Abstract
Background
Common single nucleotide polymorphisms (SNPs) at chromosome 4q25 (rs2200733, rs10033464) are associated with both lone and typical AF. Risk alleles at 4q25 have recently been shown to predict recurrence of AF after ablation in a population of predominately lone AF, but lone AF represents only 5–30% of AF cases.
Objective
To test the hypothesis that 4q25 AF risk alleles can predict response to AF ablation in the majority of AF cases.
Methods
Patients enrolled in the Vanderbilt AF Registry underwent 378 catheter-based AF ablations (median age 60 years, 71% male, 89% typical AF) between 2004 and 2011. The primary endpoint was time to recurrence of any non-sinus atrial tachyarrhythmia (atrial tachycardia, atrial flutter, or AF; [AT/AF]).
Results
Two-hundred AT/AF recurrences (53%) were observed. In multivariable analysis, the rs2200733 risk allele predicted a 24% shorter recurrence-free time (survival time ratio 0.76 95% confidence interval [CI] 0.6–0.95, P=0.016) compared with wild-type. The heterozygous haplotype demonstrated a 21% shorter recurrence-free time (survival time ratio = 0.79, 95% CI 0.62–0.99) and the homozygous risk allele carriers a 39% shorter recurrence-free time (survival time ratio = 0.61, 95% CI 0.37–1.0) (P=0.037).
Conclusion
Risk alleles at the 4q25 loci predict impaired clinical response to AF ablation in a population of predominately typical AF patients. Our findings suggest the rs2200733 polymorphism may hold promise as an as an objectively measured patient characteristic that can used as a clinical tool for selection of patients for AF ablation.
Keywords: atrial fibrillation, ablation, pulmonary vein isolation, genetics, 4q25
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and affects 2–5 million adults in the United States1 for an annual direct healthcare cost of over 6.5 billion dollars.2 It is thought that the majority of patients with AF possess a combination of common and rare genetic variants that predispose to its development, which clinically manifests in the presence of acquired cardiac or systemic disease.3 This is termed “typical” AF and is estimated to represent between 70% to greater than 95% of all AF cases.4–7 To examine the potential of genetic screening toward addressing the healthcare burden imposed by AF, it is necessary to determine the role of common genetic risk markers in modulating response to therapies, such as AF ablation, in patients with typical AF.
Over the past decade, genome wide association (GWA) studies have identified many common genetic variants associated with AF.8–10 The strongest association mapped to two common AF susceptibility single nucleotide polymorphisms (SNPS) on chromosome 4q25. This locus is near the paired-like homeodomain transcription factor 2 (PITX2) gene which codes for the development of the pulmonary veins and left to right symmetry.11 Approximately 25% of individuals of European ancestry have been found to carry the 4q25 AF risk allele and accumulating evidence suggests these individuals demonstrate impaired clinical response to a variety of AF therapies including antiarrhythmic drugs (AADs) and catheter-based AF ablation.12, 13 Recently, in a population of predominately lone AF, risk allele carriers at chromosome 4q25 demonstrated impaired response to catheter ablation.13 To address the ability of genetic risk markers to predict clinical response to AF ablation in the majority of AF patients, we sought to test the hypothesis that common AF susceptibility alleles on chromosome 4q25 (rs2200733, rs10033464) conferred an increased risk for AF recurrence after catheter-based ablation in patients with typical AF.
