Abstract
Background
Prosthetic graft infection is a major complication of peripheral vascular surgery. We investigated our institution’s experience over ten years with bypass grafts involving the femoral artery to determine the incidence and risk factors for prosthetic graft infection.
Methods
A retrospective cohort single institution review of prosthetic bypass grafts involving the femoral artery from 2001–2010 evaluated patient demographics, body mass index, comorbidities, indications, location of bypass, type of prosthetic material, case urgency, previous ipsilateral bypass or percutaneous interventions; and evaluated the incidence of graft infections, amputations, and mortality.
Results
There were 496 prosthetic grafts identified with a graft infection rate of 3.8% (n=19) at a mean follow-up of 27 months. Multivariable analysis shows that redo bypass (HR 5.8, 95% CI 2.2–15.0), active infection at time of bypass (HR 5.2, 95% CI 1.9–14.2), female gender (HR 4.5, 95% CI 1.6–12.7), and diabetes mellitus (HR 4.6, 95% CI 1.5–14.3) were significant predictors of graft infection. Graft infection was predictive of major lower extremity amputation (HR 9.8, 95% CI 3.5–27.1) as was preoperative tissue loss (HR 4.7, 95% CI 1.8–11.9). Graft infection did not predict long term mortality, however chronic renal insufficiency (HR 2.3, 95% CI 1.6–3.4), tissue loss (HR 1.4, 95% CI 1.0–1.9), and active infection (HR 2.3, 95% CI 1.6–3.4) did. Infected grafts were removed 79% of the time. Staphylococcus epidermidis (37%) and Methicillin-sensitive Staphylococcus aureus (26%) were the most common pathogens isolated.
Conclusions
Redo-bypass, female gender, diabetes, and active infection at time of bypass are associated with a higher risk for prosthetic graft infection and major extremity amputation, but do not confer an increased risk of mortality. Autologous vein for lower extremity bypass and endovascular interventions should be considered when feasible in high-risk patients.
Introduction
Surgical site infections after lower extremity bypass are common and potentially serious complications that can carry a high morbidity.1 Predictors of surgical site infections after surgical bypass include obesity, diabetes, poor preoperative functional status, a history of smoking, and female gender.2, 3 These surgical site infections occasionally lead to graft infection with rates of post-operative amputation and mortality as high as 52% and 58% respectively having been reported.4 These prosthetic graft infections are not infrequent with a reported incidence of up to 4.7%.5 Aside from the adverse effect on the patient, post-operative vascular surgical infections significantly contribute to health care costs, as they often require additional procedures and a lengthier inpatient stay.6 Reported treatments include removal of the graft as well as graft preservation with the use of vacuum assisted closure (VAC) devices, with or without muscle flap coverage.4, 7–9 Recently the trend has been to move away from graft removal with the latter options more commonly employed.7–9
There are several previous studies looking at infectious complications after bypass involving the femoral artery. However, there are few studies that distinguish wound infections from infections involving the graft. Prior larger studies that have specifically looked at infected prosthetic grafts were conducted before the identification of Methicillin-resistant Staphylococcus aureus (MRSA), did not stratify by wound location or operative indication, and did not perform statistical analysis to determine risk factors.4, 10, 11 Other previous analyses had a small sample size and included vein grafts in their analysis with only a small proportion of the cases involving prosthetic grafts.5, 12, 13
Beth Israel Deaconess Medical Center is a large referral center and university hospital with a high volume of patients with lower extremity vascular disease. Therefore we examined our ten-year experience with infected prosthetic grafts after surgical bypass involving the femoral artery to identify predictive risk factors. We also examined the impact of post-operative graft infection on amputation rates and mortality.
