Abstract
The life cycle of most viruses involves the release of particles into the extra-cellular space. Consequently, the study of virus egress as well as virus entry has focused almost exclusively on the biology of cell-free virus. However, cell-free virus spread is often very inefficient. Specific barriers, either located in the donor cell or in the target cell, prevent efficient spread by the cell-free mode. In contrast, viral spread by direct cell-cell contact is largely unaffected by most of these barriers resulting in preferential spread by cell-to-cell transmission. Virus cell-to-cell transmission allows an efficient coordination of several steps of the viral life cycle. It often involves complex inter-cellular adhesion, cellular polarity and intra-cellular trafficking. Because virus cell-to-cell transmission can involve transmission through zones of tight cell-cell contact that are resistant to neutralizing antibodies and reach a high local particle concentration, cell-to-cell transmission can contribute to the pathogenesis of viral infections.
Viruses can spread by two fundamentally distinct modes, either by diffusion through the extracellular space or by direct cell-cell contact [1–4]. Both mechanisms of viral spread have advantages and disadvantages. Transmission by cell-free virus is unrestricted by cell-cell contacts, can allow the spread across long distances within the infected host, and permits an easier spread to a new host. In contrast, spread to contacting neighboring cells via cell-to-cell transmission can be very efficient, and the exploitation of established cell-cell interactions can provide for an alternative mechanism of spread within an organism. Given that both modes of transmission have advantages and disadvantages, viruses evolved mechanisms to use either or both pathways. For example, Vaccinia virus forms different infectious particles [5]. A mature virus (MV) is released after lysis of infected cells and may promote host to host transmission by a cell-free pathway. In contrast, a double membrane-enveloped extracellular virus (EV) remains associated with the producer cell surface and spreads by cell-to-cell transmission [5,6 ].
The mode of virus transmission may also affect the viral life cycle. Cell-to-cell cytosolic connectivity is exploited by plant viruses for the transport of genomes and bypasses the need for the release of particles into the extracellular space [7]. However, in the case of most enveloped animal viruses, the two forms of transmission do not alter the viral life cycle. The observation of cell-cell fusion (syncytia) during human immunodeficiency virus (HIV) infection appears to be largely restricted to lab-adapted viruses [8–11].
Barriers in the cell-free path can enforce a contact-dependent mode of transmission
The question of why a specific virus spreads by cell-free or by cell-to-cell transmission can be better understood if one considers the requirements for spreading by a cell-free mode of transmission. Any virus should be able to spread efficiently by a cell-free mode if the following criteria are met: 1) Viral gene expression should be sufficiently high to support efficient assembly and release of new viruses, 2) cellular factors required for viral assembly and release must also be expressed at sufficient levels, 3) once assembled, viruses should be released efficiently into the extracellular space, 4) extracellular viruses need to be sufficiently stable, and 5) viruses must bind and enter efficiently to target cells. If all these factors are fulfilled, any virus should be able to spread by a cell-free mechanism. However, if any of these steps is inefficient, a barrier to cell-free spread emerges. Interestingly, while these barriers may hinder the cell-free path, they often do not interfere with cell-to-cell transmission, thereby shifting viral spread to a contact dependent mode [12]. Barriers in the cell-free path of transmission can be of a cellular nature, the consequence of antibody-mediated immune responses, or be due to anti-viral restriction factors [13–17]. The promotion of cell-to-cell transmission can be defined as either a donor cell- or a target cell-induced phenomenon.
Donor cell-induced contact dependence
Many cell types do not support viral gene expression or promote efficient viral release at levels required for efficient cell-free spread. This barrier is overcome in co-cultures of infected donor with uninfected target cells since virus assembly can be efficiently orchestrated at sites of cell-cell contact [12,18–20]. Moreover, if viral entry receptors or other proteins with an affinity for viruses remain expressed on the surface of infected cells, these proteins can prevent efficient viral release into the cell-free space. Here again cell-to-cell transmission can circumvent this barrier as long as there is a mechanism for efficient cell adhesion between infected cells and target cells that permits the transmission of viral particles [15,21]. These examples indicate that barriers that interfere with cell-free transmission may favor cell-to-cell spread. It is thus not surprising that viruses have evolved mechanisms to deliberately restrict the cell-free mode of transmission and instead promote cell-to-cell spread. A specific example is the EV form of Vaccinia virus that remains tightly associated with the surface of the producer cell. Activation of Src and Nck by an unknown receptor in the membrane of the producer cell induces actin assembly which propels the virus towards neighboring cells [6,22].