Methods
Study Population
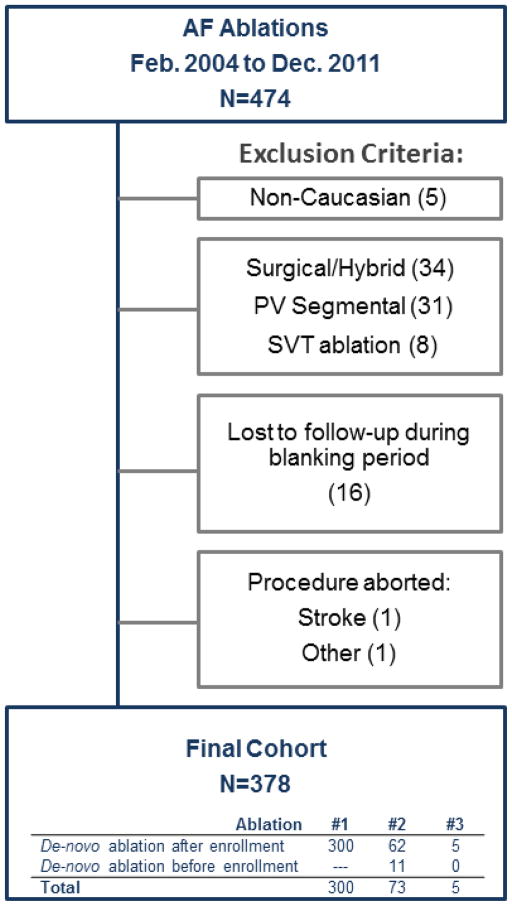
Three hundred seventy two patients enrolled in the Vanderbilt AF Registry underwent 474 catheter-based AF ablations from February, 2004 to December, 2011 (Figure 1). The Vanderbilt AF Registry is a prospective clinical and genetic database.14 Eligible ablation records were from Caucasian patients who underwent a de novo or repeat catheter-based AF ablation and were followed-up for at least 3 months. Records from de novo segmental pulmonary vein isolation procedures, surgical AF ablation, and hybrid catheter/surgical ablations were excluded. Additionally, patients lost to follow-up prior to the end of the 3-month blanking period, and ablations aborted due to intra-operative complication were excluded.
Figure 1.
Patient Eligibility diagram.
Clinical Evaluation
Patient characteristics and procedural details were entered into a central database.15 A detailed medical history was obtained through patient questioning and examination of medical records, and a physical exam was performed. Lone AF was defined as AF occurring in the absence of cardiac or systemic disease in patients less than 66 years of age. Typical AF was defined as patients with non-lone AF. Paroxysmal AF was defined as episodes lasting less than 7 days and spontaneously terminating. Non-paroxysmal AF was defined as AF episodes lasting greater than 7 days and/or requiring termination with pharmacologic or direct current cardioversion (DCCV). Total number of lifetime DCCV prior to ablation, and number of lifetime AAD trials were recorded based on patient report. Body mass index (BMI) was calculated by weight in kilograms divided by height in meters squared. Measurements of left atrial (LA) size and left ventricular ejection fraction (LVEF) were obtained from pre-ablation cardiac magnetic resonance imaging (MRI) or transthoracic echocardiogram (TTE).
Catheter Ablation
All patients received general anesthesia during the ablation. Vascular access was obtained from the right and/or left femoral veins with or without right internal jugular veins according to operator preference for coronary sinus cannulation. All ablations were performed using biplane fluoroscopy. Left atrial access was obtained using transseptal puncture under anteroposterior and left anterior oblique fluoroscopic views with assistance from intracardiac echocardiogram.
Standard peri-procedural anticoagulation strategies were used. In cases where pre-procedure therapeutic INR for at least one month was not documented, a transesophageal echocardiogram was performed prior to the ablation to document absence of left atrial thrombus. Post-procedure, heparin or low molecular weight heparin was resumed after sheath removal and hemostasis and continued until therapeutic oral anticoagulation was achieved. Anticoagulation with warfarin or dabigatran was continued for at least 3 months following ablation.
All de-novo ablations consisted of circumferential pulmonary vein antral isolation confirmed by entrance block, with additional linear ablation and ablation of non-pulmonary vein (PV) foci based on operator discretion. Wide-area circumferential antral ablation was performed with use of a 3-D electroanatomic mapping system and placement of contiguous lesions 5 to 15 mm from the PV ostia with testing for entrance and exit block. Prior to 2009, ablation was performed using an 8 French 5mm tip temperature controlled radiofrequency (RF) catheter with RF energy applied with temperatures of 55–60°C and a maximum power of 50 W for 30–45 seconds at each site. Since 2009, an 8 French 3.5 mm tip open irrigated-tip power-controlled ablation catheter was used with maximum power of 25 W on the posterior wall and 30–35 W on the anterior wall and roof. Entrance and exit block were tested utilizing high output pacing with isoproterenol and/or adenosine administration directing additional lesions as necessary to achieve complete bidirectional electrical isolation of all PVs.