Methods
We performed a retrospective cohort study of all patients undergoing prosthetic bypass grafts involving the femoral artery from 2001–2010 by the Division of Vascular and Endovascular Surgery performed at Beth Israel Deaconess Medical Center. We defined a graft infection as the presence of purulent fluid directly communicating with the graft or an exposed graft. Patient demographics, body mass index, comorbidities, indications for intervention, location of bypass, type of prosthetic material, case urgency, previous ipsilateral bypass or percutaneous interventions were recorded. The primary endpoint was graft infection, with post-operative amputation and mortality analyzed as secondary end points. The selection of the bypass conduit is surgeon dependent, however generally we preferentially use greater saphenous vein, followed by prosthetic graft for claudicants, and greater saphenous vein followed by either arm vein or lesser saphenous vein before prosthetic graft in cases of limb threat. Follow-up is variable, but in general, patients are seen three to four times per year for two years and then one to two times per year thereafter. Long-term survival was checked with the social security death index. An active infection at the time of bypass was defined as an infection in the limb in which the bypass was performed or bypass performed for management of an infected graft at another site, such as an axillary-femoral bypass performed for an infected aortic graft. We use longitudinal incisions in the groin, which we then close with two layers of running vicryl sutures followed by skin closure with staples. Ioban use was surgeon specific with most of the surgeons not using ioban and no vacuum assisted closure devices were used prophylactically. Our protocol is to administer antibiotics 60 minutes before skin incision. Our regimen has evolved over time during this study period from cefazolin alone to vancomycin and gentamicin in 2005 and to vancomycin and cefazolin in 2010 per hospital policy. These protocols did change over time in consultation with our infectious disease division based on institutional culture data Betadine was used for preparation unless there was a contraindication throughout the study period. After this study period, we switched to chlorhexidine gluconate with isopropyl alcohol as our standard preparation solution per hospital policy. Routine hibiclens use preoperative was started after the study period. For the first six years, we used razors and then switched to electrical clips thereafter. In the middle of the study period our division developed and evolved its endovascular surgery program, giving us more options to treat our patients.1
Categorical variables were analyzed by Pearson χ2 and the Fisher exact test. Treatment outcomes during the course of follow-up were analyzed using Kaplan-Meier methodology, and time-to-failure curves were compared with the log rank test and Cox proportional hazards. Univariate and multivariable Cox regression models were used to assess predictor variables for time-dependent outcomes. Statistical significance was defined as P< .05. All statistical tests were done using STATA 8 software (StataCorp, College Station, Tex).
Results
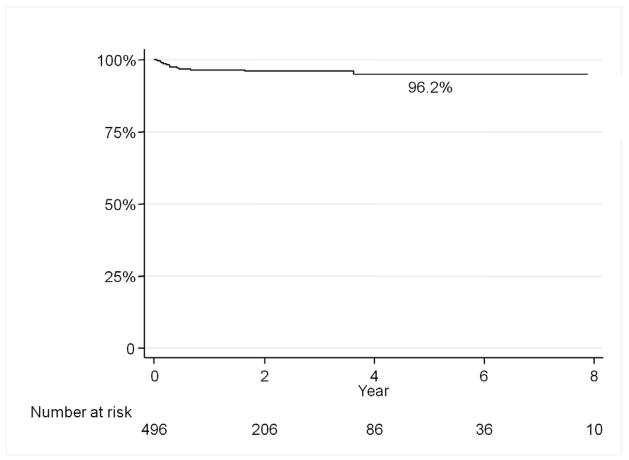
There were 496 prosthetic grafts involving the femoral artery in 478 patients from 2001–2010 performed at our institution. The average age was 69 years and 58% were male. Mean follow-up was 27 months. The graft infection rate was 3.8% (19/496) with the median time to presentation after bypass of 98 days (Figure 1). Graft infections were more common in women than men (6.8% vs 1.7%, p<0.01), DM vs non-DM (6.5% vs 1.9%, P<0.01), limb threat vs claudication (5.2% vs 2.6%, p<0.05), active infection at the time of bypass (11.7% vs 2.9%, p<0.01), redo bypass (11.5% vs 2.0%, p <0.01), and chronic renal insufficiency (6.4% vs 3.3%, p=0.05). (Table 1–3). By multivariable analysis female gender (HR 4.5, 95% CI 1.6–12.7) diabetes mellitus (HR 4.6, 95% CI 1.5–14.3), redo bypass (HR 5.8, 95% CI 2.2–15.0), and active infection (HR 2.3, 95% CI 1.6–3.4) were independent predictors of prosthetic graft infection (Table 4).
Figure 1.
