Summary
Inhibition of programmed cell death is considered to be a major aspect of tumorigenesis. Indeed, several key oncogenic transcription factors, such as NF-κB and STAT3, exert their tumor promoting activity at least in part through upregulation of survival genes. However, many cancers develop in response to chronic tissue injury, where the resulting cell death increases the tumorigenic potential of the neighboring cells. In this review, we discuss a resolution to this paradox based on cell death-mediated induction of tumor promoting inflammatory cytokines, which enhance cell survival and trigger compensatory proliferation in response to tissue injury.
Keywords: Cancer, cell death, tissue injury, inflammation, immunity, cytokines
Introduction
The idea that tissue injury and cancer are linked is not new. As far back as 1863, Rudolf Virchow hypothesized that previous tissue injury is required for tumor emergence (Mantovani et al., 2008). This hypothesis has gained recent support from clinical data revealing a strong association between chronic injury and subsequent tumorigenesis at the same site. For instance, alcohol abuse causes liver injury and increased risk of hepatocellular carcinoma (HCC). Betel nut chewing, cigarette smoking and exposure to fine silica dust cause esophageal and lung damage and promote cancer development in these tissues, whereas chronic infection with Helicobacter pylori causes gastritis and stomach cancer (El-Serag and Rudolph, 2007; Hecht, 2002; Uemura et al., 2001). How does tissue injury promote tumorigenesis? Certainly mutagenesis, genomic instability and epigenetic modifications are important in this relationship, but they are insufficient. Due to strong similarities between key features of wound healing and tumor development, including stem cell and myofibroblast activation, enhanced cell proliferation, inflammation and neoangiogenesis, it is tempting to postulate that chronic injury can result in an aberrant healing and regenerative response that ultimately promotes the expansion and progression of initiated cells. This idea has already been forwarded by Alexander Haddow who suggested that “tumor production is a possible overhealing” (Haddow, 1972) and Harold Dvorak who stated that “tumors are wounds that do not heal” (Dvorak, 1986).
Can the prevalence of inflammation be the link between tissue injury and cancer? Recent studies have highlighted the particular importance of inflammation in both processes. These studies have indicated that the role of inflammation extends far beyond protection of the injured tissue from infectious agents and removal of damaged cells. In fact, inflammation is critical to almost every phase of tissue repair and tumorigenesis. Thus, we put forth that injury causes inflammation, which in turn orchestrates wound healing and tissue regeneration. If the inflammation cannot be resolved or is chronically provoked by repetitive injury or other factors, the resulting unchecked wound healing process can promote cancer formation. This hypothesis is supported by a large body of data, which is briefly summarized below and depicted in Figure 1.
Figure 1. Tumorigenesis in response to chronic tissue injury of different types.

Tissue injury and cell death lead to activation of various inflammatory cells that secrete cytokines that promote wound healing. However, if this process is reiterated several times or inflammation fails to resolve, tissue regeneration may be dysregulated and, when combined with carcinogen exposure, can lead to malignant transformation.
It should be noted that at least several different types of inflammation associated with tumorigenesis can be delineated (Grivennikov et al., 2010). Certain types of inflammation, such as tumor elicited inflammation, defined as recruitment of immune cells into a growing tumor, occur only in later stages of tumor development. Such inflammation may not be dependent on cell death and can be elicited by chemokines secreted by the tumor (Karin, 2005), but nonetheless can still influence later aspects of tumor progression. Tumor elicited inflammation may also drive metastatic progress as recent studies of breast and prostate cancers have demonstrated (DeNardo et al., 2009; Kim et al., 2009; Luo et al., 2007; Tan et al., 2011). However, it is entirely plausible that necrosis, resulting from hypoxia at the tumor core may be one “injury” that triggers, or at least contributes to, tumor elicited inflammation. Despite the multiple facets of cancer-linked inflammation, this review is focused on the role of tissue injury and cell death as drivers for the inflammation that contributes to tumor initiation and early promotion. Other aspects of inflammation in cancer have been reviewed elsewhere (Grivennikov et al., 2010; Joyce and Pollard, 2009; Mantovani et al., 2008).
Following injury, even when no infectious agents are present, immune cells quickly migrate into the injured tissue after vasodilatation and in response to chemokine gradients, classically described as the inflammatory stage of wound healing (Velnar et al., 2009). These cells promote healing by releasing cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6 that suppress further cell death, activate stem cells and promote epithelial proliferation. For example, depletion of macrophages during the early and middle phases of skin wound repair results in attenuated epithelial proliferation and wound contraction (Lucas et al., 2010). The same cytokines are expressed by tumor-associated inflammatory cells and sometimes by cancer cells themselves, and promote cancer cell proliferation while suppressing the death of pre-malignant cells (see below). Neovascularization during wound healing is critical for providing nutrients and oxygen to the newly remodeled tissue. Following recruitment to the site of injury, inflammatory cells including macrophages and neutrophils secrete molecules such as vascular endothelial growth factor A (VEGFA) to promote blood vessel development and tissue remodeling (Bao et al., 2009). In tumors, inflammatory cells play a similar role by promoting neo-angiogenesis (Lewis et al., 2000). In particular, VEGF-secreting macrophages are critical to tumor development, as their depletion reduced vascular density in a breast cancer model (Stockmann et al., 2008). Fibroblast activation or the conversion of tissue fibroblasts into myofibroblasts, which engage in extracellular matrix (ECM) deposition and scar formation, is critical for wound healing (Wynn, 2008). The main activators of fibroblasts after tissue injury are M2-polarized macrophages that are recruited to the site of injury where they secrete factors such as platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF) and transforming growth factor β (TGF-β). In tumorigenesis, cancer associated fibroblasts (CAF) have long been thought to promote malignant progression (Orimo and Weinberg, 2006). Although the origin of CAF and their mode of activation remain controversial, immune cell secretion of TGF-β may be instrumental while CAF are essentially identical in their properties to myofibroblasts, especially in their ability to produce a large number of chemokines (Tan et al., 2011).