Target cell-induced contact dependence
Inefficient virus binding to and fusion with target cells often severely decreases the infectivity of cell-free viruses [23–26]. In contrast, viruses transferred across tight cell-cell contacts are delivered directly to their target cell receptors, thus increasing binding and entry efficiency. The ability of single cell-free viral particles to cluster sufficient receptors to promote internalization and fusion is inherently limited. During cell-to-cell transmission, this barrier is also overcome as viral entry receptors accumulate at sites of cell-cell contact, thereby facilitating viral internalization and entry [13,27,28]. For HIV, receptor signaling at these cell-cell interfaces promotes the dissolution of the cortical actin cytoskeleton to provide a more favorable environment for viral entry [29]. Altogether, these examples illustrate how barriers in donor and target cells can interfere with cell-free virus transmission. Because these barriers do not prevent cell-to-cell transmission, they can effectively enforce a contact-dependent mode of virus transmission. The poor susceptibility of T cells to cell-free HIV results in a predominately target-cell induced contact dependence for HIV transmission [12]. On the other hand, viruses like the EV form of Vaccinia and HTLV are mostly shaped by events in donor cells, thus reflecting a pronounced donor-cell induced mode of transmission [6,30–32].
The role for surface retention, cell adhesion and polarization in virus cell-to-cell transmission
The viability of cell-free viral spread via diffusion in a 3-dimensional space is dependent on the distance between the producer and target cells and the time taken to cross that distance [4]. If a virus is stable and does not decay, with sufficient time it could reach the most distant target cells (Figure 1A). In contrast, when viral particles decay or are trapped by extracellular factors, diffusion of infectious viral particles becomes very inefficient [33]. Virus retention at the surface of producer cells can overcome this limitation if it is coupled with an efficient mechanism of donor cell adhesion to target cells (Figure 1B). This retention reduces the 3-dimensional spread of virus into spreading via a very thin 2-dimensional cell-cell interface thereby dramatically increasing the local virus particle density (Figure 1B). Polarization of assembly and release would further increase the local virus density thereby increasing the efficiency of virus spreading (Figure 1C). The high local multiplicity of infection reached at sites of cell-cell contact represents a critical factor for why virus cell-to-cell transmission is more efficient than its cell-free counterpart. It permits spreading at much reduced viral and cellular costs, such as lower viral gene expression and less efficient virus release. As such, it explains why the above discussed barriers in the cell-free path are overcome by direct cell-cell contact.
Figure 1.
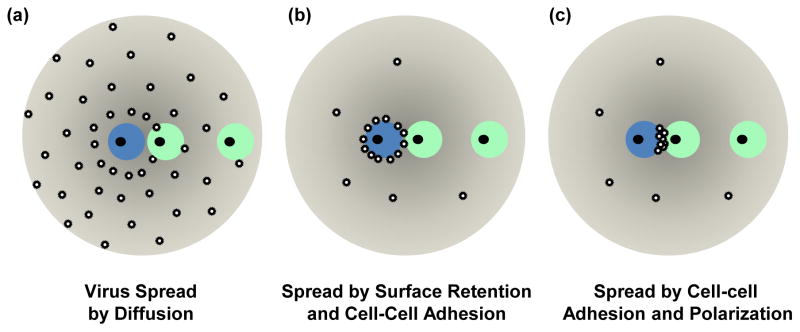
Virus cell-to-cell transmission enhances viral spreading to target cells. (a) Virus spreading by 3-dimensional diffusion creates a concentration gradient around the infected cell. The probability of a viral particle released from the infected cell (blue) to reach the target cell (green) is dependent on the travel distance and the travel time. Adjacent cells would be more efficiently infected, but distant cells can be reached with sufficient time. (b) Retention of particles on the surface of the donor cell interferes with viral spread by diffusion, but increases the local concentration of viral particles and as such increases the probability of infection of contacting neighboring target cells. Thus, any mechanism promoting cell surface retention combined with cell-cell adhesion can enhance the efficiency of viral spreading to contacting cells. (c) Virus cell-to-cell transmission becomes most efficient when particles are also polarized to the site of cell-cell contact. A mechanism involving cell-cell adhesion and polarity leads to the most efficient viral spread. The two target cells illustrate the trade-off between long-range diffusion and highly efficient local viral spread between contacting cells.
