Abstract
Introduction
Benralizumab is a monoclonal antibody that binds the α subunit of the receptor to IL-5. As IL-5 is implicated in disease states that are mediated by eosinophils, benralizumab is an attractive option for use in the management of asthma. As a result of enhanced antibody-directed cell cytotoxicity, it has enhanced eosinophil-depleting activity as compared with neutralizing monoclonal antibody directed against IL-5.
Areas covered
This review presents the available data on benralizumab, including pharmacodynamics, pharmacokinetics, preclinical data and relevant clinical studies.
Expert opinion
Our review indicates that although further investigation is necessary to demonstrate clinical benefit, benralizumab remains a promising treatment modality.
Keywords: asthma, benralizumab, IL-5, IL-5Rα, MEDI-563
1. Introduction
The purpose of this article is to review the available literature on benralizumab (Box 1), also known as MEDI-563, a humanized anti-IL-5Rα antibody and its potential use in the management of asthma.
Box 1.
Drug summary.
| Drug name | Benralizumab |
| Phase | Phase II |
| Indication | Asthma |
| Pharmacology description | Humanized monoclonal antibody |
| Route of administration | i.v. |
| Chemical structure | IgG1, anti-(human IL-5 receptor α-chain) (human-mouse monoclonal MEDI-563 heavy chain), disulfide with human-mouse monoclonal MEDI-563 kappa-chain, dimer |
| Pivotal trial(s) | [2][16] |
Pharmaprojects – copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
IL-5 is a four-helix protein, produced mainly by T-lymphocytes, that promotes eosinophilopoeisis in the bone marrow and their mobilization into the circulation. IL-5 is also involved in eosinophil activation and important for survival. It is also involved in B cell development [1,2]. In asthma, allergen challenge results in increased eosinophil activation in airways. Additionally, IL-5 expression has been shown to be lowered in response to steroids in patients with steroid responsive asthma [3]. As eosinophils are the dominating effector cells in asthma, inhibition of eosinophilia, theoretically would result in a decreased airway injury, mucus hypersecretion and bronchial hyper-responsiveness [1,2].
Neutralizing anti-IL-5 antibody experiments (with mepolizumab) were effective in inhibiting eosinophilic inflammation induced by allergen as well as the increase in circulating eosinophilia in response to allergen challenge [3]. Although mepolizumab has been seen to have an effect in a selected group of patients with severe asthma and persistent airway eosinophilia despite corticosteroid use, it has not consistently inhibited the physiologic changes associated with allergen exposure in asthma [4-6]. This subtotal efficacy is thought to be a result of incomplete depletion of eosinophils in lung tissue and bone marrow [2].
The IL-5 receptor is a heterodimer composed of two subunits. The α subunit is specific for IL-5 and the β subunit is responsible for cell signaling. IL-5Rα is expressed on human eosinophil and basophil progenitors in bone marrow cells and mature eosinophils and basophils [1,2]. Benralizumab is a fully humanized afucosylated anti-human IL5-Rα antibody that binds to an epitope on IL-5Rα which is in close proximity to the IL-5 binding site and thus inhibits IL-5 receptor signaling, independent of ligand. It also produces antibody-directed cell-mediated cytotoxicity of eosinophils and basophils and consequently depletes IL-5Rα-expressing cells. The blockade of IL-5R signaling, independent of ligand, and depletion of IL-5Rα-expressing cells may underlie enhanced clinical efficacy [2].
By contrast, mepolizumab has also been shown to result in increased IL-5Rα expression, as well as increased local production of IL-5, suggesting an endogenous autoregulatory pathway [7]. Eosinophil persistence during IL-5 blockade may possibly also be explained by other cytokines, including IL-3 and GM-CSF (granulocyte-macrophage colony-stimulating factor) [2]. Some studies have demonstrated that IL-5Rα chain is downregulated on eosinophils in the presence of IL-5 and may therefore not be affected by IL-5 depletion [8]. These varied findings may also contribute to the suboptimal clinical efficacy of neutralizing anti-IL-5 antibody and may suggest a beneficial role of receptor blockade.