Not surprisingly, somatic stem cell activation is critical for tissue repair. For example, ablation of hair follicle stem cells, leads to a complete hair loss after skin injury (Ito et al., 2005). Furthermore, pathways critical for stem cell maintenance and expansion, such as the Hedgehog and Wnt signaling pathways, are also important for injury repair and tumorigenesis (Beachy et al., 2004). Several studies have implicated inflammation in stem cell function. For example, cytokines such as TNF and IL-6 promote stem cell proliferation (Widera et al., 2006), and their transcriptional effectors NF-κB and STAT3 are involved in stem cell renewal (Matsuda et al., 1999) and cancer (Grivennikov et al., 2010). Much emphasis has recently been placed on cancer stem cells (CSC), the functional homologs of somatic stem cells that are responsible for tumor initiation and regrowth after debulking (Lobo et al., 2007). While still poorly explored, inflammation is likely to promote CSC maintenance and proliferation. Reactive oxygen species (ROS), possibly originating from inflammatory cells, can modulate self-renewal of chronic myeloid leukemia stem cells (Miyamoto et al., 2007). Furthermore, macrophages can promote CSC tumorigenicity through IL-6, STAT3 and the Hedgehog pathway (Jinushi et al., 2011) and IL-6, STAT3 and NF-κB can convert non-stem cells to CSC (Iliopoulos et al., 2011). These findings lend support to the idea that cancer and wound healing progress through similar mechanisms, many of which are affected by inflammatory cytokines. However, an even stronger parallel between inflammation-promoted wound healing and cancer may be the role of inflammation in tumor recurrence. For example, partial hepatectomy, a preferred treatment for localized HCC, is initially successful, but within 5 years recurrence rates approach 100% (Schwartz et al., 2007). Compensatory hepatocyte proliferation and HCC development depend on similar mechanisms in which IL-6 and STAT3 play key roles (He et al., 2010; Naugler et al., 2007). Similarly, the mainstay of advanced prostate cancer treatment is androgen deprivation therapy, which causes the death of androgen-dependent tumor cells. However, this therapy also promotes the recurrence of castration-resistant cancer (Gulley et al., 2003). We recently found that lymphotoxin (LT), a TNF family member expressed by tumor infiltrating B cells, plays a key role in this process (Ammirante et al., 2010). Thus, therapy-induced inflammation is likely to contribute to tumor recurrence, although it was also proposed to enhance therapeutic outcomes in other cases (Zitvogel et al., 2010).
In summary, while the complete details of the interconnection between injury, inflammation and cancer are still being unraveled, there is substantial evidence that inflammation is essential in both tissue repair and cancer. In both cases, certain forms of cell death promote inflammation and inflammatory cytokines enhance tissue repair and tumorigenesis, in part, by suppressing the death of remaining neoplastic cells.
Inflammatory vs. Non-Inflammatory Cell Death
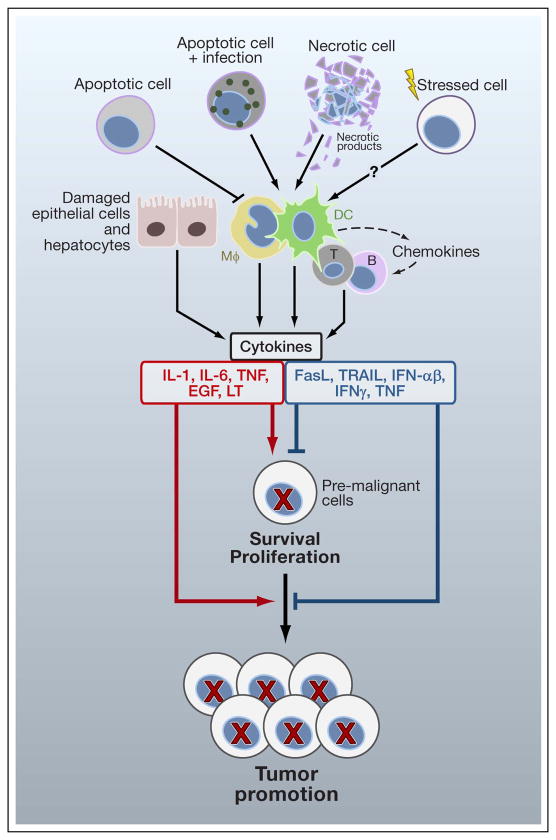
The mechanisms by which tissue injury triggers inflammation are complex and not fully understood. Amongst initial triggers of injury-induced inflammation, necrotic cell death is of particular importance. However, the early distinction between necrotic cell death being inflammatory and apoptosis being an anti-inflammatory form of cell death has become somewhat diffuse. Below we briefly review mechanisms that link local injury and cell death to induction of inflammation and cytokine production and can thereby impact tumor initiation as well as early tumor promotion (Figure 2).
Figure 2. Cell death and inflammation.
