Abstract
Impaired wound healing in the elderly represents a major clinical problem. Delineating the cellular and molecular mechanisms by which aging impairs wound healing may lead to the development of improved treatment strategies for elderly patients with non-healing wounds. Neovascularization is an essential step in wound healing, and bone marrow-derived angiogenic cells (BMDACs) play an important role in vascularization. Using a mouse full-thickness burn wound model, we demonstrate that perfusion and vascularization of burn wounds were impaired by aging and were associated with dramatically reduced mobilization of BMDACs bearing the cell surface molecules CXCR4 and Sca1. Expression of stromal-derived factor 1 (SDF-1), the cytokine ligand for CXCR4, was significantly decreased in peripheral blood and burn wounds of old mice. Expression of hypoxia-inducible factor (HIF)-1α was detected in burn wounds from young (2-month-old), but not old (2-year-old), mice. When BMDACs from young donor mice were injected intravenously, homing to burn wound tissue was impaired in old recipient mice, whereas the age of the BMDAC donor mice had no effect on homing. Our results indicate that aging impairs burn wound vascularization by impairing the mobilization of BMDACs and their homing to burn wound tissue as a result of impaired HIF-1 induction and SDF-1 signaling.
Keywords: Burn wound healing, Aging, Neovascularization, Bone marrow-derived angiogenic cells, Hypoxia-inducible factor 1, Stromal-derived factor 1
Introduction
Effective management of burn injuries represents a major public health problem. More than one million Americans require treatment each year due to burn injuries [1]. The elderly population (65 years or older) is growing rapidly in the USA and makes up approximately 15% of burn unit admissions [2]. Wound healing is impaired with aging in humans and mice [3–5]. Although burn wounds have been less well studied, burns in older adults are associated with increased morbidity and mortality [6]. Understanding the cellular and molecular mechanisms by which aging affects burn wound healing will lead to improved management of burn injuries in the elderly.
Neovascularization, which is an essential component of the wound healing process, occurs through angiogenesis and vasculogenesis [7]. Angiogenesis refers to the sprouting of new capillaries from preexisting vessels in the wound tissue, whereas vasculogenesis refers the recruitment of cells from outside of the wound that participate in de novo blood vessel formation. Aging delays vascularization and thereby contributes to delayed wound healing [8]. Vascularization is triggered by the hypoxia-induced expression of critical angiogenic factors and cytokines, including vascular endothelial growth factor (VEGF) and stromal-derived factor 1 (SDF-1; also known as CXCL12). Transcriptional activation of the genes encoding VEGF [9] and SDF-1 [10] is mediated by hypoxia-inducible factor 1 (HIF-1), which functions as a master regulator of adaptive responses to hypoxia/ischemia [11]. HIF-1 is a heterodimer composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit [12]. Compared to excisional wounds, burn wounds are characterized by reduced HIF-1α levels [13]. Hif1a+/– mice, which are heterozygous for a null (knockout) allele at the locus encoding HIF-1α, have impaired burn wound healing that is associated with impaired induction of HIF-1α and SDF-1 expression in burn wound tissue [14].
Angiogenic factors and cytokines such as VEGF and SDF-1 mediate biological effects by binding to cognate receptors (VEGFR1/VEGFR2 and CXCR4, respectively) on proximal and distant cells. The proximal cells are endothelial cells in existing wound vessels, which are activated to initiate angiogenesis. The distal cells include a population of bone marrow-derived angiogenic cells (BMDACs), which are mobilized from bone marrow and other tissues into the circulation and home to the wound, where they may differentiate into endothelial cells and thereby participate in vasculogenesis or produce angiogenic factors and thereby stimulate angiogenesis [15]. BMDACs express progenitor cell markers (such as Sca1) and receptors (such as CXCR4) that determine to which angiogenic cytokines they respond (such as SDF-1). Although BMDACs were originally described as endothelial progenitor cells, recent evidence indicates that the majority are proangiogenic myeloid cells [16]. Hif1a+/– mice have impaired mobilization of Sca1+/CXCR4+ BMDACs in response to burn wounding [14], and injection of an adenovirus encoding a constitutively active form of HIF-1α is sufficient to induce BMDAC mobilization and promote wound healing [17].
In the present study, we have utilized a mouse model that results in burn wounds of reproducible diameter and depth [18] to investigate the effect of aging on vascularization during burn wound healing in the C57BL/6 and 129 strains of mice. In both strains, aging has significant effects on wound perfusion and vascularization. We demonstrate that aging impairs BMDAC mobilization and homing, SDF-1 serum protein levels, and the expression of HIF-1α and SDF-1 in burn wounds. These results provide a cellular and molecular basis for the age-related impairment of burn wound healing.
Materials and methods
Mice
C57BL/6J and 129S1/SvImJ male mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The ages of the young and old cohorts were 2 months and 2 years, respectively. All procedures involving mice were approved by The Johns Hopkins University Animal Care and Use Committee.
Burn wound model
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), shaved on the dorsum, and depilated with Nair cream (Church & Dwight Co., Princeton, NJ). A burn wound protocol, initially established in rats [19] and then adapted for mice [18], was utilized. Briefly, a custom-made 220-g aluminum rod was heated in a 100°C water bath for 5 min. Two burns of 1.2-cm diameter each were produced on the dorsum of the animals, which together represented approximately 10% of the total body surface area. To assure burn uniformity, only the weight of the rod provided pressure to the skin surface. Contact time of 4 s measured with a metronome was chosen to produce a standardized full-thickness burn. Fluid resuscitation was performed according to the Parkland formula (4 ml/kg×% body surface area wounded) by intraperitoneal injection of normal saline within 1 h after burning. Buprenorphine (0.1 mg/kg) was administered subcutaneously for analgesia during the first 24 h.
