Abstract
Osteoporosis related fractures contribute to morbidity and mortality in U.S. patients, placing a heavy financial burden on society. Randomized clinical trials involving over 30000 subjects have established bisphosphonates’ efficacy in reducing the incidence of fragility fractures. However, as bisphosphonates are retained for years in the skeleton, reports of adverse events from prolonged use are surfacing in the literature, namely, esophageal cancer, atrial fibrillation, osteonecrosis of the jaw, and atypical fracture development. The concept of a drug holiday has been proposed to potentially reduce incidence of these adverse events. This review will highlight the benefits and risks of bisphosphonate therapy and discuss the extension data available from the bisphosphonate trials. As randomized clinical trial evidence is not yet available on who may qualify for drug holiday, this review will provide suggestions for clinicians on identification of possible candidates and monitoring during a bisphosphonate drug holiday.
Keywords: osteoporosis, bisphosphonate, drug holiday, atypical fracture
Introduction
Osteoporosis is a growing health problem in the United States. In 2002 more than 10 million Americans had diagnosed osteoporosis, 80% of whom were postmenopausal women [1]. The incidence of osteoporosis or osteopenia increases with age and by age 60 years, half of Caucasian women in this country are predicted to be affected with this disease. In 2005, 2 million fractures were attributed to osteoporosis, incurring more than $16.9 billion in medical expenses. As the number of individuals older than 50 years of age is predicted to reach 121 million people by 2025, the incidence of fractures is projected to further increase to over 3 million, incurring $25.3 billion in cost [2]. In addition, there are significant indirect costs, including loss of productivity, need for caregiver time, and use of other social services. More than half of patients will remain with some degree of permanent disability, with women carrying a 17% risk of mortality in the first year after fracture [3].
Since the 1960’s, bisphosphonates have become the most widely used agent for treatment of osteoporosis and have helped decrease incidence of osteoporosis related fractures, with a vertebral fracture risk reduction ranging from 40–70% and relative hip fracture reduction of 40–50% [4–7]. However, a number of questions have been raised recently on duration of use in light of increasing reports on the potential side effects of these medications. Common side effects include gastrointestinal intolerance, while more serious adverse events such as gastritis, esophageal cancer, osteonecrosis of the jaw, atypical femoral fractures, and atrial fibrillation have been observed. Although the results from these retrospective studies are conflicting, they have heightened awareness of the complications from long term bisphosphonate use and have led to consideration of a drug holiday in select patients. With extension trial data now available for longer use of bisphosphonates, it may be possible to discontinue bisphosphonates to prevent the adverse events while still maintaining their benefits in preventing fractures. This article will review the evidence that supports a bisphosphonate drug holiday and offer suggestions in identifying appropriate candidates and monitoring during that interval.
Adverse events associated with bisphosphonates
Oral bisphosphonates have been commonly associated with gastrointestinal intolerance and may lead to development of esophagitis, esophageal ulcers, and esophageal strictures, which could potentially heighten the risk for esophageal cancer [8]. In a UK case-control study, patients on bisphosphonates for more than 3 years had a relative risk of 2.24 of developing esophageal cancer compared to those not on bisphosphonates [9]. However, the UK General Practice Research Database study did not confirm these findings [10].
Osteonecrosis of the jaw (ONJ) is a rare complication of bisphosphonate therapy first reported in a series of 36 patients treated with high doses of IV bisphosphonates for skeletal complications of malignancy [11]. Since then, there have been additional reports of ONJ developing in patients on bisphosphonate therapy for osteoporosis though ninety percent of ONJ cases are in oncology patients receiving doses at 10-fold higher levels, usually intravenously, than those used in osteoporosis therapy. In both oral and intravenous bisphosphonate extension trials for treatment of osteoporosis, ONJ was not found to be associated with long term use of these drugs [12–14]. In an executive statement, the American Dental Association stated that the benefit provided by bisphosphonate therapy outweighed the low risk of developing ONJ [13].
The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) study raised concern regarding atrial fibrillation when the investigators noted a higher incidence of arrhythmias in the study group than in the placebo group [15]. In the Fracture Intervention Trial (FIT), a non-statistically significant increased risk of atrial fibrillation was also identified in the study group [16]. However, other extension studies, subsequent meta-analyses, and database studies did not confirm this association [12, 17, 18]. As such, the FDA did not recommend altering prescribing patterns for bisphosphonates due to this potential side effect.