1.1 Mechanism of action
The interaction of eosinophils and human IL-5 occurs through an IL-5 receptor which is expressed on eosinophils, basophils and the surface of some cultured human mast cells [9]. IL-5 has been shown to work in unanimity with GM-CSF, IL-3, IL-4, IL-13, eotaxins and other signaling proteins to induce eosinophil-mediated inflammatory responses [10]. IL-5 receptor is a heterodimer, composed of a monomeric alpha (α) and a dimeric beta (β) chain [11]. The α chain by itself specifically binds with IL-5 but with low affinity, however, the ligand binding of IL-5 and IL-5Rα is followed by hetero-oligomerization of α and β subunits that leads to further proximity of β subunit intracellular domains producing a high-affinity phenotype (Figure 1) [12]. The β chain is responsible for signal transduction pathways, the major ones being Janus-activated kinase 2 (JAK2), a protein tyrosine kinase essential in IL-5 signal transduction and STAT-3 activation [13,14].
Figure 1.
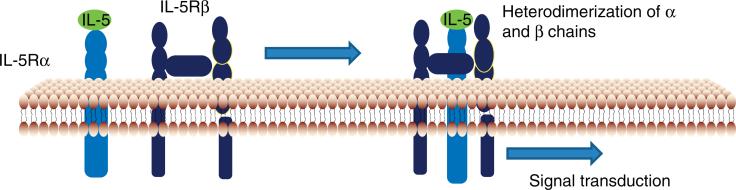
The α chain specifically binds with IL-5 with low affinity, however, the ligand binding of IL-5 and IL-5Rα is followed by hetero-oligomerization of α and β subunits that leads to further proximity of β subunit intracellular domains producing a high-affinity phenotype.
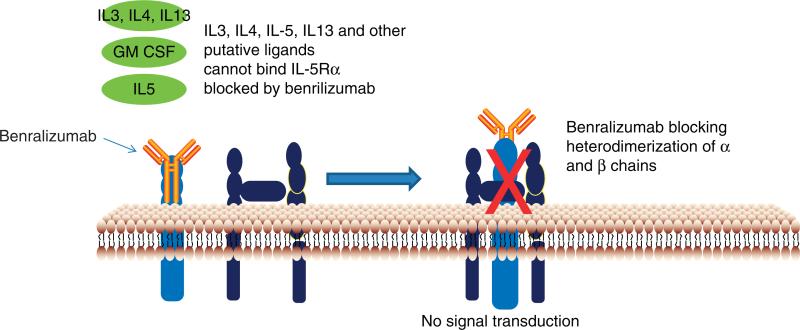
Benralizumab, also known as MEDI-563, is a humanized recombinant immunoglobulin IgG1k isotype monoclonal antibody. It has the unique property of binding with high affinity to a conformationally distinct epitope within domain 1 of the α-chain of IL-5R, and thus inhibits proliferation of IL-5-dependent cell lines, and otherwise blocks signaling through the IL-5 receptor (Figure 2) [15,16]. The other notable attribute of benralizumab is that it is afucosylated. Removal of the fucose sugar residue in the CH2 region of the oligosaccharide core of human IgG1 results in a 5- to 50-fold higher affinity to the main activating Fcγ receptor (human FcγRIIIa) expressed on natural killer (NK) cells, macrophages and neutrophils. This alteration has shown an augmented interaction of benralizumab with FcγRIIIa resulting in an amplified eosinophil apoptosis in vitro via antibody-dependent cell-mediated cytotoxicity (ADCC) functions by more than 1000-fold over the parental antibody [2].
Figure 2.
Benralizumab binds with the α chain of IL-5R, which in turn blocks the ligand binding of IL-5 as well as other putative ligands (such as IL-3, IL-4, IL-13 and GM-CSF), thereby no hetero-oligomerization of α and β subunits takes place and thus no signal transduction occurs.
1.2 Pharmacodynamics and pharmacokinetics
The pharmacokinetics (PKs) and pharmacodynamics (PDs) of benralizumab were evaluated in an open-label study, wherein single, escalating, intravenous doses (0.0003 – 3 mg/kg) of benralizumab were administered to subjects with mild atopic asthma (n = 44) over 3 – 30 minutes. The serum benralizumab concentration and blood eosinophil counts were followed over 12 weeks after dosing [17].