Repeat catheter ablation was performed for patients who experienced a recurrent atrial tachyarrhythmia after the 3-month blanking period. During the repeat ablation, PV re-isolation was performed for the presence of intact conduction into or out of a PV. Based on the presenting rhythm, additional ablation was performed with focal ablation of localized reentries, complex fractionated atrial electrogram (CFAE) ablation, and linear lesions for macroreentry circuits. Atrial tachycardias were mapped using entrainment and/or activation mapping. Based on operator discretion, additional induction maneuvers utilizing isoproterenol and/or rapid atrial pacing was performed. Ablations were performed by 1 of 6 electrophysiologists.
Follow-up
All patients were hospitalized for 24 hours after the procedure with continuous monitoring of heart rhythm. Standard clinic follow-up occurred at 3, 6, and 12 months post-ablation, with performance of ECG, detailed history, and physical exam. Ambulatory monitor use with a 48-hour Holter monitor or 7–30 day auto-trigger event monitor was routinely completed before follow-up appointments with performance of additional ambulatory monitoring to evaluate reported symptoms suggestive of recurrence. To prevent short-term recurrences, patients were discharged on AADs for at least 3 months post-ablation unless contraindicated. If free of recurrent AF, AADs were discontinued at the 3 month follow-up appointment.
Outcomes
A recurrence was defined according to the HRS/EHRA/ECAS Consensus Statement recommendations for AF ablation as any episode of non-sinus atrial tachyarrhythmia (atrial tachycardia, atrial flutter, or AF; [AT/AF]) lasting greater than 30 seconds that occurred after the 3-month post-ablation blanking period.16 Both symptomatic and asymptomatic episodes qualified as an AT/AF recurrence and were required to be documented on cardiac tracing. Date of recurrence was recorded to determine the primary endpoint which was time to first AT/AF recurrence after the 3-month blanking period. Genotyping was not performed until after completion of the study period, therefore all clinical and research personnel were blinded to risk allele status. Patients who experienced a recurrent episode of AT/AF were restarted on an AAD, and if recurrent episodes persisted despite AAD use, the patient was offered repeat ablation.
Statistical Analysis
Continuous variables are expressed as median and interquartile range (IQR). Categorical variables are presented as percentages and frequencies. Univariate analysis was performed for continuous variables using the non-parametric Mann-Whitney U test or Kruskal-Wallis H test as appropriate, and for nominal variables was performed using a Fisher’s exact test or Chi-square test as appropriate. The primary analysis tested the hypothesis that risk allele carriers at either of the two 4q25 AF SNPs (rs2200733, rs10033464) causes shorter AT/AF recurrence-free time. Both dominant and additive genetic models were employed. Multivariable survival analysis was performed to test the independent effect of carrying the risk allele at each AF susceptibility loci on AT/AF recurrence-free time. Due to the dependence between ablation records included in our database from individuals who underwent more than one ablation, a parameteric accelerated failure time log normal survival model with sandwich variance estimators was utilized rather than a Cox-Proportional Hazards Model. Unadjusted models were fit to generate the median event free time and their 95% confidence intervals. The log-normal survival model gives a beta-coefficient that equals the log survival time difference between two groups and the exponentiation of the beta coefficient generates the ratio of median survival time between two groups. The survival time ratio is the ratio of median survival time from the 4q25 wildtype genotype versus the median survival time of the 4q25 variant genotype. The survival analysis was truncated at 18 months due to decreased frequency of observations beyond 18 months of follow-up. To avoid over-fitting, covariates included in our final multivariable model were pre-specified, which means selection was based on their expected relationship to the primary determinant of AF risk allele status and the primary outcome of AT/AF recurrence-free survival time. Numbers of covariates were selected to allow 1 degree of freedom per 10 AT/AF recurrence events. The final model was adjusted for age at ablation, gender, BMI, lone AF status (yes/no), number of prior DCCV, prior AF ablation (yes/no), LA size (continuously expressed in mm), year of ablation, performing physician, cavotricuspid isthmus ablation (yes/no), and AAD discharge status (yes/no). As AF ablation is a complex procedure that during the study period evolved in many aspects including patient selection, procedural technique, and follow-up; year of ablation and performing physician were included in the final model to adjust for unidentified differences related to these factors that were not otherwise accounted for by covariates included in our model. Age, BMI, and LA size were included with a non-linear correction factor. A subgroup analysis was performed to examine whether the effect of carrying the risk allele at rs2200733 susceptibility loci persisted among the typical AF only group. A post-hoc analysis was performed to examine the independent effect of 4q25 risk allele status on AF recurrence among patients with LA enlargement > 5 cm. All analyses were performed using statistical software R version 2.14.1 (12-22-2011).