There has been mounting concern regarding development of atypical femoral fractures in patients on bisphosphonate therapy. In a Swedish population-based nationwide study, the 59 patients identified with atypical fractures had a 47% relative risk of fracture with bisphosphonate use compared to nonusers (95% CI, 25.6–87.3). Use of bisphosphonates for more than two years conferred a 67% relative risk compared to 17% in those with shorter duration of use. The difference in risk of atypical fracture between bisphosphonate users and nonusers was 5 cases per 10,000 patient-years, corresponding to a number needed to harm of 2000 per year of use. After drug withdrawal, the risk decreased by 70% per year since last use [19].
In a cohort study of the Kaiser Permanente Southern California database, 142 patients with atypical femoral fractures were identified from a pool of 1.8 million patients. Ninety percent of patients with atypical fractures had a history of bisphosphonate use ranging from 1 month to 13 years, with the incidence of atypical fractures higher with increasing duration of use. Age adjusted incidence rates of fracture increased from 1.78/100000 person/year (CI 1.5–2.0) after use of bisphosphonates for 0.1–1.9 years to an incidence rate of 113/100000 person/year (CI 69.3–156.8) with bisphosphonate exposure of 8–9.9 years [20].
A secondary analysis of the FIT, FIT Long Term Extension (FLEX), and HORIZON Pivotal Fracture Trial (HORIZON-PFT) identified 12 subtrochanteric/diaphyseal femur fractures, a rate of 2.3 per 10000 patient-years [21]. While these studies were underpowered for this outcome and the risk is low, there was an indication from published studies of an association of bisphosphonates and atypical femoral fractures. In 2010, the FDA updated the labeling of bisphosphonates with a statement that though it was not clear if these drugs were the cause of atypical fractures, these fractures have been predominantly reported in patients taking these medications. The FDA recommended patients to continue taking their medications unless told to stop by their healthcare professional [22].
Long term effects of bisphosphonates after discontinuation
The next question is whether stopping bisphosphonates to reduce the risk of complications is safe in regard to maintaining adequate prevention of developing osteoporotic fractures. Indeed, bisphosphonates are deposited in bone for at least ten years, and when bone containing bisphosphonate is resorbed, the bisphosphonate recirculates locally and systemically and is able to bind again to bone surfaces. Bone resorption continues to be inhibited over time, and the antiresorptive effect persists after the drug has been stopped [23].
Two trials, FLEX and HORIZON-PFT, evaluated the persistent effects of bisphosphonates after discontinuation (Table 1). In the FLEX trial, subjects on Alendronate for more than 5 years were randomized either to continue Alendronate therapy for a total of 10 years or to discontinue therapy after 5 years. Those who discontinued Alendronate experienced decreases in BMD without an increase nonvertebral fractures though the risk of clinically recognized vertebral fractures was higher compared to continuous Alendronate users [24]. In the latter group, the cumulative effect of Alendronate/placebo for 10 years was positive (1–3% gain of BMD), compared to a 5–10% loss expected from observational studies of untreated women of similar age [24]. Biochemical markers for bone turnover, such as serum C-terminal telopeptide of type 1 collagen, N-propeptide of type 1 collagen (Ntx), and bone-specific alkaline phosphatase, remained at levels close to baseline in the continuous treatment group, whereas the placebo group showed a gradual rise in markers over the 5 year period. Despite this, the levels in the placebo group were noted to be below pretreatment levels. Therefore, it was concluded that Alendronate maintained a residual effect after 5 years of therapy for at least another 5 years after discontinuation of the drug [24].
Table 1.
Efficacy of bisphosphonates from extension trials
| Study | Drug | Extension time | Mean difference in BMD in bisphosphonate vs placebo | Fracture risk reduction in continuous bisphosphonate group |
|---|---|---|---|---|
| FLEX trial [24] | Alendronate | 5 years | Total hip: 2.4% Femoral neck: 1.9% Lumbar spine: 3.7% |
Clinical vertebral: RR 0.45 (CI 0.24–0.85) Morphometric vertebral: RR 0.86 (CI 0.60–1.22) |
| HORIZON-PFT [25] | Zoledronic acid | 3 years | Femoral neck: 1.04% Total hip: 1.22% Lumbar spine: 2.03% |
Morphometric vertebral: OR 0.51 (CI 0.26–0.95) Clinical vertebral: HR 1.81 (CI 0.53–6.2) Nonvertebral: HR 0.99 (CI 0.7–1.5) |
In HORIZON-PFT, patients were randomized to stop therapy or to continue on yearly Zoledronic acid for 3 additional years. Although the placebo group experienced a reduction in femoral neck BMD, BMD was higher than pretreatment levels. Morphometric vertebral fractures were lower in those who remained on Zoledronic acid. Further, biochemical markers increased after 3 years off treatment but remained below pretreatment levels [25].