These considerations illustrate the trade-off between the ability of cell-free virus to reach distant cells, and highly efficient local spread to neighboring cells (Figure 1). However, viruses that use efficient cell-to-cell spread have evolved alternative mechanism of long-distance travel. For example HIV and alphaviruses exploit the migration patterns of dendritic cells to reach distant target cells [34,35].
Cell biology of virus cell-to-cell transmission
Biological models that aim to explain efficient virus cell-to-cell transmission should address aspects such as cell-cell adhesion, polarity and driving forces [3,18]. Viruses have evolved two main biological strategies to accomplish this. They may utilize existing cell-cell interactions, such as neurological or immunological synapses. Alternatively, they may deliberately establish cell-cell contacts between cells that usually do not form long-lasting contacts (Figure 2). The latter form of adhesion is often achieved by the expression of viral adhesion molecules that bind receptors on neighboring uninfected target cells. In the case of retroviruses, this role is mediated by the envelope glycoprotein [13,20,27,36]. The establishment of adhesion is followed by the ability of infected cells to polarize viral assembly and/or release to sites of cell-cell contact. Viral glycoprotein-mediated cell-cell adhesions, followed by polarized egress, are the hallmarks of the virological synapse [27,37,38]. While this concept was originally described for retroviruses, it is likely more generally applicable to other enveloped viruses [39]. The polarization of virus assembly towards the virological synapse has been documented for the murine leukemia virus (Figure 2C) [20,40]. In the case of HIV, particles on the cell surface are likely drawn toward the virological synapse [41,42]. Thus, this represents a mechanism of surface retention followed by polarization towards the cell-cell contact zone (Figure 2B). Cell-cell adhesion leads to the establishment of cellular polarity, which results in the repositioning of the microtubule organizing center and the redirection of vesicle trafficking behind the synapse. Evidence for polarized secretion of viral particles has been observed for HIV in primary T cells (Figure 3) [43]. Herpes viruses have adapted to spread in polarized neurons by exploiting bidirectional intra-cellular trafficking along microtubules [44].
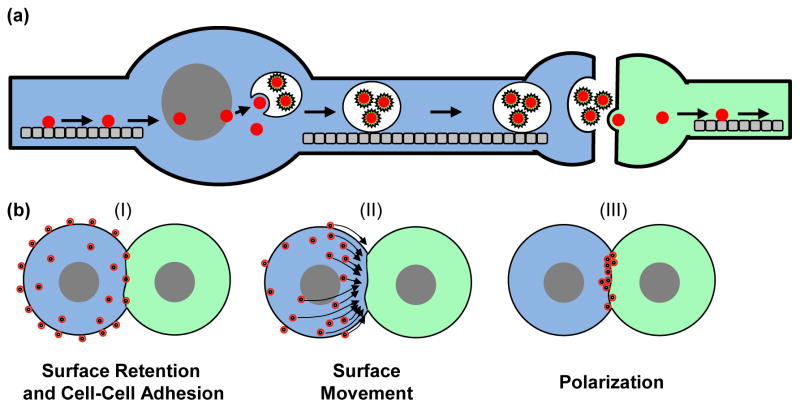
Figure 2.
Viruses evolved to utilize existing cell-cell contacts for efficient viral spreading or, alternatively, are able to deliberately establish new cell-cell contacts between the infected cell (blue) and the target cell (green). (a) Neurotropic viruses can highjack bidirectional microtubule-mediated transport in neurons to spread from one neuron to another [44]. (b) Viruses can also promote long-lived cell-cell contact between cells that typically do not engage in prolonged contacts such as T lymphocytes. Transmission can occur (I) when particles are retained on the surface of the infected cell [15,21], which then comes into contact with an uninfected cell, (II) by surface movement of assembled particles toward the site of cell-cell contact [36,40–42], or (III) by polarization of de novo viral assembly at the site of cell-cell contact [20].
