striking. In this report, the authors ruled out the TRPV1-4 channels as targets, and speculated that the TRP-like channel driving the response was in some way associated with a GPCR in that the response was attenuated by PTX. It was shown that PalGly caused calcium mobilization in the DRG cell line, F-11, as well as in primary DRGs indicating that the channel was expressed in sensory neurons. Calcium mobilization was blocked with the non-selective TRP antagonist, ruthenium red; however, the authors also showed that the more selective TRP-channel blocker, SK&F96365, was extremely effective at blocking the calcium response. In addition, PalGly induced the production of nitric oxide in the F-11 cells, which was also blocked by SK&F96365. Functionally, peripheral administration of PalGly suppressed heat-evoked firing of spinal nociceptive neurons. Figure 3 shows a comparison of the similarities of the TRPC5 response and the activity profile of PalGly in F-11 cells. Our group are currently gathering reagents to test the hypothesis that PalGly is a direct TRPC5 agonist. This may prove to be another example of how the ubiquitous TRPs and the ubiquitous N-acyl amides have co-evolved to function as a unit to transduce information about changing intra and extracellular environments.
Figure 3. Activity profiles of N-palmitoyl glycine and TRPC5.
A) Molecular structure of N-palmitoyl glycine (PalGly). B) Tabular comparison of related activity profiles for PalGly and TRPC5. These comparisons illustrate the marked similarity in the cellular signaling outcomes of either challenging cells with PalGly or activating TRPC5.
Are TRP receptors promiscuous or opportunistic?
A review of the literature about TRP receptor ligands produces numerous papers that label these receptors as ‘promiscuous’ in nature (Beech, Beech et al. , 2009, Pingle et al. , 2007). This terminology has been an oft-used adjective in pharmacological studies and prompts an ideology of a receptor that is not fully functioning without a very specific ligand. Indeed, the word promiscuous is typically defined as 1) lacking standards of selection; indiscriminate; 2) casual, random; 3) consisting of diverse, unrelated parts or individuals, confused. The example of TRPV1 activation by numerous structurally similar molecules is not likely ‘indiscriminate’ or ‘random’ in terms of its evolutionary origins. On the contrary, that the receptor is able to be activated by so many of these structurally analogous lipids suggests that it is more evolutionarily opportunistic than promiscuous. Opportunistic is typically defined as 1) exploiting chances offered by immediate circumstances; 2) taking advantage of any opportunity to achieve an end; 3) able to spread quickly in a previously unexploited habit. While this may appear to be a pedantic argument its purpose is to suggest a reframing of how both the functioning of the TRP receptors is viewed and the specificity of the lipid ligands that activate them is considered as more a specific evolutionary result than a random, non-selective occurrence.
Lipidomics studies over the past decade have demonstrated that our knowledge of the number of lipid molecules that are being synthesized, metabolized, and used in signaling has increased exponentially. Evolutionary theory would suggest that it is highly unlikely that these lipids are being synthesized to no specific end in that it would be energetically disadvantageous for the cell produce a produce a complex molecule for no purpose. Unlocking the mystery of why these lipids and these channels exist is both thrilling and daunting. Winston Churchill is credited with the following quote: “A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.” N-acyl amides are ubiquitous lipids that are produced throughout the body and have the opportunity to play key roles in cellular communication. Likewise, TRP channels are ubiquitous throughout the body and numerous studies have already demonstrated their multiple functionalities. Biological opportunism works on the principle that a system exploits its readily available resources to perform required operations for specific functions. Data presented here that show TRP channel activation by N-acyl amides provides evidence that these two systems may have evolved synergistically to perform the role of cellular sensors throughout the body.