While normal apoptotic cell death inhibits inflammation, apoptotic death of infected cells, necrotic cell death or factors released by stressed cells lead to activation of different types of immune cells, including T and B lymphocytes, macrophages (mΦ) and dendritic cells (DC). The latter produce cytokines that have either positive (red) or negative (blue) influences on cell survival and proliferation and regulate different steps of tumorigenesis, including tumor growth and progression. In addition, mΦ and DC may recruit other immune cells into the tumor microenvironment. Damaged or neighboring epithelial cells can also release various cytokines, which further modulate cell survival and growth.
It was shown that engulfment of apoptotic bodies by macrophages triggers the production of anti-inflammatory cytokines, such as IL-10 (Savill et al., 2002). The universality of this concept, however, has been challenged as apoptosis induced by particular chemotherapeutic agents, such as anthracyclines and ionizing radiation, was found to be inflammatory (Casares et al., 2005; Fucikova et al., 2011). Mechanisms that allow such agents to trigger inflammation include calreticulin translocation (Obeid et al., 2007), heat shock protein 70 and 90 translocation (Udono and Srivastava, 1994), and high mobility group box 1 (HMG1) release (Apetoh et al., 2007). These events result in dendritic cell (DC) and macrophage activation and a consequent inflammatory response (Zitvogel et al., 2010). In addition, recognition of infected apoptotic cells at sites of infection-induced injury culminates in enhanced production of IL-6, which drives differentiation of pro-inflammatory T cells (Torchinsky et al., 2009). Caspase 3-mediated cell death induced by therapy results in the release of prostaglandins, which are known to promote inflammation, but in this case directly promote the survival of residual tumor cells (Huang et al., 2011).
Unlike apoptosis, necrosis or pyroptosis [defined as cell death that is dependent on inflammatory caspase-1 activity (Bortoluci and Medzhitov, 2010)] involve rupture of the plasma membrane and release of the cell’s content, making these mechanisms of cell death naturally inflammatory (Bortoluci and Medzhitov, 2010; Zitvogel et al., 2010). Cellular components released during necrotic cell death include IL-1α, danger-associated molecular patterns (DAMP) such as calreticulin, HMG-1 and S100A8 and 9 proteins (Ghiringhelli et al., 2009; Sakurai et al., 2008) and nucleotides and nucleic acids such as ATP, RNA and DNA, whose recognition leads to DC and macrophage activation (Zitvogel et al., 2010). Although early studies have shown that neutralization of HMG1 can block cell-death-induced inflammation (Scaffidi et al., 2002), this is not universal and in many cases it has been rather difficult to block the inflammation that follows tissue injury and cancer cell death due to the release of a large number of different inflammatory triggers. Pyroptotic cell death is dependent on activation of caspases, which are also pivotal for the processing and release of IL-1 and IL-18 (Bortoluci and Medzhitov, 2010). For instance, IL-1α released by dying hepatocytes in a liver challenged with the pro-carcinogen diethyl nitrosamine (DEN) triggers IL-6 production, which activates STAT3 and promotes liver regeneration and tumor growth (He et al., 2010; Sakurai et al., 2008). Various environmental toxins are also capable of triggering cell death-induced inflammation. Components of tobacco smoke, asbestos and silica particles cause injury and cell death of the lung epithelium, thereby establishing chronic inflammation (Dostert et al., 2008), which promotes the growth of K-Ras induced lung cancer (Takahashi et al., 2010). Lipid accumulation makes hepatocytes within fatty livers more prone to cell death upon exposure to hepatotoxic stimuli (Tuncman et al., 2006) and the ensuing inflammatory response, that involves TNF and IL-6 production, promotes HCC development (Park et al., 2010).
One major factor that determines whether cell death is inflammatory or not is the rate and the extent of cell death. Slowly occurring physiological cell death may provide phagocytes with enough time to clear the resulting debris, but intense cell death during tissue injury or after cancer therapy may exceed the clearance capacity of tissue macrophages, and even when apoptotic in origin can trigger inflammation. Indeed, high doses of otherwise non-inflammatory genotoxic agents, such as cisplatin and UV irradiation, can induce necrosis and consequent inflammation (Caricchio et al., 2003; Dursun et al., 2006). Furthermore, while cell death after androgen withdrawal in prostate cancer is initially apoptotic (McKenzie and Kyprianou, 2006), androgen withdrawal triggers an inflammatory response (Ammirante et al., 2010). Most likely, extensive apoptosis results in secondary necrosis (Zitvogel et al., 2010).
Recent data suggest the involvement of additional cell intrinsic events in the release of cytokines during stress or injury. In this case, activation of inflammatory pathways either parallels cell death or can be triggered by cell stress in the absence of death. Oncogene activation (Mantovani et al., 2008), inactivation of tumor suppressor p53 (Komarova et al., 2005) and cell senescence induced by extensive DNA damage (Rodier et al., 2009) can all lead to production of the tumor promoting cytokines IL-1, IL-6 and IL-8. Often, stromal cells, such as fibroblasts, sense alterations in tissue homeostasis which precede injury and tumor development and contribute to inflammation (Bornstein et al., 2009; Stairs et al., 2011). Myofibroblast activation in response to injury results in the production of numerous inflammatory chemokines, including CXCL13, which lead to B cell recruitment into androgen-deprived prostate tumors (Ammirante et al., 2010). Undoubtedly, tissue injury and cell death are important triggers of tumor promoting inflammation (Figure 2).