Graft infection rate was 3.8% with a median time to presentation of 98 days post-operatively.
Table 1.
Patient Characteristics
| Total (percent of total with characteristic) | Graft infection rate with characteristic | Graft Infection rate without characteristic | p-value | |
|---|---|---|---|---|
| Patients | 496 | 3.8% | - | - |
| Obese (BMI>30) | 90 (18%) | 3.7% | 3.9% | 0.7 |
| Female gender | 206 (42%) | 6.8% | 1.7% | <0.01 |
| Emergent | 48 (10%) | 4.2% | 3.8% | 0.6 |
| Coronary Artery Disease | 187 (38%) | 3.7% | 3.8% | 0.7 |
| Diabetes Mellitus | 230 (46%) | 6.5% | 1.9% | <0.01 |
| Hypertension | 368 (74%) | 3.8% | 3.9% | 0.7 |
| Chronic Renal Insufficiency | 78 (16%) | 6.4% | 3.3% | 0.05 |
| Former Smoker | 180 (36%) | 3.8% | 3.8% | 0.9 |
| Current Smoker | 186 (38%) | 2.7% | 4.5% | 0.4 |
Table 3.
Limb History
| Characteristics | Total (percent with characteristic) | Graft infection rate with characteristic | Graft infection rate without characteristic | p-value |
|---|---|---|---|---|
| Limb threat | 267 (53%) | 5.2% | 2.9% | <0.05 |
| Tissue Loss | 142 (29%) | 4.2% | 3.7% | 0.7 |
| Active Infection | 51 (10%) | 11.7% | 2.9% | <0.01 |
| Prior percutaneous access | 295 (60%) | 4.4% | 3.0% | 0.7 |
| Redo Bypass | 96 (19%) | 11 (11.5%) | 2.0% | <0.01 |
Table 4.
Multivariable predictors of graft infections
| HR | 95% Confidence Interval | |
|---|---|---|
| Redo bypass | 5.8 | 2.2–15.0 |
| Active infection | 5.2 | 1.9–14.2 |
| Diabetes mellitus | 4.6 | 1.5–14.3 |
| Female gender | 4.5 | 1.6–12.7 |
The location of the bypass was not predictive of graft infection (Table 2). The type of prosthetic material also did not predict graft infection as there was no difference between dacron (3%) and polytetrafluoroethylene (PTFE) (4.3%) grafts. Previous minor ipsilateral amputation and previous percutaneous access of the ipsilateral femoral artery were not predictive of graft infection. A second bypass performed at the initial operation and concurrent stenting were also not predictive. Grafts were occluded 11% of the time in infected grafts and 13% in non-infected grafts (NS). Limbs were reintervened on post-bypass 14% of the time in non-infected grafts and 11% in infected grafts (NS). The average time between antibiotic administration and incision was 24 minutes. The rate of infection in those patients who received antibiotics greater than one hour was 11.1% (NS), however this only represents one patient. The average operating time was 167 minutes and case length was not associated with graft infection.
Table 2.
Procedures
| Bypass Type | Total (percentage undergoing procedure) | Graft infection rate with procedure | Graft infection rate with other procedures | p-value |
|---|---|---|---|---|
| Femoral-Femoral | 102 (21%) | 4.2% | 3.6% | 0.6 |
| Ilio-Femoral | 17 (3.4%) | 5.2% | 3.8% | 0.5 |
| Femoral-Popliteal | 185 (37%) | 3.2% | 3.9% | 0.9 |
| Femoral-Distal | 51 (10%) | 5.9% | 3.6% | 0.4 |
| Axillary-Femoral | 73 (15%) | 4.1% | 3.8% | 0.7 |
| Aorta-Femoral | 84 (17%) | 1.2% | 4.4% | 0.3 |
Suture lines were involved 79% of the time. Staphylococcus epidermidis was the most common isolate present in cultures from 37% of infected grafts presenting at a median of 163 days postoperatively followed by methicillin sensitive Staphylococcus aureus cultured in 26%. Surprisingly, methicillin-resistant Staphylococcus aureus was only cultured in 5% of graft infections (Table 5). The graft infections with no growth had graft exposed.
Table 5.