1.2.1 Pharmacodynamics
Benralizumab produced depletion of circulating peripheral blood eosinophils levels within 24 hours at all doses administered. This effect persisted for at least 2 to 3 months in subjects dosed in the range of 0.03 – 3 mg/kg [17].
1.2.2 Pharmacokinetics
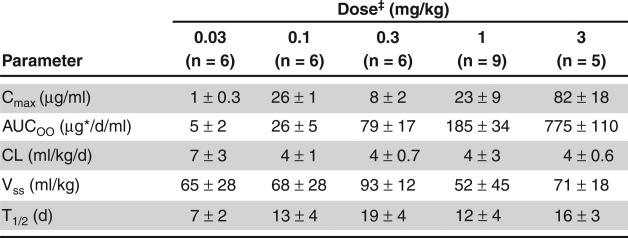
The mean maximum concentration (1 – 82 μg/ml) and mean area under the curve (5 – 775 μgd/ml) and thus the PK activity at doses of 0.03 – 3 mg/kg were approximately dose proportional.
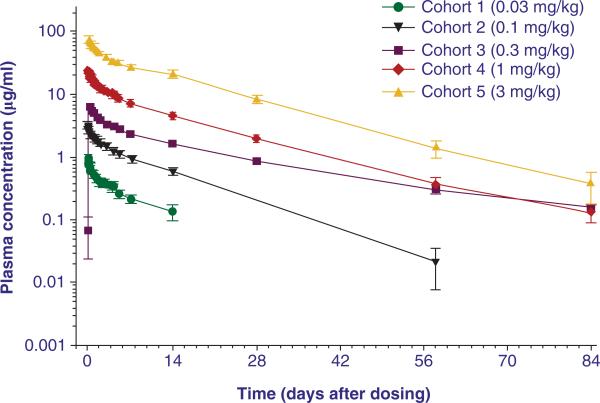
The mean volume of distribution of benralizumab (52 – 93 ml/kg) was greater than the plasma volume. This could potentially indicate binding of benralizumab to IL-5Rα-expressing blood cells as well as some penetration into extravascular tissues (Figure 3, Figure 4). The mean elimination half-life predicted was around 18 days [17].
Figure 3. Summary of non-compartmental pharmacokinetic parameters.
Reproduced from [16].
*Parameters are presented as mean ± SD.
‡Observations for cohot 6 (0.003 mg/kg) and cohort 7 (0.0003 mg/kg) were below the limit of quantitation. AUC∞: Area Area under the curve from time 0 extrapolated to infinite rtime; CL: systemic clearance; Cmax maximum concentration; T1/2: elimination half life; Vss: volume of distribution at steady state.
Figure 4. Mean (6 SD) MEDI-563 concentration–time profile.
Mean MEDI-563 plasma concentration after a single i.v. dose of 0.03, 0.1, 0.3, 1 and 3 mg/kg
Reproduced from [16].
1.3 Preclinical studies
Preclinical data in the public domain are quite limited. In fact, only one published article by the makers of benralizumab is available in addition to two abstracts submitted at national meetings.
Benralizumab binds to IL-5Rα in segment B (aa 40-61) in domain 1. A single amino acid (isoleucine at position 61) is the critical residue for benralizumab binding to human IL-5Rα. This segment is 100% conserved between human subjects and cynomolgus monkeys. Afucosylated parental anti-IL-5Rα mAb demonstrated higher mediation of eosinophil and basophil apoptosis in the presence of autologous NK cells and depleted IL-5Rα positive bone marrow mononuclear cells. Stimulation by a mixture of cytokines (RANTES, eotaxin and IL-33) did not result in evidence of eosinophil degranulation, as measured by eosinophil-derived neurotoxin (EDN) or eosinophil cationic protein (ECP) release [2].
A study done in cynomolgus monkeys demonstrated that repeated administration of benralizumab at all dose levels (0.1, 1, 10 and 30 mg/kg i.v. every 3 weeks × 12 weeks) resulted in rapid depletion (within 3 days) of peripheral eosinophil counts. These monkeys had terminal necropsy at day 67 and recovery necropsy at day 85. Bone marrow eosinophil precursors reduced 80% or greater 3 days after last dose (terminal necropsy) and remained undetectable until 18 days after the last dose in the 30 mg/kg dose group at recovery necropsy, without affecting neutro-phil precursors. Benralizumab was found to bind to the IL-5Rα with similar potency in humans and cynomolgus monkeys [2].