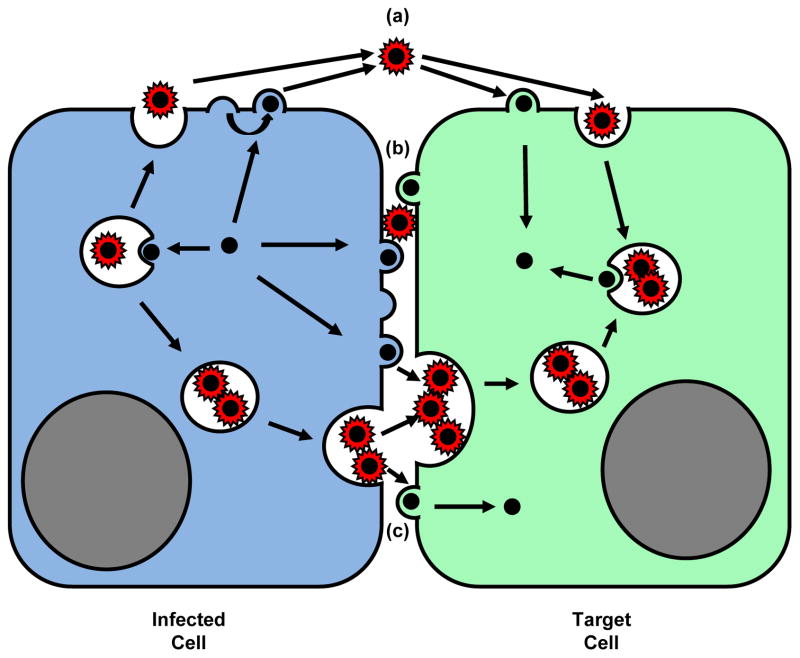
Figure 3.
Cell biology of viral spreading (I). Viral particles can be transmitted by (a) cell-free dissemination through the extracellular space [1,4] or at sites of cell-cell contact (b, c). In both cases, viruses can be released from the cell surface and enter target cells directly at the cell surface [20,27,40]. Alternatively, viruses can also assemble into vesicles that are released by fusion at the plasma membrane and enter target cells by endocytosis prior to delivery into the cytoplasm [28,37,41,43 ].
The directionality for virus cell-to-cell transmission can be provided by driving forces in the donor or target cells. In one of the simplest cases, the spreading of surface-associated viruses is explained by an affinity gradient towards a target cell that expresses the viral entry receptor [21]. The movement towards the target cell is facilitated by the ability of ligands such as growth factors or viruses bound to receptors to engage the underlying retrograde flow of filamentous actin [45–47]. Infected cells can also anchor target cell filopodia to exploit retrograde flow for efficient virus transmission (Figure 4) [36]. The EV form of Vaccinia virus uses the forces of actin assembly in the donor cell as a driving force for transmission [6]. In the case of HIV, activation of the actin cytoskeleton in the donor cell can promote the formation of filopodia that carry viral particles towards neighboring cells [48,49 ]. Thus, the actin cytoskeleton can be exploited in both donor and target cells to promote virus transmission.
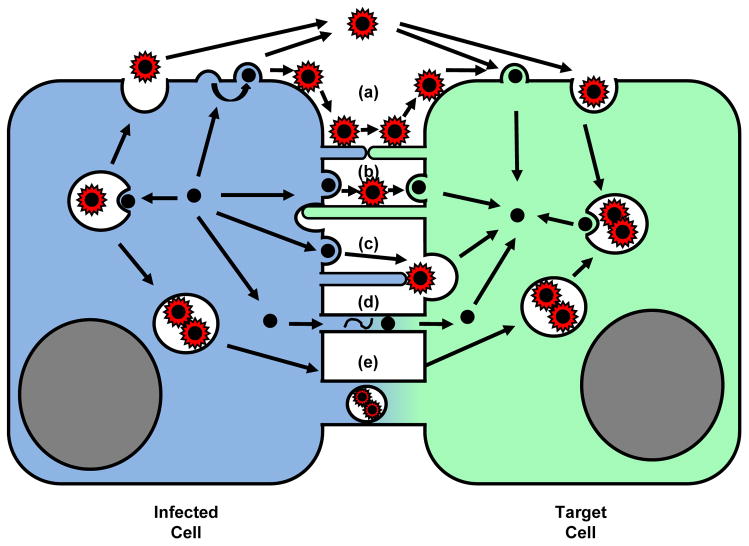
Figure 4.
Cell biology of viral spreading (II). The utilization of inter-cellular membrane bridges. (a, b) In addition to cell-free spread, viruses can remain associated with the surface of the infected cell and spread across long filopodial bridges that connect donor and target cells [21,36,51]. (c) Vaccinia virus remains associated with the producer cell to polymerize actin to propel itself towards neighboring cells [6]. (d) Cytosol-to-cytosol connectivity can promote the transmission of viral genomes or viral capsids [7]. (e) Viral particles may also be transferred across cytoplasmic connections within vesicles [53].