Wound area measurement
On days 0, 3, 7, 14, and 21, the wound borders were traced in situ onto clear acetate paper. Images were digitized at 600 dpi (Paperport 6000, Visioneer, Fremont, CA). Wound areas (in pixels) were calculated using Adobe Photoshop CS3 Image software (Adobe Systems Inc., San Jose, CA). Wound area on day 0 immediately after burn wounding was taken as 100%.
Laser Doppler perfusion imaging
Blood flow in wound areas was measured by a 632.6-nm, He–Ne scanning laser Doppler imaging device (Moor Instruments, Devon, UK), which utilizes a near-infrared laser diode to measure subcutaneous blood flow as a function of light scattering by moving red blood cells. The scanner was positioned 70 cm above each animal and scans were performed to assess blood flow in the wound. For each measurement point, a signal is generated that scales linearly with tissue perfusion defined as the product of the blood cell velocity and concentration. This signal, termed the laser Doppler perfusion index (LDPI), was represented as a two-dimensional color image on a computer screen. A photographic digital image was produced simultaneously, which allowed for direct anatomical comparison of corresponding areas of burn. For each burn, the wound site was selected by selecting an area of interest after exporting the image into the software package LDISOFT (Lisca Development AB, Lisca, Sweden). Then, the mean LDPI value within this area of interest was computed.
Flow cytometry
The percentage of CXCR4+/Sca1+ cells in the peripheral blood was determined by flow cytometry as described previously [20]. Blood samples were collected from non-burned and burned mice and were subjected to red cell lysis and FC blockade (CD16/CD32, BD Pharmingen, San Diego, CA). Mononuclear cells were incubated with phycoerythrin-conjugated monoclonal antibody against CXCR4 (2B11/CXCR4, BD Pharmingen) and FITC-conjugated monoclonal antibody against Sca1 (E13-161.7, BD Pharmingen). The live cell population in the forward-and side-scatter window was gated, and the percentage of double-positive cells was determined based on 150,000 events.
Quantitative real-time reverse transcription PCR
Total RNA was extracted from mouse non-burned skin and burn wound using TRIzol (Invitrogen), precipitated in isopropanol, and treated with DNase I (Ambion) according to the manufacturer's protocol. One microgram of total RNA was used for reverse transcription with the iScript cDNA synthesis system (BioRad Laboratories, Hercules, CA). Real-time PCR was performed using iQ SYBR Green Supermix and the iCycler Real-Time PCR detection system (BioRad Laboratories). Primers were designed using Beacon Designer software and determined to be specific by dissociation curve analysis. Expression of SDF-1 mRNA relative to hypoxanthine phosphoribosyltransferase 1 mRNA was calculated based on the comparative ΔCT method [21].
Serum SDF-1 enzyme-linked immunosorbent assay
Quantification of murine SDF-1 protein in the serum was performed using the SDF-1α murine Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Immunoblot assays
Tissue samples were harvested from burned and non-burned skin on the dorsum of the mice on day 2 as described previously [14]. Protein lysates were prepared in RIPA buffer containing Complete Protease Inhibitor (Roche Diagnostics, Indianapolis, IN) and fractionated by SDS/PAGE. Anti-HIF-1α antibody was from Novus Biologicals (Littleton, CO).
Immunohistochemistry
Burn wounds were harvested with a rim of normal skin. Specimens were fixed with immunohistochemistry zinc fixative (formalin free; BD Pharmingen) for 24 h, and 5-μm-thick paraffin sections were prepared. To prevent non-specific binding, 100 μl of blocking solution containing 2% normal rabbit serum (Jackson ImmunoResearch, West Grove, PA) was applied for 30 min, and then 100 μl of primary anti-CD31 antibody (1:50 dilution; BD Pharmingen) was applied to the sections for 1 h at room temperature. The sections were further incubated with biotinylated secondary antibody (1:500 dilution; Vector Laboratories, Burlingame, CA). Streptavidin–biotin–horse-radish peroxidase was used for signal amplification, and diaminobenzidine was used for staining (Vector Laboratories). Counterstaining was performed with hematoxylin and nuclear fast red for 30 s each. For blocking endogenous peroxidase activity, 3% H2O2 (Fisher Scientific, Fair Lawn, NJ) was used. All slides were examined in a blinded manner. CD31+ blood vessels were counted per 200× field at the center of the wound directly beneath the epithelium in samples harvested at 21 day after burn wounding. We have previously shown that staining for either CD31, which is expressed by vascular endothelial cells, or α-smooth muscle actin, which is expressed by vascular pericytes, provides similar results [14], suggesting that CD31 is an appropriate marker for blood vessels within healing wounds.
For CXCR4 immunohistochemistry, heat-induced antigen retrieval was performed after sections were deparaffinized. Endogenous peroxidase activity was blocked by incubation in 3% H2O2. Sections were incubated with rabbit anti-CXCR4 (1:150, Abcam, Cambridge, MA) primary antibody. This was followed by application of the Super PicTure Polymer Detection Kit (Invitrogen, Carlsbad, CA) and diaminobenzidine. Sections were counterstained with hematoxylin.