Genotyping
Genomic DNA was isolated from whole blood by a commercial kit (Purgene; Gentra Systems, Minneapolis, MN). Genotyping was performed for rs2200733 C>T and rs10033464 G>T using the TaqMan procedure, a plate based, one-step reaction that identifies the SNP allele during the polymerase chain reaction amplification.17 Samples that failed initial genotyping with TaqMan were resequenced using Sanger sequencing methods. All genotyping was performed by laboratory personnel blinded to all clinical data.
Results
Patient characteristics
Complete baseline patient characteristics and procedural details are presented in Tables 1 and 2. The final study cohort consisted of 311 unique patients. Two hundred thirty eight patients underwent only one ablation and 73 patients underwent two or more.
Table 1.
Patient characteristics
| All Patients (N=311) | Typical AF (N=278) | Lone AF (N=33) | |
|---|---|---|---|
| Age | 60(52,66) | 61(55,67) | 49(44,52) |
| Male | 72%(224) | 71%(198) | 79%(26) |
| Body Mass Index (kg/m2) | 30(27,35) | 30(27,35) | 27(26,34) |
| Paroxysmal AF | 47%(147) | 44%(123) | 73%(24) |
| Time since AF diagnosis (months) | 57(26,105) | 59(28,106) | 37(18,87) |
| Number of Prior Direct Current Cardioversions | 2(2,9) | 3(2,9) | 2(2,3) |
| Number of Failed Antiarrhythmic Drugs | |||
| 0 | 6%(19) | 5%(14) | 15%(5) |
| 1 | 44%(138) | 45%(125) | 39%(13) |
| 2 | 30%(93) | 29%(82) | 32%(11) |
| 3 | 14%(44) | 15%(43) | 3%(1) |
| 4 | 5%(14) | 4%(11) | 9%(3) |
| 5 | 1%(2) | 1%(2) | 0%(0) |
| 6 | 0%(1) | 0%(1) | 0%(0) |
| Previously Failed Amiodarone | 35%(110) | 37%(104) | 18%(6) |
| Coronary Artery Disease | 17%(54) | 19%(54) | 0%(0) |
| Congestive Heart Failure | 10%(30) | 11%(30) | 0%(0) |
| Left Atrial Size (mm) | 38(33,44) | 39(34,45) | 35(29,39) |
| Left Ventricular Ejection Fraction from MRI (%) | 65(56,71) | 65(56,71) | 64(59,68) |
| Genotypes | |||
| rs2200733 (4q25) | |||
| CC | 59%(184) | 62%(171) | 39%(13) |
| CT | 34%(105) | 32%(89) | 48%(16) |
| TT | 7%(21) | 6%(17) | 12%(4) |
| Failed Genotyping | 0%(1) | N/A | N/A |
| Minor Allele Frequency | 25% | 22% | 36% |
| rs10033464 (4q25) | |||
| GG | 76%(237) | 76%(212) | 76%(25) |
| GT | 22%(69) | 22%(62) | 21%(7) |
| TT | 2%(5) | 1%(4) | 3%(1) |
| Failed Genotyping | 0%(0) | N/A | N/A |
| Minor Allele Frequency | 13% | 14% | 14% |
Continuous variables are median and interquartile range. Categorical variable are percentage and frequency. For rs2200733 cytosine (C) is the wildtype and T (thymine) is the risk allele. For rs10033464 guanine (G) is the wildtype and T is the risk allele.
Table 2.