The membrane biology of cell-to-cell transmission can be complex and remains often controversial. For most animal viruses, all surface or endocytic entry events classically discussed during virus egress or virus entry of cell-free virus also apply to transmission at sites of cell-cell contact (Figure 3) [28,50]. In addition, various filopodial/nanotubulular connections have been described (Figure 4). Thin actin-rich connections can provide bridges for the movement of surface virus from cell to cell [36,42,51,52]. Direct cell-to-cell connectivity can be used for the transport of viral genomes of plant viruses thereby bypassing the need for an extracellular phase [7]. Cell-to-cell connectivity has also been proposed for the microtubule-driven exchange of vesicles carrying completely pinched-off viral particles [52,53].
Cell-to-cell transmission and viral pathogenesis
Multiple observations suggest that cell-to-cell transmission contributes to the pathogenesis of many viral infections. The ability of neurotropic viruses such as Herpes viruses to spread along neurons manifests their clinical pathogenesis. Bidirectional transport along neurons allows Herpes viruses to reach ganglions to establish latency. Yet upon activation, viruses travel back to the periphery to cause another round of acute infection [44][54]. Viral spread via tight cell-cell contacts also allows many viruses to evade neutralizing antibodies thus contributing to immune evasion [13,14,55–59]. Viral restriction factors such as TRIM5α and tetherin that effectively inhibit cell-free retroviruses are either less effective or fail entirely to inhibit cell-to-cell transmission [14,16,17,60,61]. The high local virus concentration also lowers the effectiveness of antiviral compounds as it requires a considerably higher drug concentration for effective inhibition [62–65]. Higher proviral content could result in higher genetic diversity of the viral population as recombinant variants may appear at faster rates. Thus, particularly in the case of the HIV/AIDS epidemic, two critical questions that remain to be addressed are to what extent cell-to-cell transmission contributes to viral spreading in in vivo, and if it contributes to viral persistence.
Intravital imaging of viral spreading
These considerations reinforce the importance of studying viral spreading directly within a living organism. Recently, the first visualization of the behavior of retrovirus-infected cells has been accomplished in living mice [66,67]. Intravital imaging of HIV-infected T cells in humanized mice confirms a critical role of the viral glycoprotein in adhesive interactions with uninfected cells [66]. Work in our laboratory revealed that B cells infected with MLV can indeed form virological synapses within the lymph node of living mice. Thus, both studies verify some of the main concepts of the virological synapses in vivo. These technologies will shed new light on the role of virus cell-to-cell transmission in a living organism. Finally, if virus cell-to-cell transmission is truly central to the pathogenesis of many viral infections, the development of inhibitors that directly interfere with this process may be critical to prevent the spread of viral infections.
Highlights.
Barriers in the cell-free mode of virus transmission can promote virus cell-to-cell transmission
Donor and target cell induced contact dependent viral spread
Virus cell-to-cell transmission involves cell adhesion and polarization
Virus cell-to-cell transmission is more efficient and contributes to viral pathogenesis
Acknowledgments
We thank Ari Helenius for discussions. This work was supported by the NIH R01s CA098727 & AI084096 to WM, a Cancer Research Institute postdoctoral fellowship to JBM, and a fellowship from the China Scholarship Council-Yale World Scholars in the Biomedical Sciences to PZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattentau QJ. The direct passage of animal viruses between cells. Current opinion in virology. 2011;1:396–402. doi: 10.1016/j.coviro.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–8368. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yakimovich A, Gumpert H, Burckhardt CJ, Lutschg VA, Jurgeit A, Sbalzarini IF, Greber UF. Cell-free transmission of human adenovirus by passive mass transfer in cell culture simulated in a computer model. Journal of virology. 2012;86:10123–10137. doi: 10.1128/JVI.01102-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GL, Murphy BJ, Law M. Vaccinia virus motility. Annu Rev Microbiol. 2003;57:323–342. doi: 10.1146/annurev.micro.57.030502.091037. [DOI] [PubMed] [Google Scholar]
- 6.Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 2001;11:30–38. doi: 10.1016/s0962-8924(00)01871-7. [DOI] [PubMed] [Google Scholar]