Consideration for drug holiday
The above studies demonstrate that there is a residual effect of bisphosphonates after discontinuation. Therefore, it has been suggested that physicians could consider a drug holiday after long term use of bisphosphonates in select patients. The main challenges to this decision are to determine the duration of bisphosphonate therapy, to assess who qualifies for drug holiday, and to know how long to remain off therapy. Currently, an observational study is in process to compare the effects on bone quantity and quality during a two year drug holiday after 2 years of bisphosphonate use [26]. However, until these results are available and randomized controlled clinical trials are published, physicians are asked to use their clinical judgment to determine who qualifies for drug holiday.
One group proposed to stratify patients based on their known or potential risk of fracture [27]. High risk patients include patients with prior history of fractures, low T-scores, and secondary osteoporosis due to use of chronic glucocorticoids or from diseases such as hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, or other disease states than can cause severe immobility such as multiple sclerosis. In such patients, it was recommended to continue bisphosphonate therapy for up to 10 years, and then a drug holiday could be considered. During this time, use of other agents such as Teriparatide or Raloxifene may be warranted. In moderate risk patients, they suggest drug holiday after 5–10 years and in low risk patients after 3–5 years of bisphosphonate therapy. Bisphosphonates should then be resumed if BMD decreases or the patient fractures. Duration of a drug holiday is unknown, but the authors propose 1–2 years in high risk patients, 3–5 years in moderate risk, and indefinitely in low risk patients [27].
Recently, the FDA issued an advisory to provide additional guidance to physicians [28]. The FDA analyzed the 3 long-term extension trials- FLEX, HORIZON-PFT, and VERT-MN-- which reported treatment-related increases in BMD through treatment years 6–10. Although the primary endpoint in the extension trials was BMD, the FDA analyses included both BMD and fracture outcomes (vertebral and nonvertebral). In their review of FLEX, the panel reported that the rates of vertebral and nonvertebral osteoporotic fractures were similar whether participants continued to receive Alendronate for up to 10 years or were switched to placebo. Also, the panel noted that fracture rates appear to be constant when all data on vertebral and nonvertebral osteoporotic fractures with long-term therapy are pooled from the 3 extension trials. For example, in patients who received continuous bisphosphonate treatment for at least 6 years, fracture rates ranged from 9.3–10.6%, whereas the rate for patients switched to placebo was 8.0–8.8% [28]. These data question the benefit of extended therapy with bisphosphonates after 5 years for fracture prevention. Therefore, the FDA recommended periodic assessment of a patient’s need for continued therapy, taking into account their individual risk and benefit and patient preference. Low risk patients may qualify for drug holiday after 3–5 years while patients at higher risk of fracture may benefit from continued bisphosphonate therapy [28].
The response to the FDA advisory commented that the FDA analysis focused on the composite end point of all fractures, both vertebral and non-vertebral, when the original preplanned analyses of the extension studies separated vertebral and nonvertebral fractures because of “their distinct pathogenesis and different responses to treatment” [29]. According to their analysis, risk for vertebral fractures was shown to be reduced with continued bisphosphonate therapy beyond 3–5 years while evidence was lacking for nonvertebral fractures. Data from FLEX was used to estimate the number needed to treat (for 5 additional years) to prevent one clinical vertebral fracture (in subgroups defined by BMD at the femoral neck and by prevalent vertebral fractures status at entry in FLEX) (Table 2). Those with T score of less than −2.5 appeared to benefit from continued use of bisphosphonates while those with T-score > −2.5 maintain fracture prevention during a drug holiday if they do not have a history of a vertebral fracture [30].
Table 2.