BMDAC culture
BMDACs were prepared as described previously [22]. Bone marrow was harvested from mouse femurs and tibiae. The mononuclear cell fraction was isolated with Histopaque 1083 (Sigma-Aldrich, St. Louis, MO). Cells were seeded onto vitronectin-coated 6-well plates (Corning, Lowell, MA) and cultured in endothelial basal media supplemented with a proprietary mixture of VEGF, fibroblast growth factor-2, insulin-like growth factor-1, epidermal growth factor, hydrocortisone, gentamicin, amphotericin-B, 5% FBS, and ascorbic acid (EGM2-MV; Lonza, Walkersville, PA). BMDACs were cultured for 4 days. Cell cultures were washed with phosphate-buffered saline (PBS) on day 3 to remove nonadherent cells, and fresh media was added. On day 4, cells were detached with 1 mM EDTA in PBS for intravenous (IV) injection. A total of 2×106 viable cells in saline were administered to each mouse.
Homing of BMDACs to burn wounds
BMDACs from male donor mice were administered to female recipient mice by tail vein injection 48 h after burn wounding. Normal and burn wound skin tissues were harvested 8 h after BMDAC injection and incubated in tissue lysis buffer (5 mM EDTA, 0.2% SDS, 200 mM NaCl, 100 mM Tris–HCl [pH 7.5], and 100 μg/ml proteinase K) for 18 h at 55°C. Genomic DNA was isolated and frozen at –70°C until assayed. BMDAC homing was analyzed by quantitative real-time polymerase chain reaction (qPCR) using primers for Sry (Y-linked) and Nme1 (autosomal) gene sequences [22]. Non-template controls were included in each qPCR run and were devoid of amplification. Homing was calculated as the signal ratio from burn wound/normal skin for each mouse and corrected for efficiency [23] according to the following formula [24]:
where R is the ratio of Sry signal in the burned tissue over its respective normal skin control for each mouse; E,Sry and E,Nme1 are the efficiencies for Sry and Nme1 primers respectively; and Cq is the threshold cycle value for Sry or Nme1 in the burned (b) or non-burned (n) skin.
Statistical analysis
Results are presented as mean±standard error of mean (SEM). Differences in means between groups were analyzed for significance by Student's t test or analysis of variance (ANOVA) followed by Bonferroni post hoc analysis as appropriate.
Results
Aging impairs closure, perfusion, and vascularization of burn wounds
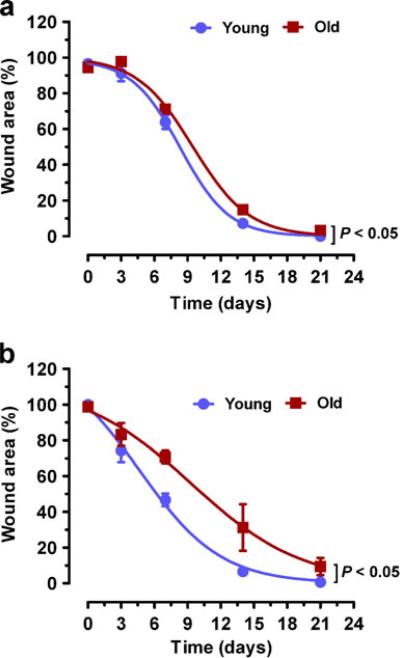
We have previously reported a murine burn wound model with uniform, graded, and reproducible thermal injury [18]. To examine the effect of aging, we used our full-thickness burn wound model and two different strains of mice. Two burns of 1.2-cm diameter were produced on the dorsum of each mouse using a heated rod with 4-s contact time. The wound area was measured on days 0, 3, 7, 14, and 21 after burning using computerized planimetry. Compared with 2-month-old (hereafter designated “young”) C57BL/6J mice, 2-year-old (hereafter designated “old”) mice showed significantly delayed wound closure (P<0.05) (Fig. 1a). Similarly, old 129S1/SvImJ mice showed significantly increased wound area compared with young mice (P< 0.05) (Fig. 1b). Thus, even though contraction of wounds is a major limitation of mouse models that may obscure differences in wound healing, our results indicate that aging delays wound closure in both C57BL/6J and 129S1/SvImJ mice.
Fig. 1.
Effect of aging on burn wound closure. a 2-month-old (young) vs 2-year-old (old) C57BL/6J mice. b Young vs old 129S1/SvImJ mice. Wound area was measured by computer-assisted planimetry on days 0, 3, 7, 14, and 21 following burn injury. Mean±SEM (n=4–8) is shown. The overall effect of aging was assessed by two-way ANOVA (brackets at right)
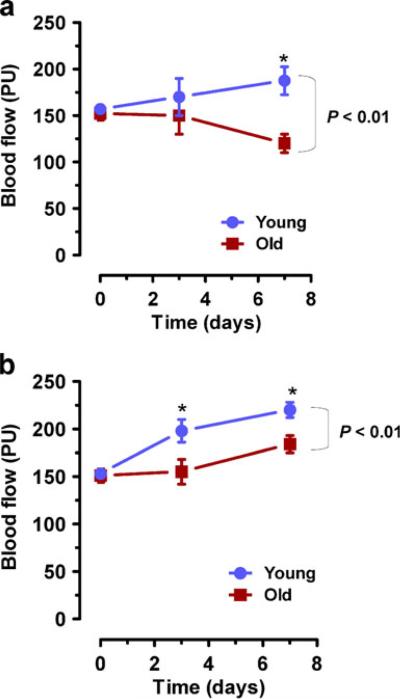
To compare tissue perfusion after burn injury, dermal blood flow was measured by serial, non-invasive LDPI in the same young and old mice that were studied in Fig. 1. We have demonstrated previously in 129S1/SvImJ mice [18] and Hif1atm1jhu mice [14] that peak blood flow occurs by day 7 after burn wounding. Compared with young C57BL/6J mice, old mice showed significantly decreased wound perfusion over the 7-day time course (P<0.01) and at the end point (P<0.05) (Fig. 2a). Likewise, aging was associated with overall decreased tissue perfusion in 129S1/SvImJ mice (P<0.01) and differences were significant on both day 3 and day 7 (P<0.05) (Fig. 2b).