- 7.Lucas WJ. Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology. 2006;344:169–184. doi: 10.1016/j.virol.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Weng J, Krementsov DN, Khurana S, Roy NH, Thali M. Formation of syncytia is repressed by tetraspanins in human immunodeficiency virus type 1-producing cells. Journal of virology. 2009;83:7467–7474. doi: 10.1128/JVI.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon-Alonso M, Yanez-Mo M, Barreiro O, Alvarez S, Munoz-Fernandez MA, Valenzuela-Fernandez A, Sanchez-Madrid F. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. Journal of immunology. 2006;177:5129–5137. doi: 10.4049/jimmunol.177.8.5129. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Orenstein J, Dimitrov D, Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 11.Moore JP, Ho DD. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9 (Suppl A):S117–136. [PubMed] [Google Scholar]
- 12.Zhong P, Agosto LM, Ilinskaya A, Dorjbal B, Truong R, Derse D, Uchil P, Heidecker G, Mothes W. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS ONE. 2012 doi: 10.1371/journal.pone.0053138. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant Mode of Human Immunodeficiency Virus Transfer between T Cells Is Mediated by Sustained Env-Dependent Neutralization-Resistant Virological Synapses. J Virol. 2007;81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. Cell-Cell Transmission Enables HIV-1 to Evade Inhibition by Potent CD4bs Directed Antibodies. PLoS pathogens. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. Most comprehensive study on the differential susceptibility of HIV cell-to-cell transmission to neutralizing antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin AG, Guatelli J, Schwartz O. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J, Riley JL. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J Virol. 2008;82:11117–11128. doi: 10.1128/JVI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DC, Huber MT. Directed egress of animal viruses promotes cell-to-cell spread. J Virol. 2002;76:1–8. doi: 10.1128/JVI.76.1.1-8.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 20•.Jin J, Sherer NM, Heidecker G, Derse D, Mothes W. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS Biol. 2009;7:e1000163. doi: 10.1371/journal.pbio.1000163. Documents the ability of a retrovirus to polarize assembly to sites of cell-cell contact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherer NM, Jin J, Mothes W. Directional spread of surface associated retroviruses regulated by differential virus-cell interactions. J Virol. 2010;87:3248–3258. doi: 10.1128/JVI.02155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodding MP, Way M. Nck- and N-WASP-dependent actin-based motility is conserved in divergent vertebrate poxviruses. Cell host & microbe. 2009;6:536–550. doi: 10.1016/j.chom.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 23.van der Schaar HM, Rust MJ, Waarts BL, van der Ende-Metselaar H, Kuhn RJ, Wilschut J, Zhuang X, Smit JM. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platt EJ, Kozak SL, Durnin JP, Hope TJ, Kabat D. Rapid Dissociation of HIV-1 from Cultured Cells Severely Limits Infectivity Assays, Causes the Inactivation Ascribed to Entry Inhibitors, and Masks the Inherently High Level of Infectivity of Virions. J Virol. 2010;84:3106–3110. doi: 10.1128/JVI.01958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27• •.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. Together with McDonald, et al. [37] and Igakura, et al. [38], this study establishes the concept of the virological synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale BM, McNerney GP, Thompson DL, Hubner W, de Los Reyes K, Chuang FY, Huser T, Chen BK. Cell-to-Cell Transfer of HIV-1 via Virological Synapses Leads to Endosomal Virion Maturation that Activates Viral Membrane Fusion. Cell host & microbe. 2011;10:551–562. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasiliver-Shamis G, Cho MW, Hioe CE, Dustin ML. Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J Virol. 2009;83:11341–11355. doi: 10.1128/JVI.01440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazurov D, Ilinskaya A, Heidecker G, Lloyd P, Derse D. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS pathogens. 2010;6:e1000788. doi: 10.1371/journal.ppat.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazurov D, Ilinskaya A, Heidecker G, Filatov A. The role of O-glycosylation and expression of CD43 and CD45 on the surface of effector T cells in HTLV-1 cell-to-cell infection. Journal of virology. 2011;10 doi: 10.1128/JVI.06993-11. 1128/JVI.06993–06911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoulouze MI, Alcover A. Can viruses form biofilms? Trends in microbiology. 2011;19:257–262. doi: 10.1016/j.tim.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald D. Dendritic Cells and HIV-1 Trans-Infection. Viruses. 2010;2:1704–1717. doi: 10.3390/v2081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. Journal of virology. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. Together with Hubner, et al. [41], both studies visualize the transfer of retroviral particles from cell to cell directly in living cells. Documents the role of thin filopodial membrane bridges in facilitating retroviral transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 38••.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. The McDonald, et al. and the Igakura, et al. studies, together with Jolly, et al. [27], establish the concept of the virological synapse. [DOI] [PubMed] [Google Scholar]