Inflammation, cytokine, cell death and survival
Paradoxically, the inflammatory cytokines that are produced in response to cell death promote tumor development by enhancing the survival of pre-malignant and fully malignant cells (Grivennikov et al., 2010). The mechanisms that lead to the upregulation of inflammatory cytokines in cancer, which can take place both in autocrine and paracrine manners, are diverse and their understanding is still rudimentary. We focus the following discussion on the relationships between cell death, cytokines and cell survival in the context of tissue injury and tumor development.
In many cases, the better understood stimuli that link local injury and cell death to inflammatory cytokine production under cancerous conditions are pathogens or commensals that are recognized by pattern recognition receptors (PRR) on immune or epithelial cells (Medzhitov, 2007). Pathogens that establish persistent infections, such as Helicobacter pylori or Hepatitis B and C viruses (HBV; HCV) can lead to the chronic production of pro-tumorigenic cytokines (Grivennikov et al., 2010). In addition to direct PRR activation, certain pathogens, for instance hepatitis B virus (HBV) and hepatitis C virus (HCV), can trigger cytokine production via induction of cell death. As mentioned above, apoptotic cells typically induce expression of IL-10 and TGF-β (Savill et al., 2002), which suppress inflammation and in some cases inhibit tumor development (Grivennikov et al., 2010). However, recognition of infected apoptotic cells results in upregulation of IL-6, which drives differentiation of pro-inflammatory T helper IL-17-producing (Th17) cells (Torchinsky et al., 2009). While this response is designed to promote host defense followed by tissue repair, it can also stimulate chronic inflammation and tumor growth, especially in cancers that develop in mucosal surfaces and epithelial barriers populated by bacteria (Torchinsky et al., 2009; Wu et al., 2009). In fact, a Th17-related cytokine signature was recently shown to be a sign of bad prognosis in colorectal cancer (Tosolini et al., 2011). In addition, components of the intestinal microflora promote tumor development in Il10−/− mice, which can be prevented by antibiotic treatment (Chichlowski et al., 2008). As discussed above, necrotic cell death caused by hypoxia, nutrient or growth factor deprivation or cancer therapy, can lead to sterile inflammation that if recurrent, can promote tumor development (Sakurai et al., 2008; Zitvogel et al., 2010).
Inflammatory cytokines are critical regulators of life vs death decisions. IL-1 and IL-6 for instance are potent inducers of cell survival and tumor development, through their ability to activate pro-survival transcription factors, such as NF-κB and STAT3. Other cytokines, such as Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL) are potent activators of caspase 8 and therefore are inducers of apoptotic cell death. However, their close relative TNF has both pro-survival and pro-death functions. Other cytokines can exert complex effects on the tumor microenvironment that may affect cell survival and death by more indirect means. According to the “cancer immunosurveillance” theory (Dunn et al., 2004), death-inducing cytokines (TRAIL, FasL, TWEAK and occasionally TNF) and cytokines that limit the proliferation of epithelial cells (type I interferon (IFN) and TGF-β) may constitute an important anti-cancer defense system. This system can eliminate transformed cells and kill metastatic seeds (Swann and Smyth, 2007). Naturally, such cytokines were considered as cancer treatments, but so far success has been rather limited. Nonetheless, genetic or pharmacological blockade of death cytokines often (but not always) increases susceptibility of mice to spontaneous and induced tumorigenesis (Swann and Smyth, 2007). For example, mice lacking membrane-bound forms of FasL or TRAIL develop sarcomas and various blood cancers (Smyth et al., 2003; Swann and Smyth, 2007). TRAIL, in combination with retinoic acid derivatives (required to upregulate TRAIL receptors on cancer cells), can kill tumors by apoptosis and limit growth of microadenomas in ApcMin mice (Zhang et al., 2010a). Transgenic mice, that overexpress TRAIL in the skin, exhibit reduced tumorigenicity in the two-step model of skin carcinogenesis (Kedinger et al., 2011). Inhibition of NF-κB activation by TNF, which acts as a pro-survival cytokine, renders metastatic colon cancer cells susceptible to the cytocidal action of TRAIL (Luo et al., 2004). Cytokines, such as IFN-γ and IL-12, rarely kill cancer cells directly, but are required for activation of immunosurveillance, including natural killer (NK) cells and cytotoxic T cells (Dunn et al., 2004). Mice deficient in components of IFN-γ signaling are susceptible to induction of sarcomas by methylcholanthrene and other malignancies, including colitis associated cancer, and also demonstrate increased rates of experimental metastasis (Shankaran et al., 2001; Swann and Smyth, 2007). However, mice lacking both IFN-γ and granulocyte macrophage-colony stimulating factor (GM-CSF) exhibit inflammation and spontaneous development of lymphomas and solid tumors due to chronic bacterial infections (Enzler et al., 2003). Type I IFNs exhibit multifaceted anti-tumorigenic effects via several distinct mechanisms. First, they restrain proliferation or induce death of pre-malignant cells, regardless whether such cells are infected with oncogenic viruses or not. Second, type I IFNs upregulate expression of stress-induced surface molecules, such as ligands for γδT cells and NK cells, as well as major histocompatibility complex (MHC) class I molecules, subjecting IFN-exposed cancer cells to enhanced immunosurveillance (Dunn et al., 2005). A third and largely unexplored mechanism of type I IFN action lies in their ability to suppress inflammation, particularly tumor associated inflammation.