Fig. 2.
Effect of aging on burn wound blood flow. a Young vs old C57BL/6J mice. b Young vs old 129S1/SvImJ mice. To measure blood flow, serial laser Doppler perfusion imaging was performed at the indicated times following burn injury. Mean±SEM (n=4–10) is shown. The overall effect of aging was assessed by two-way ANOVA (brackets at right). *P<0.05 at individual time point
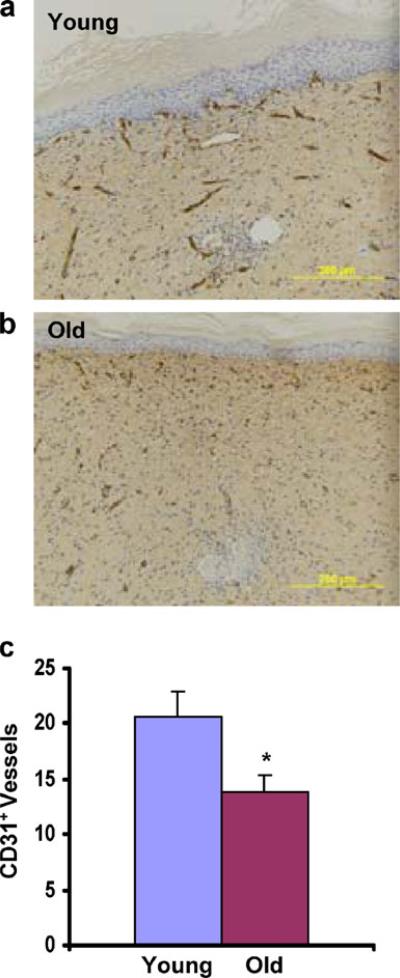
We hypothesized that the differences in perfusion reflected differences in neovascularization. Immunohistochemical analysis of wound blood vessels was performed on wound tissue harvested on day 21 from C57BL/6J mice. Sections were analyzed using an antibody that recognizes PECAM-1 (CD31), which is expressed by vascular endothelial cells. The number of CD31+ vessels within a 200× field at the center of each burn wound (Fig. 3a, b) was determined (Fig. 3c). Mean CD31+ vessel counts were significantly decreased in burn wounds from old mice compared with young mice (20±2.3 vs 13±1.5; P<0.05). The striking effects of aging on wound perfusion (Fig. 2) and wound vascularization (Fig. 3) stand in contrast to the more modest effects of aging on wound closure (Fig. 1), which may reflect the confounding effect of wound contraction that is observed in mice.
Fig. 3.
Effect of aging on burn wound vascularization. Immunohistochemical analysis of burn wounds in C57BL/6J mice on day 21 was performed using an anti-CD31 antibody. a, b Representative images from the healed center of wound from young and old mice (original magnification, 200×). c Number of CD31+ vessels per 200× field. Mean±SEM (n=7–9) is shown. *P<0.05 (Student's t test)
Aging impairs mobilization of BMDACs after burn wounding
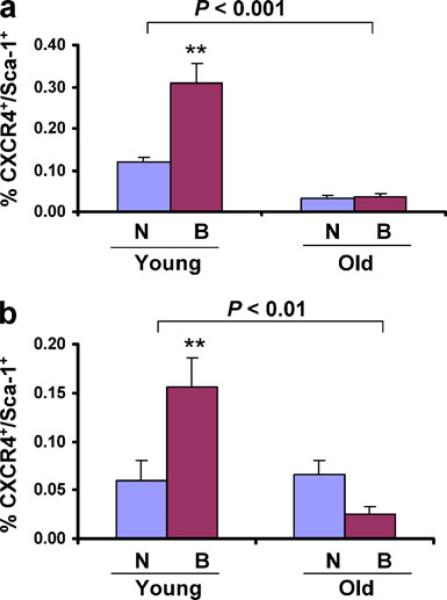
We hypothesized that the observed differences in closure, perfusion, and vascularization of wounds in old vs young mice were associated with differences in the mobilization of BMDACs. To analyze BMDAC mobilization, CXCR4+/Sca1+ cells in peripheral blood were analyzed. In non-burned C57BL/6J mice, the number of CXCR4+/Sca1+ BMDACs in peripheral blood was 3.5-fold lower in old as compared to young mice, and burn wounding induced a further increase in BMDACs in young, but not in old, C57BL/6J mice (Fig. 4a). The number of BMDACs was similar in young and old non-burned 129S1/SvImJ mice, but burn wounding led to increased BMDAC levels in young mice and decreased BMDAC levels in old mice (Fig. 4b). Thus, aging impairs the physiological mobilization of BMDACs into the circulation in response to burn wounding in both mouse strains. These results provide a cellular mechanism to account for the impaired vascularization of burn wounds that was observed in old mice (Fig. 3).
