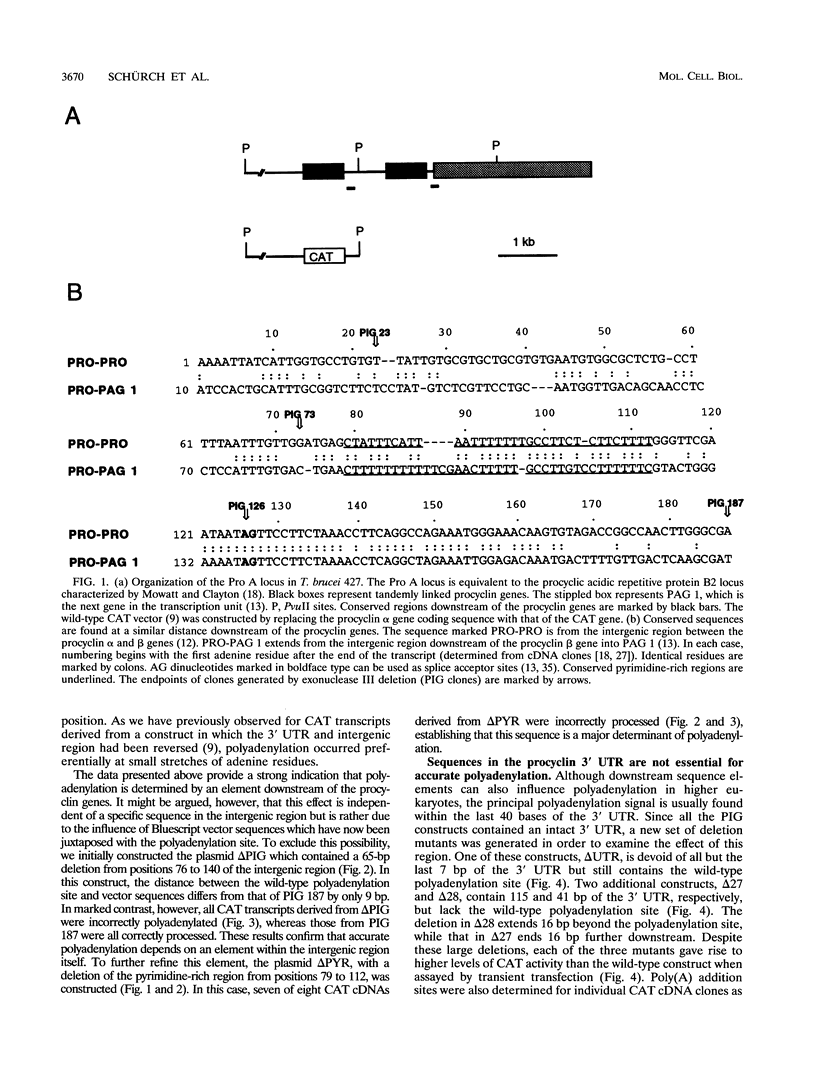
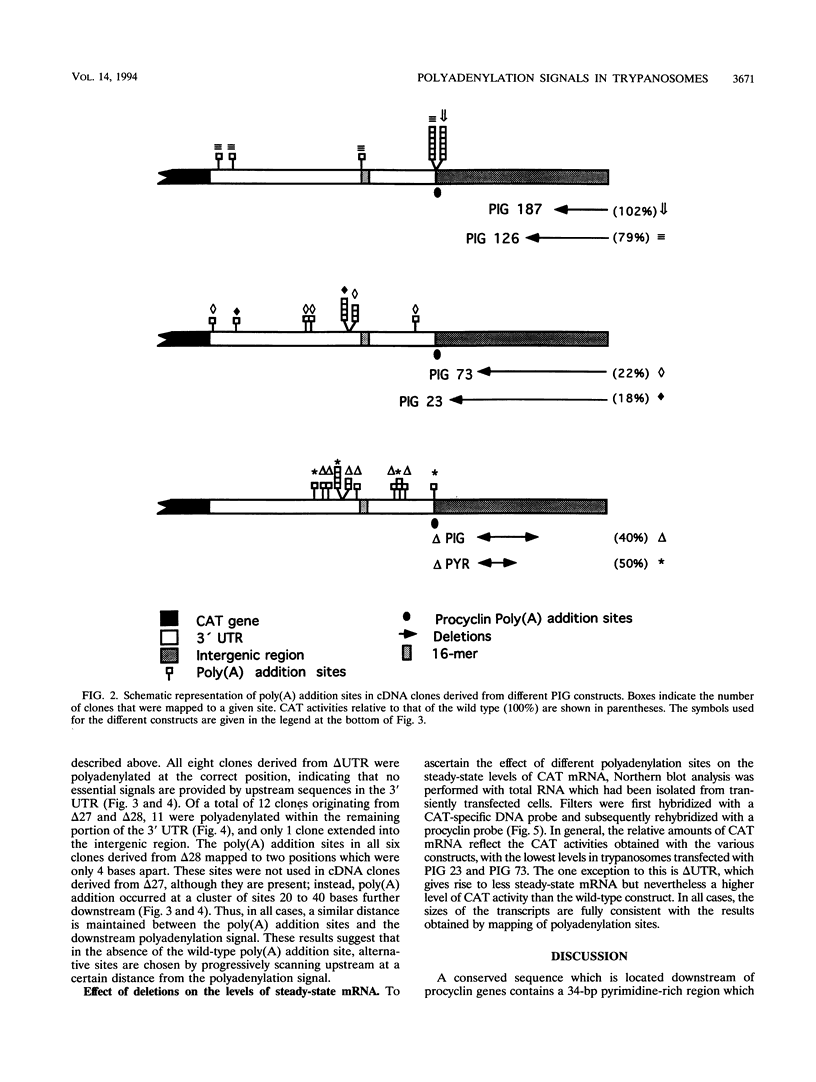
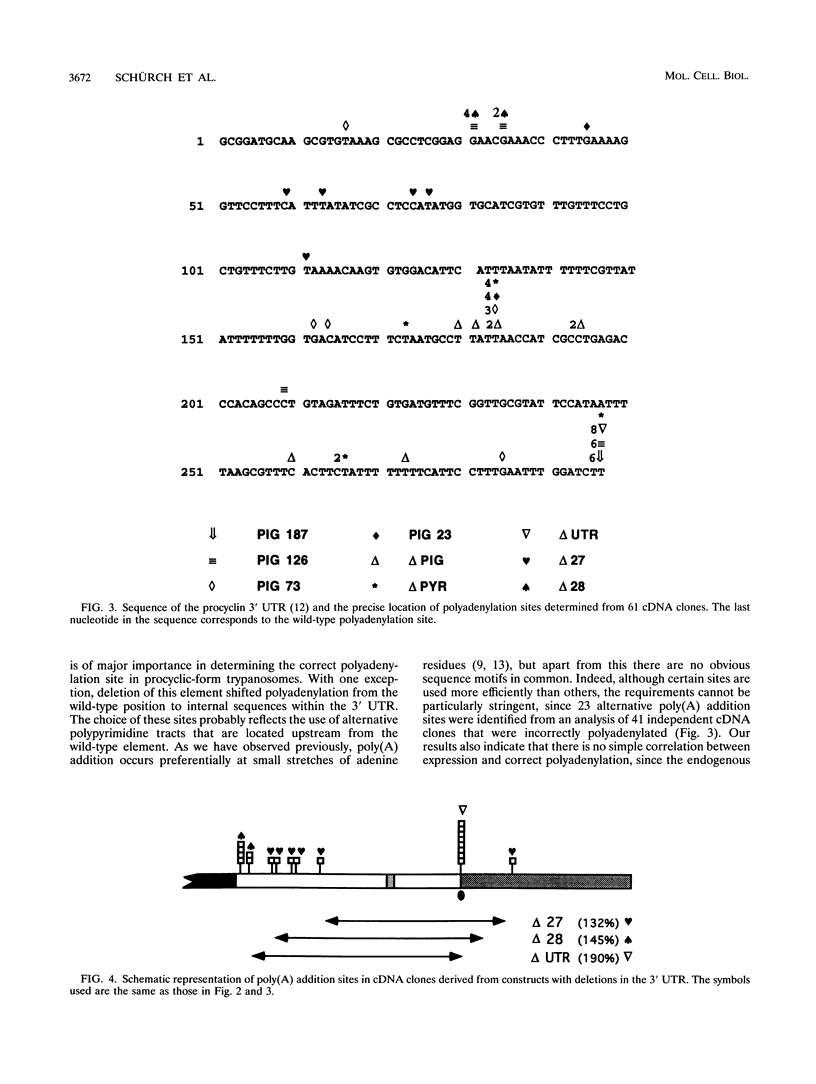
Abstract
Polycistronic precursor RNAs from trypanosomes are processed into monocistronic mRNAs by the excision of intergenic sequences and the addition of a 39-nucleotide spliced leader by trans splicing. These mRNAs are also polyadenylated, yet they do not contain the hexamer AAUAAA within their 3' untranslated regions (UTRs). To identify the signals required for the accurate polyadenylation of mRNAs, we tested the effects of deletions in either the procyclin 3' UTR or the downstream intergenic region on the polyadenylation of transcripts from a reporter gene. Deletion of the entire 3' UTR does not affect polyadenylation, but a crucial element is located in the intergenic region and includes a pyrimidine-rich sequence from positions 79 to 112 followed by an AG dinucleotide. Related motifs are also found a similar distance downstream of other genes in both the procyclin and the variant surface glycoprotein expression sites. These sequences bear a strong resemblance to splice acceptor sites, but they are generally several hundred base pairs upstream of the major splice acceptor site of the next gene in the transcription unit. There is evidence, however, that some of them can give rise to alternatively spliced transcripts with unusually long 5' UTRs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat G. J., Souza A. E., Feagin J. E., Stuart K. Transcript-specific developmental regulation of polyadenylation in Trypanosoma brucei mitochondria. Mol Biochem Parasitol. 1992 Jun;52(2):231–240. doi: 10.1016/0166-6851(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Clayton C. Developmental regulation of nuclear gene expression in Trypanosoma brucei. Prog Nucleic Acid Res Mol Biol. 1992;43:37–66. doi: 10.1016/s0079-6603(08)61043-0. [DOI] [PubMed] [Google Scholar]