Risk of clinical vertebral fracture and number needed to treat to prevent one clinical vertebral fracture for 5 years in FLEX study. Adapted from reference [29]
| Femoral neck BMD T score at start of extension trial | Number needed to treat |
|---|---|
| Women with NO previous vertebral fracture at start of FLEX study | |
| Less than or equal to −2.5 | 24 |
| Greater than −2.5 and ≤ −2.0 | 63 |
| Greater than −2.0 | 102 |
| Women WITH prevalent vertebral fracture at start of FLEX study | |
| Less than or equal to −2.5 | 17 |
| Greater than −2.5 and ≤ −2.0 | 17 |
| Greater than −2.0 | 51 |
A factor guiding determination of drug holiday is the variable anti-resorptive potency and binding affinity of each bisphosphonate due to their unique side chains. Zoledronate has the highest potency, followed by risedronate, ibandronate, and alendronate. Binding affinity is highest for zoledronate and decreases in order of magnitude for alendronate, ibandronate, and risedronate, respectively [27, 31]. This may be due to a greater affinity of alendronate and zoledronate to hydroxyapatite compared to risderonate and ibandronate [32]. The skeletal binding sites for bisphosphonate are nearly unsaturable, thereby leading to a significant accumulation of bisphosphonates while release of bisphosphonates may be small, as it partly depends on bone turnover which is reduced by use of bisphosphonates [27]. For example, after 10 years of alendronate use at a dose of 10 mg daily (70 mg weekly), the amount of alendronate released over several months or years would be equivalent to taking ¼ of the usual dose [33]. In general, zoledronate and alendronate maintain a prolonged effect after discontinuation while others such as risedronate have a more rapid offset.[32]. The available extension data confirm these findings and lead to the proposal that a holiday from zoledronate may be considered after 3 years, for alendronate after 5 years, and potentially from risedronate after 5 years, while no data is yet available for ibandronate. Another factor to consider in drug holiday is demonstration of compliance with the therapy. The decision to go on drug holiday after 3–5 years should be after assurance of continuous use of bisphosphonates during the initial therapy period.
The Endocrine Society published a statement in response to the FDA advisory, reinforcing that clinicians engage in patient-specific dialogue about the appropriateness of long-term bisphosphonate use in their patient population [34]. This discussion should take into account the length of time patients have been on bisphosphonates, their compliance with therapy, the type of bisphosphonates used, their current T score, and their risk for vertebral fracture.
Monitoring during a drug holiday
When stopping bisphosphonate therapy for a drug holiday, there are a few potential parameters to monitor for continued bone loss. Most extension trials focused on annual DEXA scans to evaluate for loss of BMD after stopping treatment. One study showed that oral Alendronate stabilized bone mass at the spine, hip, and total body for five years, with the greatest effect during the first 1–2 years on therapy. Afterwards, the BMD plateaued or declined slightly, but remained at or above baseline values by the end of 5 years [35]. Once treatment was stopped, bone loss resumed at a yearly rate of 0.5–2%, comparable to the rate of bone loss in patients on placebo. There are as yet no clear guidelines as to what point establishes a “significant decrease” enough to resume therapy again, but it is assumed that when BMD reaches pre-treatment levels, i.e. osteoporotic levels, or the patient fractures (whichever comes first), therapy should then be resumed. Therefore, annual DEXA scans should be checked while off therapy to continue monitoring for BMD changes.
Response to osteoporosis therapies can also be monitored by measuring biochemical bone turnover markers (BTMs). These can be helpful as significant changes in these levels can be appreciated even after a few weeks of initiating therapy (whereas the changes in BMD are often delayed). Prospective studies have shown that elevated resorption markers predict an increased risk of fractures (vertebral, femur neck, and other peripheral fractures) in postmenopausal women [36].
Two potential markers of bone turnover are urinary collagen type 1 N and C telopeptide, as the largest decrease in BTMs is observed with these two markers [36]. There is an inverse correlation between change in spine BMD and change in the U-NTX/Cr. In addition, patients with a decrease in U-NTX/Cr of >54% subsequently had an increase in spine BMD of greater than 4.5% [37]. The higher the urinary NTX levels, the higher likelihood of bone turnover and possible risk of fracture [38]. If these markers will be monitored during a drug holiday, then baseline levels prior to starting bisphosphonate therapy should be checked and compared to levels during therapy and during a drug holiday.
There are a few caveats to use of these BTMs. There have not been many studies to evaluate the relationship of a single marker in predicting fracture risk. There is biologic variability and inconsistencies due to sample collection and handling in addition to intra-individual variation of BTMs [39]. For example, markers of bone turnover can be affected by circadian variation and feeding [40]. In addition, fractures, renal impairment, metastatic bone disease, and secondary causes of osteoporosis such as glucocorticoid use, primary hyperparathyroidism, and thyrotoxicosis can be associated with changes in BTMs [39]. Therefore, there is lack of standardization in terms of which specific marker to use and clear cut off values for these measurements.