Fig. 4.
Effect of aging on the mobilization of bone marrow-derived angiogenic cells following burn wounding. Peripheral blood samples were collected from non-burned control mice (N) or from mice on day 3 after burn wounding (B) and analyzed by flow cytometry to determine the number of circulating CXCR4+/Sca1+ cells. a Young vs old C57BL/6 J mice. b Young vs old 129 S1/SvImJ mice. Mean±SEM (n=5–7 each) is shown. The overall effect of aging was assessed by two-way ANOVA (brackets at top); **P<0.01 vs young non-burned mice
Expression of SDF-1 and HIF-1 in response to burn wounding is impaired by aging
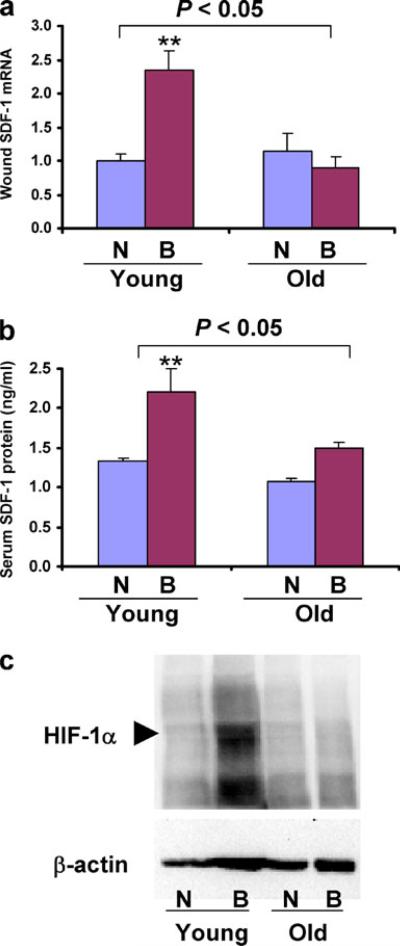
The production of SDF-1 in response to hypoxia/ischemia provides a signal for the mobilization and homing of CXCR4+/Sca1+ BMDACs. Total RNA was isolated from burned and non-burned skin samples that were harvested 2 days after burn wounding from young and old 129S1/SvImJ mice, and SDF-1 mRNA levels were determined by reverse transcription (RT)-qPCR. Mean mRNA levels were not significantly different in the non-burned skin of young vs old mice. However, in young mice, SDF-1 mRNA levels were significantly increased after burn wounding, whereas in old mice, no increase in SDF-1 mRNA levels was observed (Fig. 5a). Thus, aging has no effect on the basal production of SDF-1 mRNA but has a major effect on the expression of SDF-1 mRNA in response to burn wounding.
Fig. 5.
Effect of aging on the expression of SDF-1 and HIF-1α in response to burn wounding. a SDF-1 mRNA expression in the burn wounds. RNA was isolated from the skin of non-burned mice (N) or from the burn wound (B) of young and old 129S1/SvImJ mice on day 2. SDF-1 RNA levels were analyzed by RT-qPCR. Mean±SEM (n=3–4 each) is shown. The overall effect of aging was assessed by two-way ANOVA (brackets at top); **P<0.01 vs Young N. b SDF-1 protein levels in serum samples collected from the same mice analyzed above were determined by ELISA. Mean±SEM (n=3–4 each) is shown. The overall effect of aging was assessed by two-way ANOVA (brackets at top); **P<0.01 vs Young N. c HIF-1α protein levels in burn wounds of young and old mice. On day 2 after burn, non-burned and burned tissues were harvested and aliquots of protein lysates were subjected to immunoblot assays using antibodies against HIF-1α and β-actin
To determine whether differences in SDF-1 mRNA levels in burn wounds are translated into differences in levels of circulating SDF-1 protein that can serve as a signal for mobilization and homing, we analyzed serum SDF-1 levels on day 2 after burn wounding in young and old 129S1/SvImJ mice. Serum SDF-1 protein levels were significantly increased in young, but not in old, mice (Fig. 5b). The significantly decreased SDF-1 mRNA and protein levels in old as compared to young mice provide a molecular basis for the impaired mobilization of CXCR4+/Sca1+ BMDACs.
In a previous study, we demonstrated that in young Hif1a+/– mice, which are heterozygous for a null allele at the locus encoding HIF-1α, serum SDF-1 protein levels do not increase after burn wounding due to a failure to induce HIF-1α expression in burn wounds [14]. Based on these findings, we hypothesized that the impaired expression of SDF-1 in old 129S1/SvImJ mice after burn wounding is associated with an aging-associated defect in HIF-1α expression. HIF-1α protein levels were analyzed in the non-burned and burned skin samples harvested on day 2 after burn wounding from young and old 129S1/SvImJ mice by immunoblot assay. In young mice, HIF-1α protein levels were not detected in the non-burned tissue, whereas after burn wounding, strong expression of HIF-1α protein was detected (Fig. 5c). In contrast, there was no detectable increase in HIF-1α protein levels in old 129S1/SvImJ mice after burn wounding. The impaired induction of HIF-1α protein levels in the burn wounds provides a molecular basis for the marked reduction of SDF-1 expression and thus for the failure of BMDAC mobilization in old mice.
