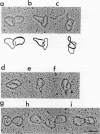
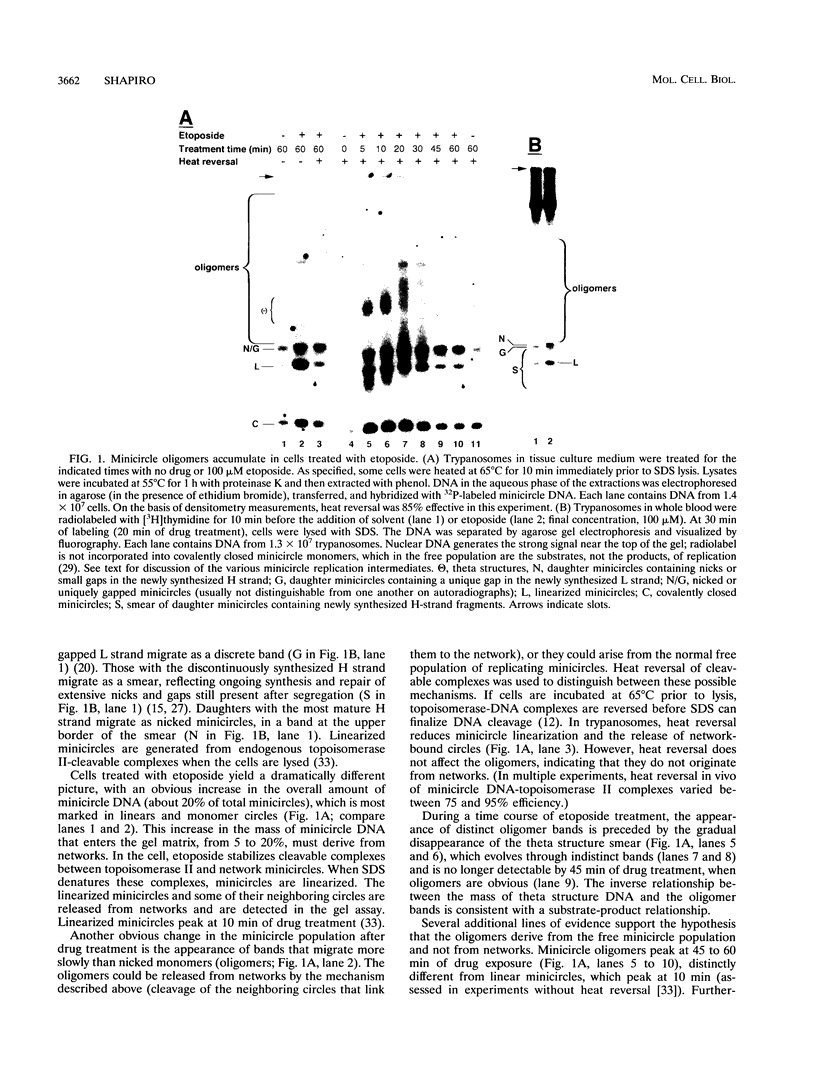
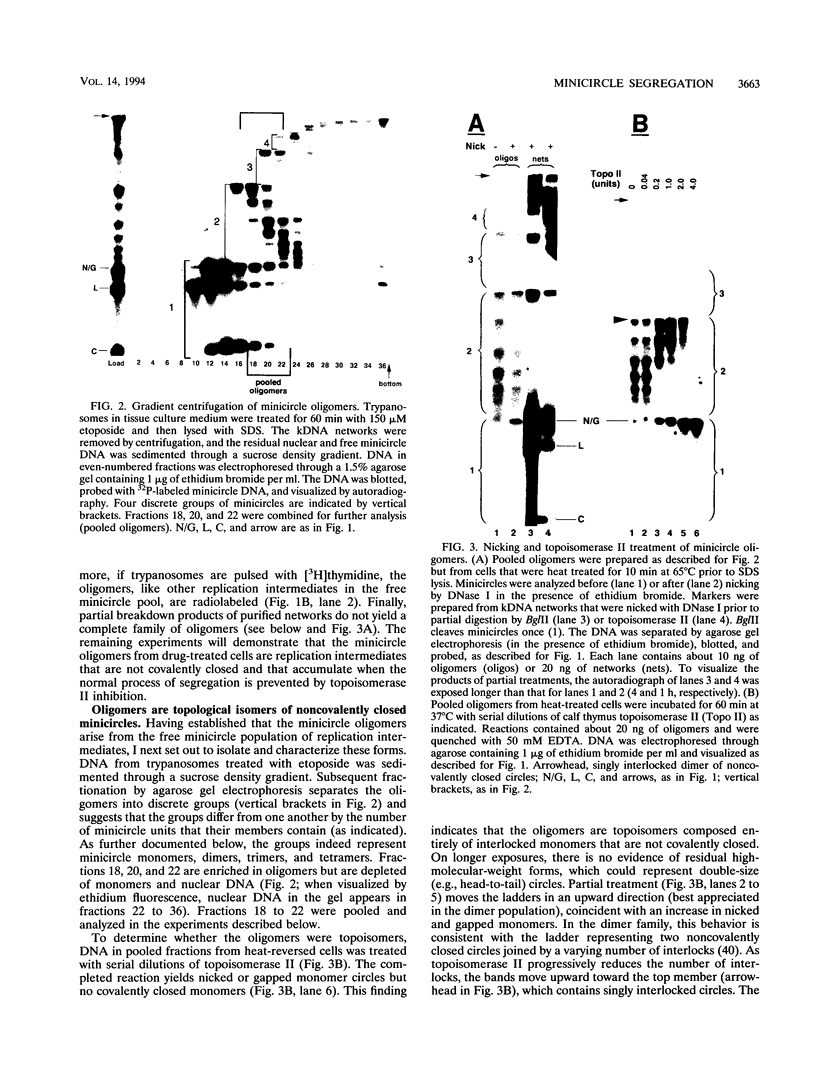
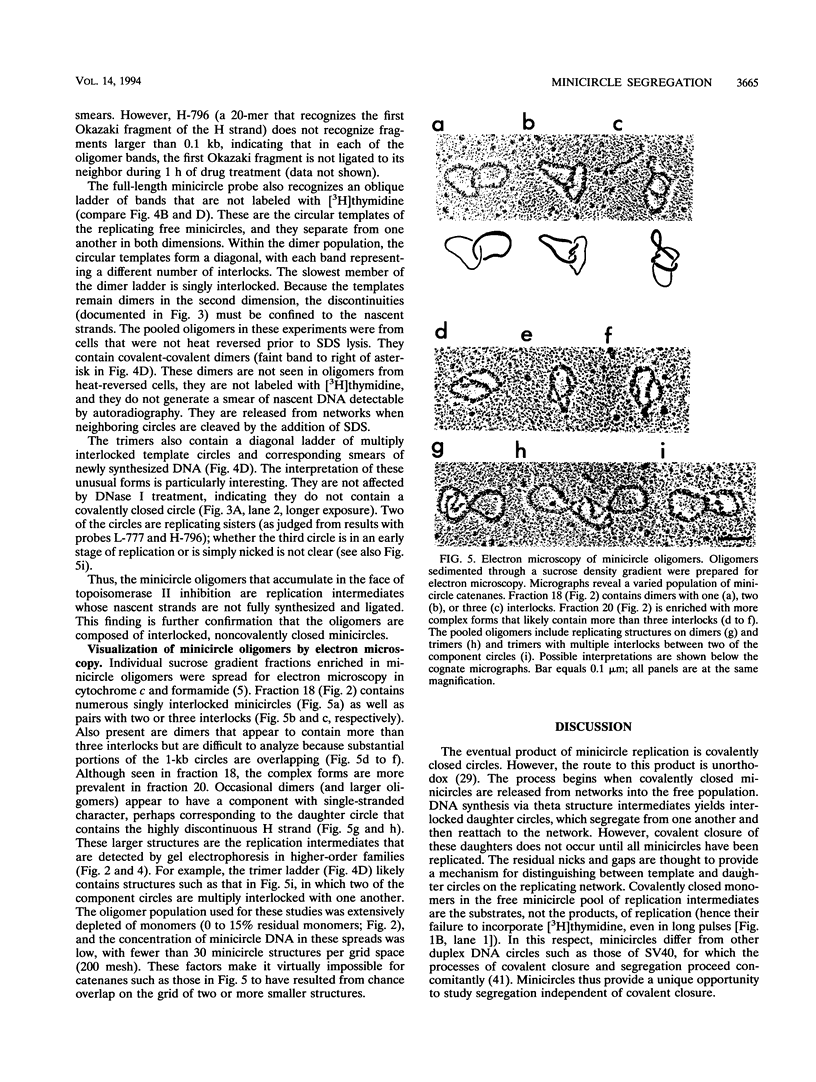
Abstract
Etoposide, a nonintercalating antitumor drug, is a potent inhibitor of topoisomerase II activity. When Trypanosoma equiperdum is treated with etoposide, cleavable complexes are stabilized between topoisomerase II and kinetoplast DNA minicircles, a component of trypanosome mitochondrial DNA (T. A. Shapiro, V. A. Klein, and P. T. Englund, J. Biol. Chem. 264:4173-4178, 1989). Etoposide also promotes the time-dependent accumulation of small minicircle catenanes. These catenanes are radiolabeled in vivo with [3H]thymidine. Dimers are most abundant, but novel structures containing up to five noncovalently closed minicircles are detectable. Analysis by two-dimensional gel electrophoresis and electron microscopy indicates that dimers joined by up to six interlocks are late replication intermediates that accumulate when topoisomerase II activity is blocked. The requirement for topoisomerase II is particularly interesting because minicircles do not share the features postulated to make this enzyme essential in other systems: for minicircles, the replication fork is unidirectional, access to the DNA is not blocked by nucleosomes, and daughter circles are extensively nicked and (or) gapped.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Dean F. B., Cozzarelli N. R. Mechanism of strand passage by Escherichia coli topoisomerase I. The role of the required nick in catenation and knotting of duplex DNA. J Biol Chem. 1985 Apr 25;260(8):4984–4994. [PubMed] [Google Scholar]
- DiGate R. J., Marians K. J. Identification of a potent decatenating enzyme from Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):13366–13373. [PubMed] [Google Scholar]
- Douc-Rasy S., Kayser A., Riou J. F., Riou G. ATP-independent type II topoisomerase from trypanosomes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7152–7156. doi: 10.1073/pnas.83.19.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Fragoso S. P., Goldenberg S. Cloning and characterization of the gene encoding Trypanosoma cruzi DNA topoisomerase II. Mol Biochem Parasitol. 1992 Oct;55(1-2):127–134. doi: 10.1016/0166-6851(92)90133-5. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Evidence for the reversibility of cellular DNA lesion induced by mammalian topoisomerase II poisons. J Biol Chem. 1989 Jun 15;264(17):9713–9715. [PubMed] [Google Scholar]