TNF, as its name indicates, was discovered as a death cytokine that induces lysis of sarcoma cells (Carswell et al., 1975). Binding of TNF to its type I receptor (TNFR1) can lead to caspase 8 activation, which can trigger apoptosis, or receptor-interacting protein (RIP) 3 activation, which can lead to necrosis (Kaiser et al., 2011; Oberst et al., 2011). In addition, TNFR1 engagement leads to IκB kinase (IKK) and NF-κB activation, which inhibit apoptosis, as well as c-jun N-terminal kinase (JNK) activation which can potentiate both forms of cell death and stimulate production of ROS (Chang et al., 2006; Ventura et al., 2004). TNF can induce the regression of certain tumors when administered at high doses, most likely through the killing of endothelial cells and thereby starving the tumors of blood supply (Balkwill, 2009). However, there is also ample evidence that TNF is a potent tumor promoter (Balkwill, 2009). Importantly, TNF can directly activate pro-survival NF-κB signaling in cancer cells, as demonstrated in a model of inflammation-dependent cholestatic liver cancer (Pikarsky et al., 2004). A similar effect has been demonstrated for the TNF-related cytokine lymphotoxin (LT), whose overexpression in liver leads to development of HCC in a manner dependent on IKKβ signaling (Haybaeck et al., 2009). TNF can also stimulate the proliferation of pre-malignant cells, most likely through its ability to activate AP-1 transcription factors (Balkwill, 2009). In addition, TNF has a potent pro-tumorigenic effect in ovarian and colitis-associated cancer, via indirect effects on immune and stromal cells in the microenvironment (Charles et al., 2009; Popivanova et al., 2008). A pro-tumorigenic effect has also been demonstrated for the TNF family member BAFF, whose overexpression in Eμ-Myc transgenic mice leads to rapid development of chronic lymphocytic leukemia (Zhang et al., 2010b). Thus, the pro-tumorigenic effect of anti-apoptotic cytokines is not limited to solid malignancies and is also observed in tumors of hematopoietic origin.
Another well-established pro-tumorigenic cytokine is IL-6 (Naugler and Karin, 2008). IL-6 exerts its pro-tumorigenic activities by enhancing the survival and proliferation of pre-malignant cells, as first demonstrated in colitis associated cancer (Becker et al., 2004; Bollrath et al., 2009; Grivennikov et al., 2009). Most of the pro-tumorigenic effects of IL-6 depend on activation of STAT3, a transcription factor found to be activated in many cancers, including those of the colon, stomach, breast and prostate (Yu et al., 2009). STAT3 exerts its pro-survival and anti-apoptotic activities through transcriptional activation of classical pro-survival genes, such as Bcl-XL, as well as genes involved in maintenance of epithelial sheet integrity and antimicrobial immunity such as Hsp70 and RegIII (Bollrath et al., 2009). Loss of barrier integrity as demonstrated by the deletion of p120-catenin, an adhesion protein whose expression is downregulated in human gastric cancer, can result in epithelial cell death and formation of a pro-tumorigenic and inflammatory microenvironment (Stairs et al., 2011). Similar effects have been observed during the development of pancreatic cancer (Fukuda et al., 2011; Lesina et al., 2011). The exact mechanisms by which loss of adhesion proteins results in cell death are not entirely clear, but it is tantalizing to speculate that diminished cell adhesion can lead to cell death by anoikis, a type of cell death caused by the loss of cell to cell contact. Taken together, cytokines produced in response to injury and cell death have a profound effect on cell death vs. survival decisions, thereby contributing to tumor initiation, growth, progression and metastasis, as well as to the response to anti-tumor therapy.
Liver cancer- a death driven cancer
Two important hallmarks of cancer are uncontrolled cell proliferation and the ability to avoid programmed cell death (Hanahan and Weinberg, 2000). Accordingly, apoptotic cell death is believed to be a major tumor suppressive mechanism. Indeed, many of the examples outlined above support this concept. However, tumor suppression by apoptosis is not universal and several cancers, especially liver cancer, are caused by cell death. To induce neoplasia, oncogenic mutations or epigenetic modifications need to be fixed within subsequent cell generations and therefore genetically altered cells have to divide before giving rise to cancer (Figure 3). While this is not a major obstacle in rapidly renewing epithelia, many tissues, such as the liver, exhibit very low rates of cell proliferation. In fact, adult liver parenchymal cells are synchronized at the G0 phase of the cell cycle and can be evoked to proliferate only in response to tissue damage or debulking (Luedde and Schwabe, 2011). Not surprisingly, HCC, the major form of adult liver cancer, almost invariably develops in the context of chronic liver inflammation linked to injury and cell death and caused by infection with HBV or HCV, chronic alcohol consumption, excessive hepatosteatosis or exposure to environmental toxins (El-Serag and Rudolph, 2007). Initially, it was assumed that NF-κB activation may promote the development of chemically-induced HCC by suppressing p53-induced apoptosis, as found in tissue culture studies (Tergaonkar et al., 2002). We were therefore surprised to find that inactivation of liver NF-κB through the cell type specific ablation of IKKβ leads to a marked enhancement of hepatocarcinogenesis in DEN-treated mice (Maeda et al., 2005). Similar observations were made in mice with a hepatocyte-specific deletion of the stress activated protein kinase p38α (Hui et al., 2007; Sakurai et al., 2008). IKKβ-mediated NF-κB and p38α-regulated Hsp25 expression are critical for prevention of excessive ROS accumulation and thus are important for the survival of pericentral hepatocytes that are engaged in DEN metabolism (Maeda et al., 2005; Sakurai et al., 2008). Thus, in the absence of either NF-κB or p38α, DEN-exposed hepatocytes undergo excessive cell death, resulting in an enhanced regenerative response and compensatory proliferation of surviving hepatocytes. We have therefore proposed that enhanced compensatory proliferation is the major cause of augmented HCC development in both IKKβ- and p38α-hepatocyte specific knockout mice (Karin, 2006). The regenerative response that leads to compensatory hepatocyte proliferation depends on the release of IL-1α by dying hepatocytes and the activation of the adaptor MyD88-dependent IL-1R signaling in resident liver macrophages (Kupffer cells), leading to IL-6 production by the latter (Naugler et al., 2007; Sakurai et al., 2008). Strikingly, hepatocyte specific deletion of IKKγ (NEMO), the regulatory subunit of the IKK complex (Luedde et al., 2007) or TAK1, an upstream kinase required for NF-κB activation (Bettermann et al., 2010; Inokuchi et al., 2010) result in spontaneous liver damage, inflammation and hepatosteatosis which eventually lead to HCC development in the absence of any exogenous carcinogen. Curiously, tumor development in the absence of TAK1 is reported to be NEMO-dependent and TAK1 can gain pro-tumorigenic activity in the absence of NEMO, underscoring their potential role as both tumor promoters and tumor suppressors (Bettermann et al., 2010). These opposing activities of TAK1 and NEMO are likely to be NF-κB independent and may involve activation of other transcription factors, such as AP-1 and SMADs.