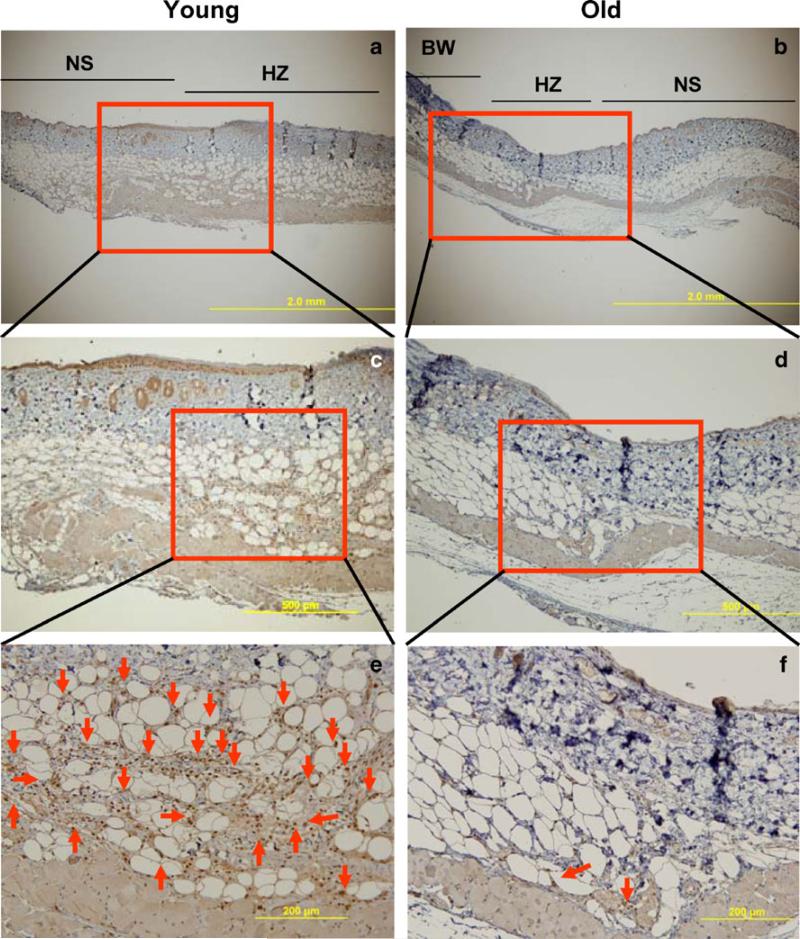
Aging is associated with a reduction of CXCR4+ cells in burn wounds
Our results indicate that aging impairs the mobilization of BMDACs bearing CXCR4 due to a failure to produce the cytokine SDF-1, which is the ligand that binds to CXCR4, and activates mobilization. We hypothesized that in addition to impaired mobilization of BMDACs, the homing of CXCR4+ cells to burn wounds is impaired by failure to induce the homing signal, SDF-1, in old mice. To test this hypothesis, we performed immunohistochemical analysis of burn wounds using an antibody against CXCR4 (Fig. 6). When sections from burn wounds of young 129S1/SvImJ mice on day 3 were analyzed, an abundance of CXCR4+ cells were observed in subcutaneous tissue below the healing zone of burn wounds (Fig. 6e). In contrast, there were few CXCR4+ cells observed in burn wounds from old mice (Fig. 6f). These results are consistent with the hypothesis that aging impairs both the mobilization and homing of CXCR4+ cells to burn wounds. Furthermore, the distant location of these cells relative to the wound suggests that they promote vascularization through paracrine effects rather than through direct incorporation into neovessels within the wound.
Fig. 6.
Effect of aging on the recruitment of CXCR4+ cells to burn wounds. Tissue was harvested from young and old 129S1/SvImJ mice on day 3 after burn wounding and sections were analyzed by immunohistochemistry using anti-CXCR4 antibody. a, b Representative sections of wound and adjacent areas (BW burn wound, NS normal skin, HZ healing zone; original magnification, 40×). c, d Boxed areas in a and b are shown at higher power (original magnification, 100×). e, f Boxed areas in c and d are shown at higher power (original magnification, 200×); red arrow indicates CXCR4+ cell
Aging impairs homing of BMDACs after burn wounding
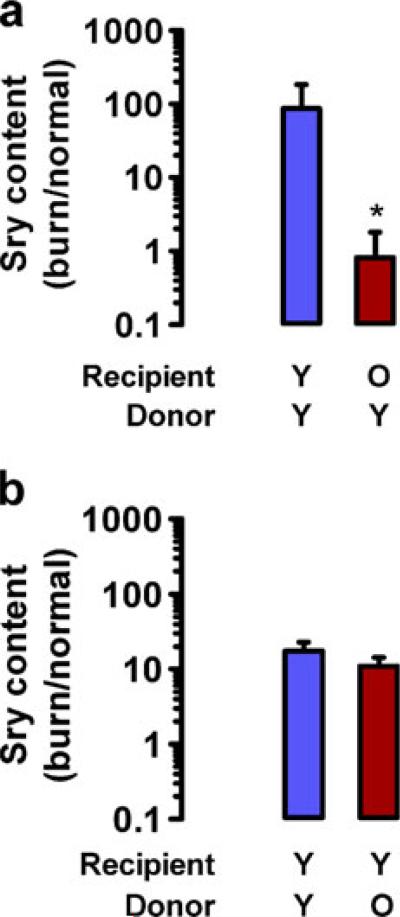
Whereas the immunohistochemical data are consistent with the hypothesis that homing of BMDACs to burn wounds is impaired with aging, BMDACs are likely to represent only a subset of myeloid cells that express CXCR4. To directly analyze homing of BMDACs, we isolated bone marrow cells from young male donor mice and cultured them for 4 days under endothelial growth conditions to select for BMDACs [21, 22], which were injected intravenously into young or old female recipient mice 48 h after burn wounding. To detect homing of BMDACs into the burn wound, non-burned and burned tissues were harvested 8 h after IV injection, DNA was isolated, and qPCR was performed using primers from the Sry gene, which is located on the Y chromosome and is therefore a marker for BMDACs from donor male mice. In young recipient female mice, there was a >87-fold increase in the homing of BMDACs to burned as compared to non-burned skin, whereas in the old recipient mice the ratio of homing to burned vs non-burned skin was 0.8-fold, indicating a complete loss of homing in old recipient mice (Fig. 7a).