Procedural details
| All Ablations (N=378) | Typical AF (N=338) | Lone AF (N=40) | |
|---|---|---|---|
| Year of ablation | |||
| 2004 | 3%(12) | 3%(10) | 5%(2) |
| 2005 | 5%(19) | 4%(15) | 10%(4) |
| 2006 | 9%(34) | 9%(30) | 10%(4) |
| 2007 | 11%(43) | 12%(39) | 10%(4) |
| 2008 | 15%(56) | 15%(50) | 15%(6) |
| 2009 | 16%(60) | 16%(55) | 12%(5) |
| 2010 | 26%(99) | 27%(92) | 18%(7) |
| 2011 | 15%(55) | 14%(47) | 20%(8) |
| Performing Physician | |||
| 1 | 23%(88) | 23%(78) | 25%(10) |
| 2 | 12%(47) | 12%(40) | 18%(7) |
| 3 | 13%(48) | 13%(43) | 12%(5) |
| 4 | 18%(66) | 18%(62) | 10%(4) |
| 5 | 31%(115) | 30%(102) | 32%(13) |
| 6 | 3%(13) | 4%(12) | 2%(1) |
| Mitral annulus linear ablation | 25%(95) | 25%(85) | 25%(10) |
| LA roof linear ablation | 45%(171) | 46%(157) | 35%(14) |
| CFAE ablation | 12%(44) | 13%(43) | 2%(1) |
| Cavotricuspid isthmus ablation | 22%(84) | 22%(76) | 20%(8) |
| Discharged on AAD | 86%(317) | 90%(35) | 85%(282) |
CFAE=complex fractionated atrial electrogram.
Samples were successfully genotyped at a call rate ≥ 99% for both SNPs. Complete haplotype and minor allele frequencies are presented in Table 1. Risk allele carriers at rs2200733 were more likely to have lone AF and demonstrated a graded effect with respect to proportion of patients with lone AF depending on number of risk allele copies (CC: 7% vs. CT: 15% vs. TT: 22%, P=0.01). No difference existed with respect to proportion of patients with lone AF among risk allele carriers at rs10033464.
Response to AF Ablation
Two-hundred patients experienced a qualifying AT/AF recurrence after ablation (10.8 months, 95% CI 9.4, 12.2) for an overall recurrence rate of 53% (200/378). Among the 300 patients undergoing de-novo ablation, 165 experienced a recurrence (median 10.2 months, 95% CI 7.4, 12.8) for a rate of 55% (165/300). Among the 78 patients undergoing repeat ablation, 35 experienced a recurrence (median 11.3 months, 95% CI 7.8, NA) for a recurrence rate of 45% (35/78). Overall, 71.2% of patients either completed 18 months follow-up or achieved the primary end-point of AT/AF recurrence. Patients were censored due to less than 18-months had elapsed since date of ablation, or they were lost to follow-up. The 28.8% of censored patients were evenly distributed throughout the follow-up protocol such that 7.9% were censored between 3–6 months, 11.9% between 6–12 months, and 9.8% between 12–18 months. No difference existed in rate of censoring between 4q25 genotypes.
Univariate Analysis of Risk Allele Status on Response to Ablation
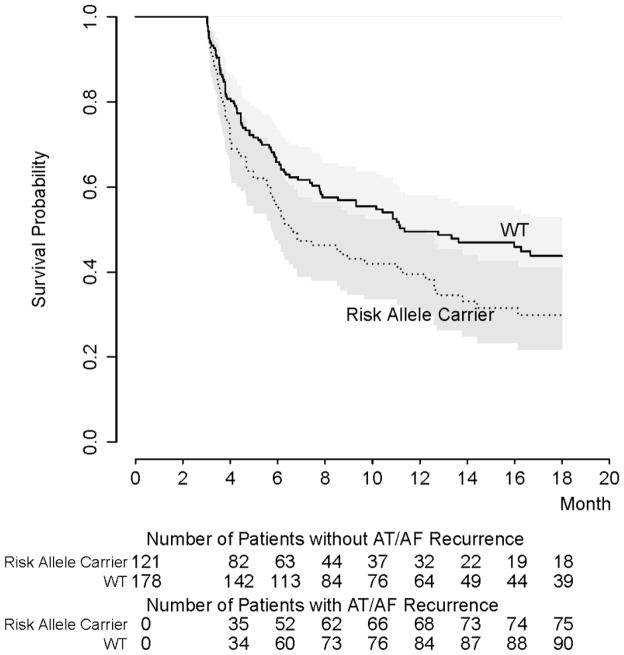
Examining rs2200733, the rate of AT/AF recurrence among risk allele carriers was 58% versus 50% for wild-type (WT) patients (P=0.113), with a significantly shorter unadjusted time to recurrence (9.3 months [95% CI 7.8, 11.1] vs. 11.8 months [95% CI 9.9, 14], P=0.036). In subgroup analysis when restricted to the 299 patients undergoing de-novo ablation who were successfully genotyped at rs2200733, the effect of rs2200733 risk alleles on earlier recurrence was found to persist (6.7 months [95% CI 5.8,12.2] vs. 11.4 months [95% CI 8.6,NA], P=0.018, Log rank test) (Figure 2), but the study was not powered to detect a difference when restricted to the 78 patients undergoing repeat ablation (8.5 months [95% CI 6.6, NA] vs. 12.7 [95% CI 7.0, NA], P=0.91 Log rank test). No significant difference was detected in overall recurrence rate (53% vs. 53%, P=0.98) or recurrence-free time in risk allele carriers at rs10033464 (10.8 months [95% CI 9.3, 12.5] vs.10.8 months [95% CI 8.3, 14.2], P=0.97).