Biopsy specimens from patients with asthma, using immunohistochemical methods demonstrated that all eosinophils were bound by MEDI-563 after incubation [2].
Prior case studies have raised concerns for the association of eosinophil and basophil deficiency with recurrent infections and this potential adverse effect will require further clarification in human studies [2].
1.4 Clinical studies
1.4.1 Phase I clinical studies
The mechanism of action and potency of benralizumab were investigated by in vitro studies. Benralizumab was found to mediate the lysis of IL-5R transfected cells with a 200-fold higher potency in comparison with the parental mAb and a 10-fold higher binding affinity to human FcγRIIIa manifesting in an enhanced ADCC. All eosinophils from bronchial biopsies of mild asthmatics expressed IL-5Rα [2].
The safety and biological activity of benralizumab were assessed in an open-label first-in-human study, wherein six subjects with mild asthma and absence of corticosteroids, received a single intravenous dose of 0.03 mg/kg and were followed for 84 days [16]. A robust eosinopenia for at least 84 days was demonstrated in all six subjects and an overall similar pattern was followed by circulating basophils. Benralizumab was found to be well tolerated with that single dose, with mild adverse effects.
To further evaluate the safety and tolerability of benralizumab, seven cohorts of six patients with mild atopic asthma each were given single escalating intravenous doses (0.0003 –3 mg/kg) of benralizumab [13]. The results indicated a rapid (within 24 h) and significant peripheral blood eosinopenia which was maintained for 56 – 84 days. Asthma symptom scores and β2-agonist use remained low and pulmonary function did not decrease during the study. Fractional exhaled nitric oxide, a marker of airway inflammation remained unchanged. Nasopharyngitis (27.3%), reduced neutrophil count (34.1%), increased creatine phosphokinase (CPK) (25%) and increased cholesterol were the most common adverse effects reported that reverted without sequelae. No deaths or serious adverse effects were reported [16].
The PK and PD relationships for benralizumab were also evaluated in the same study [16]. The PK activity was dose proportional at doses of 0.03 – 3 mg/kg. The elimination half-life was approximately 18 days. The maximal stimulation effect of benralizumab on eosinophil elimination was approximately 170-fold higher than the baseline elimination rate. The maximal inhibition effect of benralizumab on eosinophil production was approximately 90% of the baseline eosinophils production rate.
1.4.2 Phase II studies
A Phase IIa study has been reported in adult asthmatics to evaluate the safety and tolerability profiles of multiple doses of benralizumab administered subcutaneously [17]. In this study, subjects received three monthly s.c. injections of benralizumab (25 mg [7], 100 mg [6] and 200 mg [6], or placebo [6]) on days 0, 28 and 56. Within a few days of administration, peripheral blood eosinophils were found to be substantially depleted and continued to remain the same for at least 160 days in all cohorts. Furthermore, benralizumab displayed an acceptable safety profile.
The PKs and immunogenic potential of benralizumab after multiple subcutaneous doses in adult asthmatics were also evaluated in the above Phase IIa study [17]. Benralizumab displayed linear PKs. Both area under curve (AUC) and maximal concentration (Cmax) increased in a dose-proportional manner. Confirming the previous study, the terminal half-life was roughly 18 days. Out of 19 subjects treated with multiple doses of benralizumab, 4 exhibited evidence of immunogenicity (as measured by anti-drug antibodies).
1.4.3 Ongoing studies
A Phase II multicenter, randomized, double-blind, placebo-controlled study is under progress to evaluate the safety and efficacy of intravenously administered benralizumab on asthma control following acute exacerbations in adults. The study will evaluate the effect of two i.v. dose regimens of benralizumab on the proportion of adult subjects with asthma exacerbations who require an urgent healthcare visit for treatment of an acute asthma exacerbation. There is another ongoing study designed to evaluate the effects of benralizumab on eosinophil counts in airway mucosal biopsies 28 days after completion of dosing in adults with atopic asthma.