Fig. 7.
Effect of recipient or donor age on homing of BMDACs to burn wounds. BMDACs from donor male C57BL/6J mice were administered by tail vein injection 48 h after burn wounding of recipient female C57BL/6J mice. Normal skin and burn wound were harvested 8 h after injection. DNA was extracted and BMDAC homing was measured using qPCR for the Y chromosome-specific Sry gene relative to the autosomal Nme1 gene. Homing was expressed as the signal ratio from burn wound/normal skin. Mean±SEM (n=3–6 each) is shown. Statistical significance was assessed by two-way ANOVA with Bonferroni post hoc comparisons. *P<0.01 vs young recipients. a BMDACs from young (Y) donors were injected into Y or old (O) recipients. b BMDACs from Y or O donors were injected into Y recipients
To investigate whether the age of the BMDAC donor had any effect on homing, we injected BMDACs from young or old male donors into young female recipients 48 h after burn wounding. There was no significant difference in the homing of BMDACs from young vs old donors (homing ratio of 17.4 [young] vs 10.9 [old], P>0.05; Fig. 7b). The selective homing defect of old recipient mice is consistent with the observed defect in HIF-1 → SDF-1 signaling in burn wounds of old mice that is demonstrated in Fig. 5.
Discussion
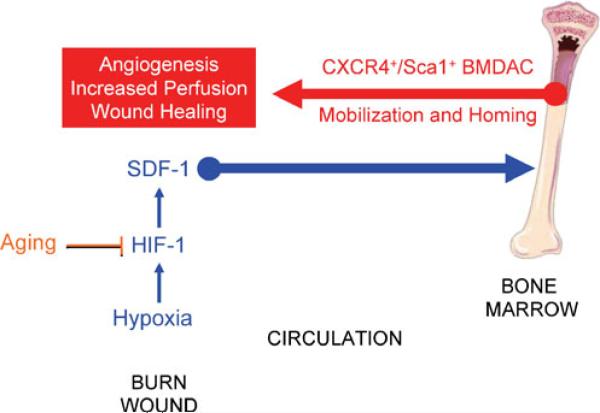
Several factors have been implicated as playing a role in the effect of aging on wound healing, including an excessive inflammatory response and matrix degradation [25], altered energy metabolism [26], decreased granulation tissue [27], and impaired vascularization [8, 27]. A deficit in BMDACs in the granulation tissue of ischemic skin wounds was associated with significantly delayed wound closure [28]. In this study, we provide evidence that impaired HIF-1 → SDF-1 signaling in the burn wounds of old mice is a primary defect resulting in impaired BMDAC mobilization and homing to the wound, which in turn leads to impaired wound vascularization (Fig. 8). These findings are clinically relevant, as studies of burn patients have demonstrated that circulating BMDACs are significantly correlated with levels of SDF-1 in peripheral blood [29].
Fig. 8.
Effect of aging on burn wound healing. Aging impairs the induction of HIF-1α, reduces SDF-1 expression, and impairs mobilization of BMDACs to circulation and their homing to wound, thereby resulting in diminished angiogenesis, reduced tissue perfusion, and delayed wound healing
This work complements previous studies in which we have demonstrated that: impaired HIF-1 induction in mice with partial HIF-1α deficiency blocks SDF-1 expression in burn wounds and the mobilization of CXCR4+/Sca1+ BMDACs [14]; aging impairs the induction of HIF-1 and downstream target genes encoding angiogenic growth factors in excisional wounds of diabetic mice [17]; and aging or partial HIF-1α deficiency impairs the recovery of blood flow following femoral artery occlusion by impairing the expression of genes encoding multiple angiogenic growth factors [21]. Furthermore, work by others has also demonstrated that in a non-diabetic excisional wound model, the age-related impairment in wound healing and vascularization is also associated with reduced expression of HIF-1α and SDF-1 [27]. In an ischemic flap model, HIF-1-mediated SDF-1 expression in hypoxic tissue was found to induce the migration and homing of circulating CXCR4+ cells to ischemic tissue [10]. In this model, the effect of aging on homing was also found to be restricted to the recipient mice [30]. Thus, in several different models of wound healing, HIF-1 → SDF-1 → BMDAC signaling appears to play a critical role. HIF-1 may play other important roles in wound healing, such as regulating keratinocyte proliferation in the hypoxic wound environment [31], which may serve as a means of limiting O2 consumption until vascularization (and increased O2 delivery) is established.