- Kim R. A., Wang J. C. Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. J Biol Chem. 1992 Aug 25;267(24):17178–17185. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Low R. L., Kaguni J. M., Kornberg A. Potent catenation of supercoiled and gapped DNA circles by topoisomerase I in the presence of a hydrophilic polymer. J Biol Chem. 1984 Apr 10;259(7):4576–4581. [PubMed] [Google Scholar]
- Melendy T., Ray D. S. Novobiocin affinity purification of a mitochondrial type II topoisomerase from the trypanosomatid Crithidia fasciculata. J Biol Chem. 1989 Jan 25;264(3):1870–1876. [PubMed] [Google Scholar]
- Melendy T., Ray D. S. Purification and nuclear localization of a type I topoisomerase from Crithidia fasciculata. Mol Biochem Parasitol. 1987 Jun;24(2):215–225. doi: 10.1016/0166-6851(87)90108-3. [DOI] [PubMed] [Google Scholar]
- Melendy T., Sheline C., Ray D. S. Localization of a type II DNA topoisomerase to two sites at the periphery of the kinetoplast DNA of Crithidia fasciculata. Cell. 1988 Dec 23;55(6):1083–1088. doi: 10.1016/0092-8674(88)90252-8. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- Pasion S. G., Hines J. C., Aebersold R., Ray D. S. Molecular cloning and expression of the gene encoding the kinetoplast-associated type II DNA topoisomerase of Crithidia fasciculata. Mol Biochem Parasitol. 1992 Jan;50(1):57–67. doi: 10.1016/0166-6851(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Pérez-Morga D. L., Englund P. T. Microtechnique for electron microscopy of DNA. Nucleic Acids Res. 1993 Mar 11;21(5):1327–1328. doi: 10.1093/nar/21.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch C. A., Perez-Morga D., Cozzarelli N. R., Englund P. T. The absence of supercoiling in kinetoplast DNA minicircles. EMBO J. 1993 Feb;12(2):403–411. doi: 10.1002/j.1460-2075.1993.tb05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Riou G. F., Gabillot M., Douc-Rasy S., Kayser A., Barrois M. A type I DNA topoisomerase from Trypanosoma cruzi. Eur J Biochem. 