- Coquelet H., Steinert M., Pays E. Ultraviolet irradiation inhibits RNA decay and modifies ribosomal RNA processing in Trypanosoma brucei. Mol Biochem Parasitol. 1991 Jan;44(1):33–42. doi: 10.1016/0166-6851(91)90218-u. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- Cross G. A., Manning J. C. Cultivation of Trypanosoma brucei sspp. in semi-defined and defined media. Parasitology. 1973 Dec;67(3):315–331. doi: 10.1017/s0031182000046540. [DOI] [PubMed] [Google Scholar]
- Cully D. F., Ip H. S., Cross G. A. Coordinate transcription of variant surface glycoprotein genes and an expression site associated gene family in Trypanosoma brucei. Cell. 1985 Aug;42(1):173–182. doi: 10.1016/s0092-8674(85)80113-6. [DOI] [PubMed] [Google Scholar]
- Dorn P. L., Aman R. A., Boothroyd J. C. Inhibition of protein synthesis results in super-induction of procyclin (PARP) RNA levels. Mol Biochem Parasitol. 1991 Jan;44(1):133–139. doi: 10.1016/0166-6851(91)90229-y. [DOI] [PubMed] [Google Scholar]
- Hehl A., Vassella E., Braun R., Roditi I. A conserved stem-loop structure in the 3' untranslated region of procyclin mRNAs regulates expression in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):370–374. doi: 10.1073/pnas.91.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Van der Ploeg L. H. Requirement of a polypyrimidine tract for trans-splicing in trypanosomes: discriminating the PARP promoter from the immediately adjacent 3' splice acceptor site. EMBO J. 1991 Dec;10(12):3877–3885. doi: 10.1002/j.1460-2075.1991.tb04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig-Martin E., Yamage M., Roditi I. A procyclin-associated gene in Trypanosoma brucei encodes a polypeptide related to ESAG 6 and 7 proteins. Mol Biochem Parasitol. 1992 Oct;55(1-2):135–145. doi: 10.1016/0166-6851(92)90134-6. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König E., Delius H., Carrington M., Williams R. O., Roditi I. Duplication and transcription of procyclin genes in Trypanosoma brucei. Nucleic Acids Res. 1989 Nov 11;17(21):8727–8739. doi: 10.1093/nar/17.21.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Smith H. Q., Rusche L., Beverley S. M. Coupling of poly(A) site selection and trans-splicing in Leishmania. Genes Dev. 1993 Jun;7(6):996–1007. doi: 10.1101/gad.7.6.996. [DOI] [PubMed] [Google Scholar]
- Lu Y., Hall T., Gay L. S., Donelson J. E. Point mutations are associated with a gene duplication leading to the bloodstream reexpression of a trypanosome metacyclic VSG. Cell. 1993 Feb 12;72(3):397–406. doi: 10.1016/0092-8674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Polymorphism in the procyclic acidic repetitive protein gene family of Trypanosoma brucei. Mol Cell Biol. 1988 Oct;8(10):4055–4062. doi: 10.1128/mcb.8.10.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Wisdom G. S., Clayton C. E. Variation of tandem repeats in the developmentally regulated procyclic acidic repetitive proteins of Trypanosoma brucei. Mol Cell Biol. 1989 Mar;9(3):1332–1335. doi: 10.1128/mcb.9.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Tebabi P., Pays A., Jefferies D., Steinert M., Koenig E., Williams R. O., Roditi I. Trypanosoma brucei: constitutive activity of the VSG and procyclin gene promoters. EMBO J. 1990 Oct;9(10):3145–3151. doi: 10.1002/j.1460-2075.1990.tb07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Tebabi P., Pays A., Coquelet H., Revelard P., Salmon D., Steinert M. The genes and transcripts of an antigen gene expression site from T. brucei. Cell. 1989 Jun 2;57(5):835–845. doi: 10.1016/0092-8674(89)90798-8. [DOI] [PubMed] [Google Scholar]
- Pellé R., Murphy N. B. Stage-specific differential polyadenylation of mini-exon derived RNA in African trypanosomes. Mol Biochem Parasitol. 1993 Jun;59(2):277–286. doi: 10.1016/0166-6851(93)90225-m. [DOI] [PubMed] [Google Scholar]