Bacteria Isolated
| Bacteria Isolated | |
|---|---|
| Staphylococcus epidermidis | 37% |
| Methicillin-sensitive Staphylococcus aureus | 26% |
| Enterococcus | 10% |
| Methicillin-resistant Staphylococcus aureus | 5% |
| Pseudomonas aeruginosa | 5% |
| Streptococcus pyogenes | 5% |
| Klebsiella pneumoniae | 5% |
| Corynebacterium | 5% |
| Polymicrobial | 10% |
| No Growth | 10% |
All amputations after graft infection occurred during the readmission for the graft infection and were all within 30 days of diagnosis of graft infection. The median length of stay for the readmission for graft infection was 11 (range 8–94) days. There were no additional complications in 53% of the patients with graft infections. Excluding major amputations, other complications included one patient with diabetic ketoacidosis, two had minor amputations, and one had an acute myocardial infarction and ultimately expired. One patient, who had an above the knee amputation, had recurrent infections requiring further debridement. Eleven out of the 19 patients that were discharged went home with services and the others went to rehabilitation facilities. All patients without amputations were fully ambulatory at the time of discharge. Seventy-four percent of infected grafts (14/19) were removed, however in the last three years of this study period, only 50% (3/6) of the infected grafts were removed. Preservation of the grafts in these cases utilized a sartorius muscle flap in one case and all had VAC devices placed. All preserved grafts were successfully salvaged and none went on to amputation. Vascular reconstruction was possible and performed in 50% (7/14) of the patients who had grafts removed, none of whom went on to have a major amputation. The remaining limbs were not reconstructed as they were not salvageable secondary to the extent of infection and lack of conduit. Five of seven patients (71%) without vascular reconstruction who had grafts removed went on to major lower extremity amputation.
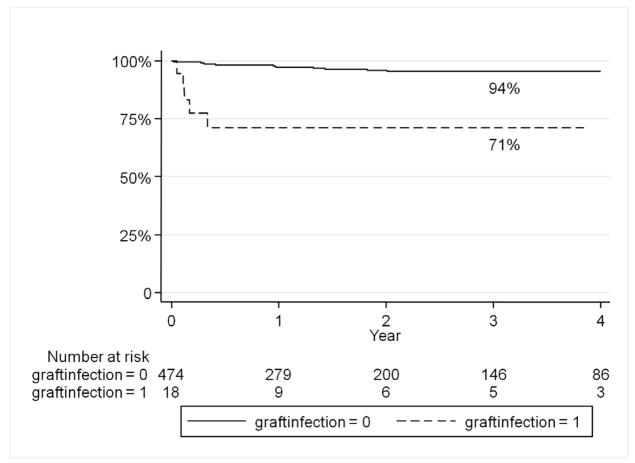
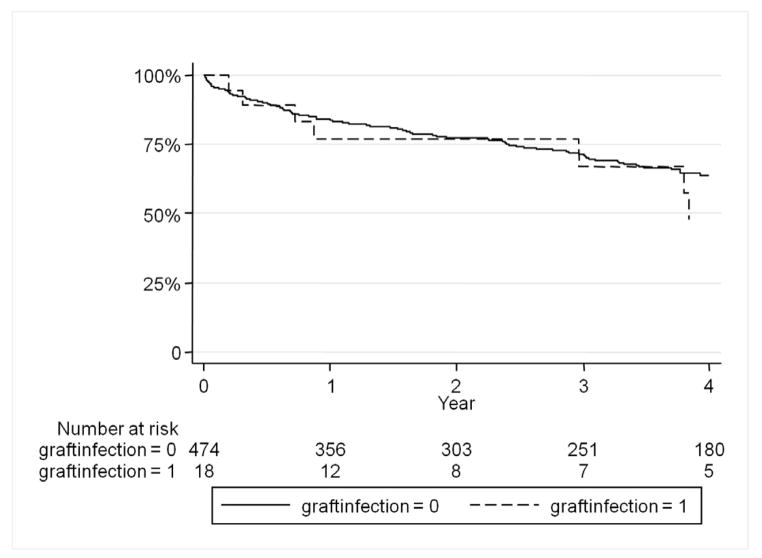
Graft infection was predictive of major lower extremity amputation (HR 9.8, 95% CI 3.5–27.1). Freedom from amputation was 94% without graft infection and 71% in those with graft infections at one year (Figure 2). Preoperative tissue loss in the ipsilateral limb was also predictive of amputation (HR 4.7, 95% CI 1.8–11.9). Surprisingly, graft infection did not predict an increase in long-term mortality (Figure 3). However chronic renal insufficiency (HR 2.3, 95% CI 1.6–3.4), tissue loss (HR 1.4, 95% CI 1.0–1.9), and active infection (HR 2.3, 95% CI 1.6–3.4) were predictive of overall late mortality.