Supplementary Material
Acknowledgments
This work was supported by NIH DA032150.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baez-Nieto D, Castillo JP, Dragicevic C, Alvarez O, Latorre R. Thermo-TRP Channels: Biophysics of Polymodal Receptors. In: Islam MS, editor. Transient Receptor Potential Channels: Springer Netherlands. 2011. pp. 469–490. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 204:227–237. doi: 10.1111/j.1748-1716.2011.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko R, Illényi L, Kelemen D, Papp R, Papp A, Bartho L. Pulmonary, Gastrointestinal and Urogenital Pharmacology: Use and limitations of three TRPV-1 receptor antagonists on smooth muscles of animals and man: A vote for BCTC. European Journal of Pharmacology. 2012;674:44–50. doi: 10.1016/j.ejphar.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Hu SSJ, Burstein S, Walker JM. Chapter 8 Novel Endogenous N[hyphen (true graphic)]Acyl Glycines: Identification and Characterization. In: Gerald L, editor. Vitamins & Hormones. Academic Press; 2009. pp. 191–205. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. British Journal of Pharmacology. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. THE VANILLOID RECEPTOR: A Molecular Gateway to the Pain Pathway. Annual Review of Neuroscience. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, et al. N-Oleoyldopamine, a Novel Endogenous Capsaicin-like Lipid That Produces Hyperalgesia. Journal of Biological Chemistry. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Signal transduction. Hot and cold TRP ion channels. Science (New York, NY) 2002;295:2228–2229. doi: 10.1126/science.1070766. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Letters. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- Denda S, Denda Mitsuhiro, Inoue Kaori, Hibino Toshihiko. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. Journal of Dermatology. 2010;57:108–113. doi: 10.1016/j.jdermsci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Devane WAHL, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Blumberg PM, Szallasi A. Review: Endovanilloid signaling in pain. Current Opinion in Neurobiology. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- Hardie RC. A brief history of trp: commentary and personal perspective. Pflugers Arch. 461:493–498. doi: 10.1007/s00424-011-0922-9. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Current Pharmaceutical Biotechnology. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. Review: From worm to man: three subfamilies of TRP channels. Trends in Neurosciences. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Hiu-Yee K, Ching-On W, Zhen-Yu C, Tak-Wah Dominic C, Yu H, Xiaoqiang Y. Molecular and Cellular Pharmacology: Stimulation of histamine H2 receptors activates TRPC3 channels through both phospholipase C and phospholipase D. European Journal of Pharmacology. 2009;602:181–187. doi: 10.1016/j.ejphar.2008.10.054. [DOI] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. 'Entourage' effects of N-palmitoylethanolamide and Noleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology and Therapeutics. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Qiu Lei, Ding Li, Wang Shaozhan, Wang Jing, Quangang Zhua, Song Fengyu, Hu Jinhong. Ginsenoside Rb1 and paeoniflorin inhibit transient receptor potential vanilloid-1-activated IL-8 and PGE2 production in a human keratinocyte cell line HaCaT. International Immunopharmacology. 2010;10:1279–1283. doi: 10.1016/j.intimp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee S-Y, Kang C-J, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circulation Research. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Gamper N, Beech DJ. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets. 12:724–736. doi: 10.2174/138945011795378568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl FA, Jacob TA, Ganley OH, Ormond RE, Meisinger MAP. The identification of N-2(hydroxyethyl)-palmitamide as a naturally occuring anti-inflammatory agent. J Am Chem Soc. 1957;79:5577–5578. [Google Scholar]
- Lee Y, Kang S, Lee S, Kong K, Lee J, Kim E, et al. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Archives of Dermatological Research. 2011;303:727–736. doi: 10.1007/s00403-011-1153-9. [DOI] [PubMed] [Google Scholar]
- Loïc L, Mohamed T, James WP., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, et al. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. The Journal Of Biological Chemistry. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- McHugh D, McMaster RS, Pertwee RG, Roy S, Mahadevan A, Razdan RK, et al. Novel compounds that interact with both leukotriene B4 receptors and vanilloid TRPV1 receptors. J Pharmacol Exp Ther. 2006;316:955–965. doi: 10.1124/jpet.105.095992. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl l-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular Pain. 2005;1:1. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. European Journal of Pharmacology. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation Properties of Heterologously Expressed Mammalian TRPV2. Journal of Biological Chemistry. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- Rimmerman N, Bradshaw HB, Hughes HV, Chen JS-C, Hu SS-J, McHugh D, et al. N-Palmitoyl Glycine, a Novel Endogenous Lipid That Acts As a Modulator of Calcium Influx and Nitric Oxide Production in Sensory Neurons. Molecular Pharmacology. 2008;74:213–224. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. Research report: [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Research. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-Regulated Class of N-Acyl Taurines That Activates TRP Ion Channels†. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- Saito S, Fukuta N, Shingai R, Tominaga M. Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs. PLoS Genetics. 2011;7:1–11. doi: 10.1371/journal.pgen.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Christianson JA, Chen XW, La J-HH, Davis BM, Albers KM, et al. Synergistic Role of TRPV1 and TRPA1 in Pancreatic Pain and Inflammation. Gastroenterology. 2011;140:1283–1291. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144:187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Starowicz KCL, Di Marzo V. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des. 2008:42–54. 42–54. doi: 10.2174/138161208783330790. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. Journal of Neurobiology. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Voets T, Droogmans G, Nilius B. Invertebrate TRP proteins as functional models for mammalian channels. Pflügers Archiv European Journal of Physiology. 2004;449:213–226. doi: 10.1007/s00424-004-1314-1. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang LC, Lv YW, Ji Y, Yan XJ, Xue JP. Involvement of the nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway in the antihyperalgesic effects of bovine lactoferrin in a model of neuropathic pain. Brain Res. 2008;1209:1–7. doi: 10.1016/j.brainres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William AL, Sohag NS, Anthony PA. Review: Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582. doi: 10.1016/j.ceca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Villereal ML. Functional significance of human trp1 and trp3 in store-operated Ca(2+) entry in HEK-293 cells. American Journal Of Physiology Cell Physiology. 2000;278:C526–C536. doi: 10.1152/ajpcell.2000.278.3.C526. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Yutaka H, Yoshitaka O. TRP channels are involved in mediating hypercapnic Ca2+ responses in rat glia-rich medullary cultures independent of extracellular pH. Cell Calcium. 2010;48:124–132. doi: 10.1016/j.ceca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, et al. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci U S A. 108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H-h, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.