Figure 3. The critical pro-tumorigenic function of cell death.
Sentinel immune cells are present in normal tissue, but they do not produce inflammatory cytokines and do not promote tumorigenesis. (A) Injury and cell death induce inflammation which precedes or accompanies tumor development, while immune cells and epithelial cell mutations without injury do not cause cancer (1) Normal tissue (2). Inflammation results in robust tissue infiltration by immune cells, but without extensive tissue injury and cell death, tumor promotion does not take place. (3) Tissue injury and epithelial cell death in combination with inflammation result in an enhanced regenerative response, leading to the proliferation of stem cells that may harbor oncogenic mutations. Immune cells, in turn, maintain chronic inflammation and cause additional tissue injury. This leads to tumor initiation and promotion and further enhancement of tumor progression and metastasis and modulation of anti-tumor therapy response by inflammation (4). (B) NB Inflammation can regulate tumor promotion, progression and metastasis by other mechanisms, which do not invoke tissue damage and cell death. These mechanisms rely on cancer cells recruiting immune cells to the tumor microenvironment as a part of tumor-elicited inflammation.
Consistent with the pro-tumorigenic function of hepatocyte death, mice that are specifically devoid of the death receptor Fas on hepatocytes exhibit a substantial decrease in DEN-induced hepatocarcinogenesis (Chen et al., 2010). To fully appreciate the importance of these findings, it should be noted that in many other cell types, especially in lymphocytes and their precursors, Fas has been shown to act as a tumor suppressor (Peter et al., 2007). It should also be noted that administration of DEN to mice that are older than one month of age does not lead to HCC induction, unless combined with a tumor promoter, such as phenobarbital or carbon tetrachloride (CCl4) (Maeda et al., 2005). Congruently, feeding of mice with a high fat diet (HFD) for 3 months prior to DEN administration greatly enhanced DEN-induced ROS accumulation and hepatocyte death, resulting in HCC induction in the absence of other tumor promoters (Park et al., 2010). In addition to enhancing DEN-induced liver damage, consumption of HFD results in elevated expression of TNF and IL-6, both of which promote HCC development (Park et al., 2010). Paradoxically, while inducing hepatocyte proliferation IL-6 also potentiates DEN-induced hepatocyte death (Naugler et al., 2007). The latter effect may be due to IL-6-induced neutrophil recruitment. Such observations may also explain why hepatocyte-specific Stat3 null mice are protected from DEN-induced HCC (He et al., 2010), but are highly susceptible to HCC induction upon challenge with CCl4, a liver-damaging agent that does not cause HCC by itself (Wang et al., 2011). A number of retrospective studies suggest that IL-6 is also involved in the pathogenesis of human HCC and inflammatory liver adenomas (Naugler and Karin, 2008; Rebouissou et al., 2009).
As mentioned above, NF-κB signaling has a dual role in hepatocarcinogenesis. While enhancing DEN-induced HCC development, inhibition of liver NF-κB activity can attenuate inflammation-induced HCC that is not accompanied by obvious liver injury (Haybaeck et al., 2009; Pikarsky et al., 2004). Indeed, there is substantial heterogeneity in respect to NF-κB activation in human HCC. While the majority of HCCs appear to be devoid of nuclear RelA, (a subunit of the NF-κB multiprotein complex) about 25% of such tumors exhibit clear NF-κB activation, suggesting that even in human liver, NF–κB can assume both anti-tumorigenic and pro-tumorigenic functions (He et al., 2010).
Cell death and skin cancer
Death-promoted tumorigenesis is not unique to the liver as injury can play both tumor suppressive and tumor promoting effects in the skin. Squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are the most prominent non-melanoma forms of skin cancer and two of the most common cancers worldwide (Domingo and Baron, 2008). Inflammatory processes are important for induction, promotion and progression of both SCC and BCC. However, as the incidence of these cancers is higher in immunocompromised individuals, the immune system and inflammation may have dual roles in skin carcinogenesis. Death promoting environmental challenges, including UV irradiation, tobacco smoke and betel nut chewing are major contributors to the etiology of SCC (Rudolph and Zelac, 2004). The skin consists of different cell types and serves as a critical barrier that protects us from microbes, harmful chemicals and dehydration. Skin integrity is maintained by the ongoing division of stem cells and transient amplifying cells in its basal layer, which give rise to more differentiated cell types that eventually undergo enucleation to form the cornified cell bodies that make up the outermost layer.