Figure 2.
Graft infection predicts major amputation of the affected limb.
Figure 3.
There is no significant difference in long-term survival between those with infected grafts and those without infected grafts.
Discussion
The infection rate of prosthetic grafts involving the groin in our series was 3.8% at a mean follow-up of 27 months. There was no difference in the incidence in graft infection over the ten years. Predictors of graft infection were redo bypass, active infection at time of bypass, female gender, and diabetes mellitus. Graft infection was predictive of major lower extremity amputation, as was preoperative tissue loss. However graft infection did not predict mortality. Over time we have been increasingly managing graft infections without graft removal.
Our graft infection rate is similar to previously published rates ranging from 4.3–4.7%.5,10 Chang et al looked at predictors of graft infections, the majority of which were vein grafts, and found that only operative time was predictive of graft infections after lower extremity bypass.5 No other studies looked at predictors of graft infections.
The association of female gender with SSI and with prosthetic graft infection as noted in our series may be due to a higher fat distribution in the lower extremities or incontinence causing wound contamination or other factors.14,15 We also found diabetes to be predictive of graft infection, which is not surprising as it is also associated with surgical site infections not only in the lower extremities, but also in other surgical procedures.16 Perioperative tight glucose control in diabetics is protective against cardiovascular complications, however it has not been shown to decrease wound complications most likely due to the deleterious effects of diabetes aside from hyperglycemia.17,18
The presence of an active infection at the time of the operation was also predictive of prosthetic graft infection, most likely due to contamination during the procedure or perioperative period. This has previously shown to be a risk for surgical site infections.2 A redo operation was also predictive of graft infection, likely secondary to scarring and poor vascular supply to the surgical bed. This was not previously shown to be a predictive factor for surgical site or graft infections.2,3,5 Surprisingly body mass index was not predictive of graft infection as it is closely linked to surgical site infection after lower extremity bypass.2 We also did not find an association with smoking, or length of operation. However, the lack of associations in our series may have been due to a type II error due to the small number of infected grafts.
Graft infection was predictive of amputation, as some patients who required graft removal had no reconstructive options or the limbs were not salvageable at the time of presentation with graft infection. Mertens et al showed that infrainguinal prosthetic graft infection had a 40% amputation risk at one year.11 However, prior studies looking at graft infections have not analyzed if graft infection is an independent predicator of major lower extremity amputation.4,5,10,11 Cryopreserved veins are an alternative conduit option to avoid amputation that has been reported with some success. 19 We did not use it in this study period, however we have subsequently used it with short term success.
Our management for these graft infections has changed over time. In the first seven years of our study, all but one graft were removed. However in the later three years 50% were preserved by debridement with placement of a VAC dressing, with or without local rotational muscle flap closure. This parallels the national trend which has shown success with graft preservation by using both sartorius or gracilis muscle flaps with and without VACs devices.7–9, 19 Our grafts that had sartorius muscle flap coverage and VAC coverage alone were successfully salvaged as has been previously shown with both vein and prosthetic grafts with complete success with sartorius flaps and over 90% success with VACs alone with two failures from bleeding at the anastomosis and a recurrent infection previously reported.8,9 In the past two decades the incidence of MRSA has greatly increased and has become an important pathogen in vascular surgical site infections, contributing to up to 33% of surgical site infections in vascular surgery patients.21–23 Surprisingly it was seen in only 5% of our graft infections, with Staphylococcus epidermidis, MSSA, and Enterococcus being more prevalent.