The International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine recommend use of serum C-terminal telopeptide of type 1 collagen (s-CTX) as a marker of bone resorption and serum procollagen type 1 N propeptide (s-PINP) as a marker of bone formation. Both have been shown to have a more standardized, automated assay and reference range with well characterized biological and analytical variability. They have been studied in both fracture prevention and monitoring response to osteoporosis therapy [41].
Evaluating the change in BTMs in conjunction with annual DEXA scans may help guide the physician as to when to resume therapy again from a drug holiday. Currently, the appropriate duration of drug holiday is unknown. Fracture protection may wane after 3–5 years off bisphophonate though there yet may still be some residual effects [24, 25, 30]. One potential approach, though unvalidated, is to reassess after 1–3 years to see if CTX levels are in the mid range of young adults and to then reinitiate bisphosphonate therapy [14]. Physicians may opt to restart bisphosphonate therapy sooner than expected if BTMs levels were to significantly increase or reach pre-treatment levels and/or a decrease in BMD on DEXA scans is noted. On the other hand, if the markers are minimally changed from treatment levels and the BMD does not show a significant change, then potentially one may continue the holiday beyond 1–3 years [39, 42].
Conclusions
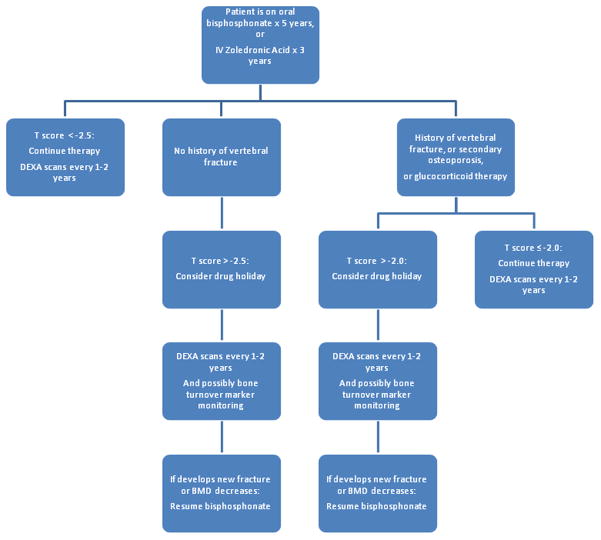
Though bisphosphonates are commonly used in prevention of fracture and treatment of osteoporosis in the United States, there remains much controversy surrounding its potential side effects from prolonged use. Until further evidence is published, clear recommendations regarding the length of use and drug holidays are not available. Based on current data, a potential algorithm to guide physicians is proposed (Figure 1). If the patient is at minimal risk for fractures, then a drug holiday potentially may continue with annual monitoring. If the patient has moderate to high risk of fractures, then the drug holiday can vary from 2–5 years depending on their BMD/BTM and fracture status. Furthermore, each bisphosphonate is unique, and the above recommendations may differ for each drug. Finally, it is uncertain at this point whether a drug holiday will prevent the possible complications such as gastrointestinal cancers, atypical fractures, ONJ, and atrial fibrillation.
Figure 1.
Proposed algorithm for selection of candidates for bisphosphonate drug holiday and principles of monitoring
In conclusion, physicians treating patients with osteoporosis should have a clear discussion with patients prior to starting therapy, explaining the benefits and possible risks involved. The decision on duration of therapy needs to be tailored to the individual patient, with annual assessments of their risk factors, BMD response to therapy, and potentially bone turnover markers. In this way, serious adverse events potentially may be prevented while at the same time striving to maximize benefit from the effective therapies for osteoporosis.