As in the liver, chronic stress and injury, as well as defects in normal wound healing can cause skin cancer (Rudolph and Zelac, 2004). For instance, repetitive exposure to UV radiation causes skin injury, cell death and leads to tumor development. Although UV radiation is a potent DNA damaging agent and a mutagen, not all of its effects depend on DNA damage. The TGF-β-SMAD4 signaling pathway is required for efficient epithelial wound healing and a conditional deletion of SMAD4 in the epidermis results in severe defects in skin wound healing that are accompanied by spontaneous skin lesions, inflammation and SCC (Owens et al., 2010). Likewise and as seen in liver, inhibition of epidermal NF-κB activation results in enhanced skin tumorigenesis (Dajee et al., 2003). However, ablation of the NF-κB activating cytokine TNF inhibits two-stage skin cancer carcinogenesis (Moore et al., 1999). As discussed above, TNF is likely to exert its pro-tumorigenic activity through the transcription factor AP-1 while ablation of the transcription factor c-Jun inhibits skin tumorigenesis (Schonthaler et al., 2011). However, ablation of both c-Jun and JunB results in psoriatic inflammation (Schonthaler et al., 2011). Notably, despite being a chronic inflammatory condition linked to increased production of TNF, psoriasis is associated with protection from skin tumorigenesis (Nickoloff et al., 2005), presumably because psoriasis does not cause excessive tissue injury and cell death (see below). Another transcription factor involved in psoriasis is STAT3, which can be activated by a large variety of cytokines, including EGF, HB-EGF, IL-22 and IL-6, which can be particularly induced by stresses and cell injury. STAT3 integrates pro-survival signals from these cytokines and its ablation in the epidermis inhibits two-stage skin carcinogenesis (Kataoka et al., 2008).
Skin damage can result in the release of self-antigens and DAMPs, leading to activation of innate and adaptive immunity and stimulation of T and B cells responses (de Visser et al., 2005). In K14-HPV16 transgenic mice, which overexpress human papilloma virus (HPV) 16 proteins E6 and E7 in keratinocytes and spontaneously develop skin papillomas, antibody-producing B cells lead to activation of mast cells via Fc receptors, thereby triggering a local inflammatory response that stimulates tumor progression (Andreu et al., 2010; de Visser et al., 2005). In a two-step skin carcinogenesis model, B cells producing IL-10 promote papilloma growth by inactivating anti-tumor immunity represented by cytotoxic T cells and the anti-tumorigenic cytokine IFN-γ (Schioppa et al., 2011). The DAMPs HMGB1 and B2 are upregulated during SCC progression, and may be required for establishment of chronic inflammation, cancer invasion and metastasis (Sharma et al., 2008). The identity of the antigens which activate the adaptive immune response in skin injury and cancer remains unknown, although self-antigens, such as chitinase-like proteins (Qureshi et al., 2011) and antigens derived from the skin bacterium Staphylococcus aureus (Daniel et al., 2003) have been implicated. T cell responses play a dual role in skin tumorigenesis. While IL-12 and IFN-γ-driven cytotoxic T cells inhibit initiation and progression of skin cancer, IL-23 and IL-17 mediated responses promote skin tumorigenesis (Langowski et al., 2006).
As mentioned above, certain types of chronic skin inflammation, such as psoriasis or atopic dermatitis, do not increase the risk of skin cancer development. It is tempting to speculate that they fail to do so because no prominent tissue damage and subsequent regeneration are inflicted under these conditions. It is also plausible that chronic injury also increases the exposure of normal cells to environmental mutagens and carcinogens, which are normally kept at bay by the skin’s barrier function. Given the physiology and anatomy of the epidermis, which consists of stem cells, rapidly dividing basal cells and differentiated cells, which eventually undergo enucleation and death, a key question is when and not whether these keratinocytes will eventually die. Therefore, keratinocyte death is probably not a major suppressor of skin tumorigenesis. Rather, the continuous death of differentiated keratinocytes results in expansion of transient amplifying cells, some of which may harbor oncogenic mutations, thereby promoting tumorigenesis. Thus, unless cell death is restricted to transformed cells harboring oncogenic mutations, it is not universally tumor suppressive. In the case of melanoma, tumor development does not seem to depend on earlier tissue injury and cell death while tumor-cell specific NF-κB activation promotes tumorigenesis (Yang et al., 2010). On the other hand, activation of melanocyte precursors by UVB light, a typical stress stimulus, and IFNγ, promotes the recruitment of immune cells and enhances melanoma development in an animal model (Zaidi et al., 2011). Here, IFNγ provides pro-survival and pro-tumorigenic signals to initiated melanocytes.