- Revelard P., Lips S., Pays E. A gene from the VSG expression site of Trypanosoma brucei encodes a protein with both leucine-rich repeats and a putative zinc finger. Nucleic Acids Res. 1990 Dec 25;18(24):7299–7303. doi: 10.1093/nar/18.24.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I., Pearson T. W. The procyclin coat of African trypanosomes (or the not-so-naked trypanosome). Parasitol Today. 1990 Mar;6(3):79–82. doi: 10.1016/0169-4758(90)90216-q. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Bishop D., Gottesdiener K., Van der Ploeg L. H. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 1989 Dec 20;8(13):4259–4263. doi: 10.1002/j.1460-2075.1989.tb08611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi K., Deflorin J., Seebeck T. The major component of the paraflagellar rod of Trypanosoma brucei is a helical protein that is encoded by two identical, tandemly linked genes. J Cell Biol. 1989 Oct;109(4 Pt 1):1695–1709. doi: 10.1083/jcb.109.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E., Matthews K. R., Tschudi C. Temporal order of RNA-processing reactions in trypanosomes: rapid trans splicing precedes polyadenylation of newly synthesized tubulin transcripts. Mol Cell Biol. 1993 Jan;13(1):720–725. doi: 10.1128/mcb.13.1.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Wong S., Morales T. H., Neigel J. E., Campbell D. A. Genomic and transcriptional linkage of the genes for calmodulin, EF-hand 5 protein, and ubiquitin extension protein 52 in Trypanosoma brucei. Mol Cell Biol. 1993 Jan;13(1):207–216. doi: 10.1128/mcb.13.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk J. C., Ouellette M., ten Asbroek A. L., Kieft R., Bommer A. M., Clayton C. E., Borst P. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 1990 Sep;9(9):2791–2801. doi: 10.1002/j.1460-2075.1990.tb07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