Footnotes
Disclosure
The authors reported no potential conflicts of interest relevant to this article.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.National Osteoporosis Foundation. America’s bone health: The state of osteoporosis and low bone mass in our nation. Washington, DC: 2002. [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Forsen L, Sogaard AJ, Meyer HE, et al. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–8. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 5.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 6.Chesnut IC, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 7.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 8.Haber SL, McNatty D. An evaluation of the use of oral bisphosphonates and risk of esophageal cancer. Ann Pharmacother. 2012;46:419–23. doi: 10.1345/aph.1Q482. [DOI] [PubMed] [Google Scholar]
- 9.Green J, Czanner G, Reeves G, et al. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardwell CR, Abnet CC, Cantwell MM, et al. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657–63. doi: 10.1001/jama.2010.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 12.Mellstrom DD, Sorensen OH, Goemaere S, et al. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462–8. doi: 10.1007/s00223-004-0286-7. [DOI] [PubMed] [Google Scholar]
- 13.Hellstein JW, Adler RA, Edwards B, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–51. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 14.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 16.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 17.Rhee CW, Lee J, Oh S, et al. Use of bisphosphonate and risk of atrial fibrillation in older women with osteoporosis. Osteoporos Int. 2012;23:247–54. doi: 10.1007/s00198-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 18.Barrett-Connor E, Swern AS, Hustad CM, et al. Alendronate and atrial fibrillation: a meta-analysis of randomized placebo-controlled clinical trials. Osteoporos Int. 2012;23:233–45. doi: 10.1007/s00198-011-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364:1728–37. doi: 10.1056/NEJMoa1010650. This study demonstrated the association of atypical femoral fractures with longer duration of bisphosphonate use. [DOI] [PubMed] [Google Scholar]
- 20.Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–50. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 21•.Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–71. doi: 10.1056/NEJMoa1001086. This was a secondary analysis of 3 RCTs showing that atypical fractures were a rare occurrence and were not associated with use of bisphosphonates. [DOI] [PubMed] [Google Scholar]
- 22.Fosamax, Fosamax Plus D, Actonel, Actonel with calcium, Boniva, Atelvia, and Reclast. Oct 13, 2010. FDA: Bisphosphonates (Osteoporosis drugs): Label change- Atypical fractures update. [Google Scholar]
- 23.Gibaldi M, Perrier D. Clinical Pharmacology of Alendronate Sodium. In: Gibaldi M, Perrier D, editors. Pharmacokinetics. New York: Marcel Dekker; 1982. pp. 145–198. [Google Scholar]
- 24.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 25••.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:243–54. doi: 10.1002/jbmr.1494. This study provided extension data for Zoledronic acid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed November 2012]; NCT01406613: Resolution of effect of bisphosphonates on bone in postmenopausal osteoporosis. www.Clinicaltrials.gov.
- 27.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–65. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 28•.Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis--where do we go from here? N Engl J Med. 2012;366:2048–51. doi: 10.1056/NEJMp1202619. This is the FDA advisory which reviewed extension data and discussed the concern regarding atypical femoral fractures. [DOI] [PubMed] [Google Scholar]
- 29••.Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012;366:2051–3. doi: 10.1056/NEJMp1202623. This is the response to FDA advisory and provides guidance in selecting appropriate candidates for drug holiday. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010;25:976–82. doi: 10.1002/jbmr.11. [DOI] [PubMed] [Google Scholar]
- 31.Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 32.Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Rodan G, Reszka A, Golub E, et al. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004;20:1291–300. doi: 10.1185/030079904125004475. [DOI] [PubMed] [Google Scholar]
- 34.The Endocrine Society Recommends Patient-Centered Approach to Long-Term Bisphosphonate Use for the Treatment of Osteoporosis: A review of the May 2012 FDA Analysis. 2012. [Google Scholar]
- 35.Ravn P, Weiss SR, Rodriguez-Portales JA, et al. Alendronate in early postmenopausal women: effects on bone mass during long-term treatment and after withdrawal. Alendronate Osteoporosis Prevention Study Group. J Clin Endocrinol Metab. 2000;85:1492–7. doi: 10.1210/jcem.85.4.6549. [DOI] [PubMed] [Google Scholar]
- 36.Bieglmayer C, Dimai HP, Gasser RW, et al. Biomarkers of bone turnover in diagnosis and therapy of osteoporosis: A consensus advice from an Austrian working group. Wien Med Wochenschr. 2012 doi: 10.1007/s10354-012-0133-9. [DOI] [PubMed] [Google Scholar]
- 37.Baxter I, Rogers A, Eastell R, et al. Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2097-4. [DOI] [PubMed] [Google Scholar]
- 38.Looker AC, Bauer DC, Chesnut CH, 3rd, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int. 2000;11:467–80. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Vasikaran S. Current recommendations for laboratory testing and use of bone turnover markers in management of osteoporosis. Ann Lab Med. 2012;32:105–12. doi: 10.3343/alm.2012.32.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenora J, Ivaska KK, Obrant KJ, et al. Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int. 2007;18:1297–305. doi: 10.1007/s00198-007-0379-z. [DOI] [PubMed] [Google Scholar]
- 41.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 42•.Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. These are current endocrine guidelines on management of osteoporosis. [DOI] [PMC free article] [PubMed] [Google Scholar]