Concluding Remarks
Chronic tissue damage and inflammation have long been suspected for their ability to promote cancer development and progression, but only recently was the incriminating “smoking gun” identified through the extensive use of physiologically relevant mouse models. Importantly, the experimental evidence obtained in mice is strongly supported by correlative and retrospective analysis of human clinical and epidemiological data. One of the earliest experiments that underscored the importance of tissue injury and cell death in tumorigenesis was the demonstration that v-Src cannot induce cancer unless accompanied by tissue injury and subsequent regeneration (Sieweke et al., 1990). Likewise, experimental pancreatic injury is required to unravel the oncogenic potential of activated K-Ras in the postnatal pancreas (Guerra et al., 2007). In lung cancer, tissue injury inflicted by tobacco smoke contributes to disease development, while inhibition of cell death related pathways (JNK) or death-induced pro-inflammatory cytokines (TNF and IL-6) dampens tumor progression (Takahashi et al., 2010). Disruption of epithelial homeostasis by deletion of p120 catenin induces esophageal cancer (Stairs et al., 2011). Even inflammatory bowel diseases, such as ulcerative colitis (UC) and Crohn’s disease (CD), that are characterized by massive epithelial damage and inflammation are known to elevate colon cancer risk (Saleh and Trinchieri, 2011). Likewise, mucosal injury induced by dextrane sulfate sodium (DSS) or by oxazolone is required for induction of colitis-associated cancer in mice treated with the pro-carcinogen azoxymethane (AOM) (Greten et al., 2004; Schiechl et al., 2011). Taken together, these and many other experiments illustrate the pro-tumorigenic activity of cell death and together with clinical and epidemiological findings support the notion that a substantial fraction of all cancer cases are likely to be initiated and promoted by chronic tissue injury (Figure 3). These conclusions also suggest that therapeutic approaches that minimize injury and restore normal tissue homeostasis can be used in cancer prevention.
Acknowledgments
A.K. was supported by a postdoctoral fellowship from the American Cancer Society. S.G. was supported by Crohn’s and Colitis Foundation of America (CDA #2693); K99 Pathway to Independence award from NIDDK NIH (1K99DK088589-01A1) and a UCSD DDRDC Pilot Grant (DK080506). M.K. is an American Cancer Society Research Professor and research in his lab is supported by the NIH and the American Association for Cancer Research.
Footnotes
The authors also apologize to those whose papers were not cited due to space restrictions and declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, Andersen P, Gross N, Olson S, et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 2009;119:3408–3419. doi: 10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010;67:1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–5786. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, Peter ME. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58:534–541. [PMC free article] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, Hanahan D. Immune enhancement of skin carcinogenesis by CD4+ T cells. J Exp Med. 2003;197:1017–1028. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo DS, Baron ED. Melanoma and nonmelanoma skin cancers and the immune system. Adv Exp Med Biol. 2008;624:187–202. doi: 10.1007/978-0-387-77574-6_15. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol. 2006;291:F578–587. doi: 10.1152/ajprenal.00455.2005. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–1219. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature medicine. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Gulley J, Figg WD, Dahut WL. Treatment options for androgen-independent prostate cancer. Clin Adv Hematol Oncol. 2003;1:49–57. [PubMed] [Google Scholar]
- Haddow A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Adv Cancer Res. 1972;16:181–234. doi: 10.1016/s0065-230x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O’Sullivan B, He Z, Peng Y, Tan AC, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nature genetics. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Inflammation and cancer: the long reach of Ras. Nat Med. 2005;11:20–21. doi: 10.1038/nm0105-20. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Kim DJ, Carbajal S, Clifford JL, DiGiovanni J. Stage-specific disruption of Stat3 demonstrates a direct requirement during both the initiation and promotion stages of mouse skin tumorigenesis. Carcinogenesis. 2008;29:1108–1114. doi: 10.1093/carcin/bgn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger V, Muller S, Gronemeyer H. Targeted expression of tumor necrosis factor-related apoptosis-inducing ligand TRAIL in skin protects mice against chemical carcinogenesis. Mol Cancer. 2011;10:34. doi: 10.1186/1476-4598-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, et al. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19:1030–1032. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Owens P, Engelking E, Han G, Haeger SM, Wang XJ. Epidermal Smad4 deletion results in aberrant wound healing. Am J Pathol. 2010;176:122–133. doi: 10.2353/ajpath.2010.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AM, Hannigan A, Campbell D, Nixon C, Wilson JB. Chitinase-like proteins are autoantigens in a model of inflammation-promoted incipient neoplasia. Genes Cancer. 2011;2:74–87. doi: 10.1177/1947601911402681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R, Zelac DE. Squamous cell carcinoma of the skin. Plast Reconstr Surg. 2004;114:82e–94e. doi: 10.1097/01.prs.0000138243.45735.8a. [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schiechl G, Bauer B, Fuss I, Lang SA, Moser C, Ruemmele P, Rose-John S, Neurath MF, Geissler EK, Schlitt HJ, et al. Tumor development in murine ulcerative colitis depends on MyD88 signaling of colonic F4/80+CD11b(high)Gr1(low) macrophages. J Clin Invest. 2011;121:1692–1708. doi: 10.1172/JCI42540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl1):i109–112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest. 2008;26:843–851. doi: 10.1080/07357900801954210. [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248:1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature’s TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a bona fide tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer research. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes & development. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lafdil F, Wang L, Park O, Yin S, Niu J, Miller AM, Sun Z, Gao B. Hepatoprotective versus Oncogenic Functions of STAT3 in Liver Tumorigenesis. Am J Pathol. 2011;179:714–724. doi: 10.1016/j.ajpath.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Splittgerber R, Yull FE, Kantrow S, Ayers GD, Karin M, Richmond A. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. The Journal of clinical investigation. 2010;120:2563–2574. doi: 10.1172/JCI42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, Lynch PM, Moyer MP, Wen XF, Wu X. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010a;464:1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kater AP, Widhopf GF, 2nd, Chuang HY, Enzler T, James DF, Poustovoitov M, Tseng PH, Janz S, Hoh C, et al. B-cell activating factor and v-Myc myelocytomatosis viral oncogene homolog (c-Myc) influence progression of chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:18956–18960. doi: 10.1073/pnas.1013420107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]