Figure 2.
Kaplan-Meier curve of rs2200733 risk allele carriers versus wild-type (WT) demonstrates shorter AT/AF recurrence-free survival time for patients undergoing de-novo ablation only (N=299). Gray area defines 95% confidence interval (CI) for survival plot (P = 0.018 by Log-rank test).
Multivariable Analysis of Risk Allele Status on Response to Ablation
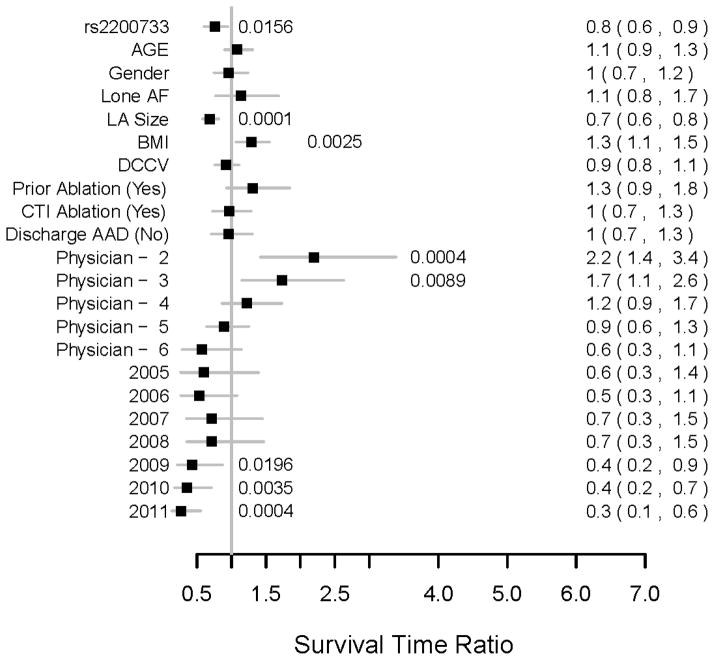
In the primary analysis of the study, using a dominant genetic model the rs2200733 risk allele was found to predict a statistically significant 24% shorter recurrence-free time after ablation (survival time ratio = 0.76, 95% CI 0.6–0.95, P=0.016) (Figure 3). LA size (P=0.0001) and BMI (P=.0025) were patient characteristics also found to independently predict recurrence-free time. Performing physician (P=0.0013), and year of ablation (P=0.0002) were found to be significant confounders affecting recurrence-free time that were also adjusted for in this multivariable model. The association between rs2200733 remained significant in the typical AF only subgroup (P=0.027)
Figure 3.
For the final multivariable analysis of rs2200733, the median survival time ratios with 95% CIs for all covariates are displayed. Significant P-values are displayed adjacent to plots. Median survival time ratios for continuous variables were calculated comparing 3rd versus 1st quartile values (Age 66.8 vs. 53 years, LA diameter 44 vs. 34 mm, BMI 34.4 vs. 27.0 kg/m2). Physician 1 and 2004 were used as the reference values for their respective ordinal covariate categories. DCCV=direct current cardioversion; CTI=cavotricuspid isthmus; AAD=antiarrhythmic drug.