There are several limitations to our study. It is a single center retrospective review. There was no standardization for the choice of conduit, rather it was surgeon dependent and based somewhat on personal preference which could cause selection bias as patients that are viewed as high risk may have more vein options exhausted before using a prosthetic. However, this study is the largest statistical analysis of prosthetic grafts involving the femoral artery and is the only study to look at predictors of infections exclusively in prosthetic grafts.
Conclusions
Prosthetic graft infections were seen in 3.8% of bypass grafts involving the femoral artery. Redo-bypass, female gender, diabetes, and active infection at time of bypass are associated with a higher risk for graft infection which results in subsequent early major extremity amputation, but does not confer an increased risk of mortality. Alternate sources of vein and endovascular interventions should be considered preferentially in these high-risk patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siracuse JJ, Giles KA, Pomposelli FB, Hamdan AD, Wyers MC, Chaikof EL, Nedeau AE, Schermerhorn ML. Long-term results for primary bypass versus primary angioplasty/stent for intermittent claudication due to superficial femoral artery occlusive disease. J Vasc Surg. 2012 Apr;55(4):1001–7. doi: 10.1016/j.jvs.2011.10.128. Epub 2012 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Siracuse JJ, Schermerhorn ML. Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005–2007. Ann Vasc Surg. 2010 Jan;24(1):48–56. doi: 10.1016/j.avsg.2009.05.003. Epub 2009 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg. 2011 Aug;54(2):433–9. doi: 10.1016/j.jvs.2011.01.034. Epub 2011 Mar 31. [DOI] [PubMed] [Google Scholar]
- 4.Edwards WH, Jr, Martin RS, 3rd, Jenkins JM, Edwards WH, Sr, Mulherin JL., Jr Primary graft infections. J Vasc Surg. 1987 Sep;6(3):235–9. [PubMed] [Google Scholar]
- 5.Chang JK, Calligaro KD, Ryan S, Runyan D, Dougherty MJ, Stern JJ. Risk factors associated with infection of lower extremity revascularization: analysis of 365 procedures performed at a teaching hospital. Ann Vasc Surg. 2003 Jan;17(1):91–6. doi: 10.1007/s10016-001-0337-8. Epub 2003 Jan 15. [DOI] [PubMed] [Google Scholar]
- 6.Vogel TR, Dombrovskiy VY, Carson JL, Haser PB, Lowry SF, Graham AM. Infectious complications after elective vascular surgical procedures. J Vasc Surg. 2010 Jan;51(1):122–9. doi: 10.1016/j.jvs.2009.08.006. discussion 129–30. Epub 2009 Dec 2. [DOI] [PubMed] [Google Scholar]
- 7.Dosluoglu HH, Loghmanee C, Lall P, Cherr GS, Harris LM, Dryjski ML. Management of early (<30 day) vascular groin infections using vacuum-assisted closure alone without muscle flap coverage in a consecutive patient series. J Vasc Surg. 2010 May;51(5):1160–6. doi: 10.1016/j.jvs.2009.11.053. Epub 2010 Mar 31. [DOI] [PubMed] [Google Scholar]
- 8.Morasch MD, Sam AD, 2nd, Kibbe MR, Hijjawi J, Dumanian GA. Early results with use of gracilis muscle flap coverage of infected groin wounds after vascular surgery. J Vasc Surg. 2004 Jun;39(6):1277–83. doi: 10.1016/j.jvs.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Landry GJ, Carlson JR, Liem TK, Mitchell EL, Edwards JM, Moneta GL. The sartorius muscle flap: an important adjunct for complicated femoral wounds involving vascular grafts. Am J Surg. 2009 May;197(5):655–9. doi: 10.1016/j.amjsurg.2008.12.020. discussion 659. Epub 2009 Mar 24. [DOI] [PubMed] [Google Scholar]
- 10.Jensen LJ, Kimose HH. Prosthetic graft infections: a review of 720 arterial prosthetic reconstructions. Thorac Cardiovasc Surg. 1985 Dec;33(6):389–91. doi: 10.1055/s-2007-1014176. [DOI] [PubMed] [Google Scholar]