- 39.Aubert M, Yoon M, Sloan DD, Spear PG, Jerome KR. The virological synapse facilitates herpes simplex virus entry into T cells. Journal of virology. 2009;83:6171–6183. doi: 10.1128/JVI.02163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin J, Li F, Mothes W. Viral determinants of polarized assembly for the murine leukemia virus. Journal of virology. 2011;85:7672–7682. doi: 10.1128/JVI.00409-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. Together with Sherer, et al. [36], both studies visualize the transfer of retroviral particles from cell to cell directly in living cells. Documents the surface flux of HIV particles into the cell-cell contact zone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudnicka D, Feldman J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple tragets through polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolly C, Welsch S, Michor S, Sattentau QJ. The regulated secretory pathway in CD4(+) T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS pathogens. 2011;7:e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A. 2001;98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burckhardt CJ, Greber UF. Virus movements on the plasma membrane support infection and transmission between cells. PLoS Pathog. 2009;5:e1000621. doi: 10.1371/journal.ppat.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–4852. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal A, Iemma TL, Shih I, Newsome TP, McAllery S, Cunningham AL, Turville SG. Mobilization of HIV Spread by Diaphanous 2 Dependent Filopodia in Infected Dendritic Cells. PLoS pathogens. 2012;8:e1002762. doi: 10.1371/journal.ppat.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 52.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 53.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. Journal of Neuroimmune Pharmacology. 2011;6:658–675. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roizman B, Knipe DM. Herpes Simplex viruses and their replication. In: Knipe DM, editor. Fields Virology. 4. Vol. 1 Lippincott Williams & Wilkins; 2001. p. 1123. [Google Scholar]
- 55.Ganesh L, Leung K, Lore K, Levin R, Panet A, Schwartz O, Koup RA, Nabel GJ. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Black FL, Melnick JL. Microepidemiology of poliomyelitis and herpes-B infections: spread of the viruses within tissue cultures. J Immunol. 1955;74:236–242. [PubMed] [Google Scholar]
- 57.Gupta P, Balachandran R, Ho M, Enrico A, Rinaldo C. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J Virol. 1989;63:2361–2365. doi: 10.1128/jvi.63.5.2361-2365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merz DC, Scheid A, Choppin PW. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980;151:275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips DM. The role of cell-to-cell transmission in HIV infection. Aids. 1994;8:719–731. doi: 10.1097/00002030-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Celestino M, Calistri A, Del Vecchio C, Salata C, Chiuppesi F, Pistello M, Borsetti A, Palu G, Parolin C. Feline tetherin is characterized by a short N-terminal region and is counteracted by the feline immunodeficiency virus envelope glycoprotein. Journal of virology. 2012;86:6688–6700. doi: 10.1128/JVI.07037-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhl BD, Sloan RD, Donahue DA, Bar-Magen T, Liang C, Wainberg MA. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology. 2010;7:115. doi: 10.1186/1742-4690-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. Multiploid inheritance of HIV-1 during cell-to-cell infection. Journal of virology. 2011;85:7169–7176. doi: 10.1128/JVI.00231-11. Demonstrates the concept of high multiplicity of infection during virus cell-to-cell transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Permanyer M, Ballana E, Ruiz A, Badia R, Riveira-Munoz E, Gonzalo E, Clotet B, Este JA. Antiretroviral Agents Effectively Block HIV Replication after Cell-to-Cell Transfer. Journal of virology. 2012;86:8773–8780. doi: 10.1128/JVI.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 65.Mori K, Haruyama T, Nagata K. Tamiflu-resistant but HA-mediated cell-to-cell transmission through apical membranes of cell-associated influenza viruses. PloS one. 2011;6:e28178. doi: 10.1371/journal.pone.0028178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012 doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Sewald X, Gonzalez DG, Haberman A, Mothes W. In vivo imaging of virological synapses. 2012 doi: 10.1038/ncomms2338. Submitted. The Murooka, et al. [66] and Sewald, et al. [67] represent the first in vivo studies on retroviral spreading that confirm key concepts of the virological synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]