1983 Aug 15;134(3):479–484. doi: 10.1111/j.1432-1033.1983.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Englund P. T. Replication of kinetoplast DNA in Trypanosoma equiperdum. Minicircle H strand fragments which map at specific locations. J Biol Chem. 1989 Jan 15;264(2):823–830. [PubMed] [Google Scholar]
- Ryan K. A., Englund P. T. Synthesis and processing of kinetoplast DNA minicircles in Trypanosoma equiperdum. Mol Cell Biol. 1989 Aug;9(8):3212–3217. doi: 10.1128/mcb.9.8.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Griffith J. D., Englund P. T. A knotted free minicircle in kinetoplast DNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5844–5848. doi: 10.1073/pnas.85.16.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Englund P. T. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc Natl Acad Sci U S A. 1990 Feb;87(3):950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Klein V. A., Englund P. T. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J Biol Chem. 1989 Mar 5;264(7):4173–4178. [PubMed] [Google Scholar]
- Shlomai J., Zadok A., Frank D. A unique ATP-dependent DNA topoisomerase from trypanosomatids. Adv Exp Med Biol. 1984;179:409–422. doi: 10.1007/978-1-4684-8730-5_42. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Snapka R. M., Powelson M. A., Strayer J. M. Swiveling and decatenation of replicating simian virus 40 genomes in vivo. Mol Cell Biol. 1988 Feb;8(2):515–521. doi: 10.1128/mcb.8.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Strauss P. R., Wang J. C. The TOP2 gene of Trypanosoma brucei: a single-copy gene that shares extensive homology with other TOP2 genes encoding eukaryotic DNA topoisomerase II. Mol Biochem Parasitol. 1990 Jan 1;38(1):141–150. doi: 10.1016/0166-6851(90)90214-7. [DOI] [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases: why so many? J Biol Chem. 1991 Apr 15;266(11):6659–6662. [PubMed] [Google Scholar]
- Wasserman S. A., Cozzarelli N. R. Biochemical topology: applications to DNA recombination and replication. Science. 1986 May 23;232(4753):951–960. doi: 10.1126/science.3010458. [DOI] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]