2. Expert opinion and conclusions
Benralizumab is a humanized IgG1k class monoclonal antibody that recognizes an epitope on the IL-5Rα chain that is close to the binding side of authentic IL-5. Binding of benralizumab inhibits ligand-induced signaling through the IL-5 receptor. This mechanism is distinct from neutralizing antibodies against IL-5, in that other putative IL-5 receptor ligands could induce signaling and eosinophil activation in the presence of an IL-5 antibody, but would not be expected to induce signaling in the setting of an anti-receptor antibody (such as benralizumab). The PKs are consistent with other humanized monoclonal antibodies, and the elimination of fucose residues improves binding to the IgG receptor, which appears to enhance efficacy. Although the preclinical data are intriguing, the available clinical data are sparse. Further investigation, in formal randomized clinical trials, will be essential to define the role, if any, of benralizumab in the management of allergic inflammatory diseases such as asthma.
Apart from asthma, eosinophils are also implicated in other disease states including hypereosinophilic syndrome (HES), eosinophilic esophagitis, nasal polyposis and Churg–Strauss syndrome. Mepolizumab has been shown to have somewhat positive results in HES [4]. Since benralizumab blocks the IL-5Rα receptor thereby preventing the binding of all putative IL-5 ligands, it could manifest in better outcomes than mepolizumab in HES. Thus, studies need to be done to establish the better efficacy of benralizumab in diseases like HES as well.
Acknowledgment
This review was conducted at the University of Texas Medical Branch at Galveston supported in part by grant UL1RR029876 from the National Center for Research Resources, National Institutes of Health. A Ghazi and A Trikha contributed equally to this work.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.Lotvall J, Pullertis T. Treating asthma with Anti-IgE or Anti IL-5. Curr Pharm Des. 1999;5:757–70. [PubMed] [Google Scholar]
- 2.Kolbeck R, Kozhich A, Koike M, et al. Medi-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(1):1344–53. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kotsimbos ATC, Hamid Q. IL5 and IL-5 receptor in Asthma. Mem Inst Oswaldo Cruz. 1997;92(Suppl II):75–91. doi: 10.1590/s0074-02761997000800012. [DOI] [PubMed] [Google Scholar]
- 4.Busse W, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol. 2010;125(4):803–13. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Hadar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 7.Stein ML, Villanueva JM, Buckmeier BK, et al. Anti IL-5 (mepolizumab) therapy reduces eosinophil activations ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121(6):1473–83. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as Anti-Interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 9.Migita M, Yamaguchi N, Mita S, et al. Characterization of the human IL-5 receptors on eosinophils. Cell Immunol. 1991;133:484–97. doi: 10.1016/0008-8749(91)90120-z. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Tavernier J, Devos R, Cornelis S, et al. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–84. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 12.Zaks-Zilberman M, Harrington AE, Ishino T, Chaiken IM. Interleukin-5 receptor subunit oligomerization and rearrangement revealed by fluorescence resonance energy transfer imaging. J Biol Chem. 2008;283:13398–406. doi: 10.1074/jbc.M710230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogata N, Kouro T, Yamada A, et al. JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and beta c subunit, respectively, and are activated upon IL-5 stimulation. Blood. 1998;91:2264–71. [PubMed] [Google Scholar]
- 14.Takaki S, Kanazawa H, Shiiba M, Takatsu K. A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor alpha chain and its function in IL-5-mediated growth signal transduction. Mol Cell Biol. 1994;14:7404–13. doi: 10.1128/mcb.14.11.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koike M, Nakamura K, Furuya A, et al. Establishment of humanized anti-interleukin-5 receptor alpha chain monoclonal antibodies having a potent neutralizing activity. Hum Antibodies. 2009;18:17–27. doi: 10.3233/HAB-2009-0198. [DOI] [PubMed] [Google Scholar]
- 16.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti--IL-5 receptor antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–44. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Gossage D, Geba G, Gillen A, et al. A multiple ascending subcutaneous dose study of MEDI-563, A humanized anti-IL-5RA monoclonal antibody, in adult asthmatics (clinicaltrails.gov Identifier: NCT00783289).. Annual Congress of the European Respiratory Society (ERS); 2010. [Google Scholar]