Our findings offer insight into the pathophysiology of burns in the elderly and point to potential targets for developing new therapeutic strategies. Recent studies have demonstrated that increasing HIF-1α expression either by gene therapy or by administering a chemical inducer promoted angiogenesis in diabetic mouse wound model [17, 32]. Moreover, HIF-1α gene therapy combined with BMDAC therapy in a limb ischemia model in aged mice showed improved recovery of perfusion and limb salvage, whereas either therapy alone was ineffective [22]. Further studies are warranted to investigate whether HIF-1α and BMDAC replacement therapy may be useful for treating burn wounds in elderly patients.
Acknowledgments
This work was supported by NIH grant P20-GM078494, the Johns Hopkins Institute for Cell Engineering, and the Hendrix Burn Fund of Johns Hopkins University. We thank Karen Padgett of Novus Biologicals Inc. for generously providing anti-HIF-1 antibody.
Footnotes
X. Zhang and K. Sarkar contributed equally to this work.
Disclosure statement G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine. All authors confirm that there is no conflict of interest associated with this publication.
Contributor Information
Xianjie Zhang, Hendrix Burn Laboratory, Department of Surgery, Johns Hopkins Bayview Medical Center, Baltimore, MD 21224, USA.
Kakali Sarkar, Vascular Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Sergio Rey, Vascular Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; McKusick-Nathans Institute of Genetic Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Raul Sebastian, Hendrix Burn Laboratory, Department of Surgery, Johns Hopkins Bayview Medical Center, Baltimore, MD 21224, USA.
Efstathia Andrikopoulou, Hendrix Burn Laboratory, Department of Surgery, Johns Hopkins Bayview Medical Center, Baltimore, MD 21224, USA.
Guy P. Marti, Hendrix Burn Laboratory, Department of Surgery, Johns Hopkins Bayview Medical Center, Baltimore, MD 21224, USA
Karen Fox-Talbot, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Gregg L. Semenza, Vascular Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA gsemenza@jhmi.edu Department of Pediatrics, Oncology, Radiation Oncology, and Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; McKusick-Nathans Institute of Genetic Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
John W. Harmon, Hendrix Burn Laboratory, Department of Surgery, Johns Hopkins Bayview Medical Center, Baltimore, MD 21224, USA jharmon2@jhmi.edu
References
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Staley M, Richard R. The elderly patient with burns: treatment considerations. J Burn Care Rehabil. 1993;14:559–565. doi: 10.1097/00004630-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Tomic-Canic M, Entero H, Hanflik AM, Wang VM, Fallon JT, Ehrlich HP. The synergism of age and db/db genotype impairs wound healing. Exp Gerontol. 2007;42:523–531. doi: 10.1016/j.exger.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 5.Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992;112:293–297. [PubMed] [Google Scholar]
- 6.Pham TN, Kramer CB, Wang J, Rivara FP, Heimbach DM, Gibran NS, Klein MB. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J Burn Care Res. 2009;30:30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg. 2005;39:293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 8.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- 9.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiol (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 12.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwacha MG, Nickel E, Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1α expression. Mol Med. 2008;14:628–633. doi: 10.2119/2008-00069.Schwacha. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Liu L, Wei X, Tan YS, Tong L, Chang R, Ghanamah MS, Reinblatt M, Marti GP, Harmon JW, Semenza GL. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1α heterozygous-null mice after burn wounding. Wound Repair Regen. 2010;18:193–201. doi: 10.1111/j.1524-475X.2010.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horrevoets AJ. Angiogenic monocytes: another colorful blow to endothelial progenitors. Am J Pathol. 2009;174:1594–1596. doi: 10.2353/ajpath.2009.090198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, Nastai M, Semenza GL, Harmon JW. Age-dependent impairment of HIF-1α expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Wei X, Liu L, Marti GP, Ghanamah MS, Arshad MJ, Strom L, Spence R, Jeng J, Milner S, Harmon JW, Semenza GL. Association of increasing burn severity in mice with delayed mobilization of circulating angiogenic cells. Arch Surg. 2010;145:259–266. doi: 10.1001/archsurg.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DE, Phillips TM, Jeng JC, Rizzo AG, Roth RT, Stanford JL, Jablonski KA, Jordan MH. Microvascular assessment of burn depth conversion during varying resuscitation conditions. J Burn Care Rehabil. 2001;22:406–416. doi: 10.1097/00004630-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1α enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci USA. 2009;106:18769–18774. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 22.Rey S, Lee K, Wang CJ, Gupta K, Chen S, McMillan A, Bhise N, Levchenko A, Semenza GL. Synergistic effect of HIF-1α gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci USA. 2009;106:20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Manhas N, Raghubir R. Energy metabolism during cutaneous wound healing in immunocompromised and aged rats. Mol Cell Biochem. 2004;259:9–14. doi: 10.1023/b:mcbi.0000021339.34784.81. [DOI] [PubMed] [Google Scholar]
- 27.Loh SA, Chang EI, Galvez MG, Thangarajah H, El-ftesi S, Vial IN, Lin DA, Gurtner GC. SDF-1α expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123:65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 28.Bauer SM, Goldstein LJ, Bauer RJ, Chen H, Putt M, Velazquez OC. The bone marrow-derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J Vasc Surg. 2006;43:134–141. doi: 10.1016/j.jvs.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 29.Fox A, Smythe J, Fisher N, Tyler MP, McGrouther DA, Watt SM, Harris AL. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95:244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 30.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1α stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 31.Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, Kuppusamy P, Friedman A, Sen CK. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs wound closure in a murine model of ischemic wounds. Proc Natl Acad Sci USA. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of HIF-1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636–645. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]