- 11.Mertens RA, O’Hara PJ, Hertzer NR, Krajewski LP, Beven EG. Surgical management of infrainguinal arterial prosthetic graft infections: review of a thirty-five-year experience. J Vasc Surg. 1995 May;21(5):782–90. doi: 10.1016/s0741-5214(05)80009-6. discussion 790–1. [DOI] [PubMed] [Google Scholar]
- 12.Henke PK, Bergamini TM, Rose SM, Richardson JD. Current options in prosthetic vascular graft infection. Am Surg. 1998 Jan;64(1):39–45. discussion 45–6. [PubMed] [Google Scholar]
- 13.Pedersen G, Laxdal E, Hagala M, Aune S. Local infections after above-knee prosthetic femoropopliteal bypass for intermittent claudication. Surg Infect (Larchmt) 2004 Summer;5(2):174–9. doi: 10.1089/sur.2004.5.174. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Zhou J, Waring ME, Parker DR, Eaton CB. Abdominal obesity and peripheral vascular disease in men and women: a comparison of waist-to-thigh ratio and waist circumference as measures of abdominal obesity. Atherosclerosis. 2010 Jan;208(1):253–7. doi: 10.1016/j.atherosclerosis.2009.06.027. Epub 2009 Jul 8. [DOI] [PubMed] [Google Scholar]
- 15.Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998 Apr;46(4):473–80. doi: 10.1111/j.1532-5415.1998.tb02469.x. Review. [DOI] [PubMed] [Google Scholar]
- 16.Koutsoumbelis S, Hughes AP, Girardi FP, Cammisa FP, Jr, Finerty EA, Nguyen JT, Gausden E, Sama AA. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011 Sep 7;93(17):1627–33. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam B, Panzica PJ, Novack V, Mahmood F, Matyal R, Mitchell JD, Sundar E, Bose R, Pomposelli F, Kersten JR, Talmor DS. Continuous perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery: a prospective, randomized trial. Anesthesiology. 2009 May;110(5):970–7. doi: 10.1097/ALN.0b013e3181a1005b. [DOI] [PubMed] [Google Scholar]
- 18.Shrikhande GV, Scali ST, da Silva CG, Damrauer SM, Csizmadia E, Putheti P, Matthey M, Arjoon R, Patel R, Siracuse JJ, Maccariello ER, Andersen ND, Monahan T, Peterson C, Essayagh S, Studer P, Guedes RP, Kocher O, Usheva A, Veves A, Kaczmarek E, Ferran C. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLoS One. 2010 Dec 6;5(12):e14240. doi: 10.1371/journal.pone.0014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujitani RM, Bassiouny HS, Gewertz BL, Glagov S, Zarins CK. Cryopreserved saphenous vein allogenic homografts: an alternative conduit in lower extremity arterial reconstruction in infected fields. J Vasc Surg. 1992 Mar;15(3):519–26. [PubMed] [Google Scholar]
- 20.Taylor SM, Weatherford DA, Langan EM, 3rd, Lokey JS. Outcomes in the management of vascular prosthetic graft infections confined to the groin: a reappraisal. Ann Vasc Surg. 1996 Mar;10(2):117–22. doi: 10.1007/BF02000754. [DOI] [PubMed] [Google Scholar]
- 21.Nasim A, Thompson MM, Naylor AR, Bell PR, London NJ. The impact of MRSA on vascular surgery. Eur J VascEndovasc Surg. 2001 Sep;22(3):211–4. doi: 10.1053/ejvs.2001.1429. [DOI] [PubMed] [Google Scholar]
- 22.Taylor MD, Napolitano LM. Methicillin-resistant Staphylococcus aureus infections in vascular surgery: increasing prevalence. Surg Infect (Larchmt) 2004 Summer;5(2):180–7. doi: 10.1089/sur.2004.5.180. [DOI] [PubMed] [Google Scholar]
- 23.Herrera FA, Kohanzadeh S, Nasseri Y, Kansal N, Owens EL, Bodor R. Management of vascular graft infections with soft tissue flap coverage: improving limb salvage rates--a veterans affairs experience. Am Surg. 2009 Oct;75(10):877–81. doi: 10.1177/000313480907501003. [DOI] [PubMed] [Google Scholar]