Using additive genetic modeling, rs2200733 predicted shorter recurrence-free time (P=0.037) by 21% for the heterozygous risk allele haplotype (survival time ratio = 0.79, 95% CI 0.62–0.99) and 39% for the homozygous risk allele haplotype (survival time ratio = 0.61, 95% CI 0.37–1.0). The rs10033464 risk allele did not predict shorter recurrence-free time (P=0.47).
Discussion
In this study we demonstrated that the 4q25 AF risk allele rs2200733 independently predicted recurrence of AT/AF after catheter ablation. Our findings are from a highly generalizable, non-selected group of AF ablation patients with predominately typical AF undergoing both de-novo and repeat procedures. This extends the finding that common genetic variation at the 4q25 AF risk loci modulate clinical response to AF ablation in patients with lone AF to the majority of AF patients who possess significant comorbid risk factors.13
Increased LA size is a patient characteristic that is well recognized to predict clinical response to AF ablation and in clinical practice an LA diameter of 5.0 to 5.5 cm is used by many physicians as a cutoff to determine AF ablation eligibility.16 Our findings demonstrate that LA size and the rs2200733 risk allele confer independent effects, and among a small subset of patients with LA diameter > 5 cm who underwent AF ablation, the addition of rs2200733 risk allele status was found to increase recurrence rate from 40% to 87.5% (P=0.04, Figure 4). This suggests that rs2200733 may hold promise as another objectively measured patient characteristic that can be used along with LA size as a clinical tool to select patients for AF ablation.
Figure 4.
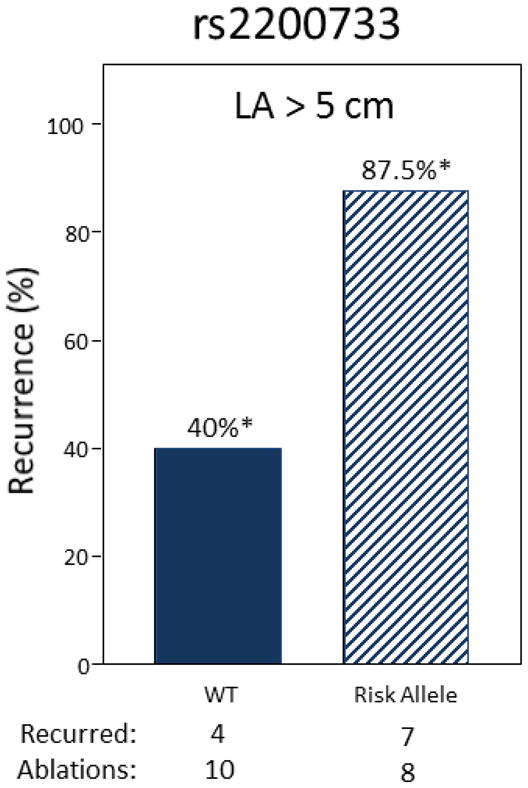
Among patients with left atrial (LA) diameter > 5 cm, possession of a risk allele at rs2200733 increases rate of AT/AF recurrence from 40% to 87.5% (*P=0.04, Fisher’s Exact Test).
Comparison to Prior Studies
In 2010, the effect of the 4q25 AF risk alleles on 6-month recurrence outcomes after catheter-based AF ablation was examined in a population of patients with predominately lone (83%), paroxysmal (78%) AF.13 Both rs2200733 and rs10033464 were found to predict an approximate 2.5-fold increased odds of recurrence by 6-months. Compared to typical AF, lone AF occurs in younger patients without any predisposing cardiac or systemic comorbid conditions and is believed to occur due to genetic determinants of larger effect size. Lone AF represents only 5–30% of all AF cases and it was not known whether the observed effect of 4q25 AF risk alleles to modify response to ablation persisted in the majority of AF cases which are represented by typical AF.
We demonstrated in our population of predominately typical AF patients, that the 4q25 risk allele at rs2200733 conferred a similar modest-sized effect on impaired response to catheter-based AF ablation. Unlike the previous study, rs10033464 was not found to significantly predict impaired response, but this is in agreement with other studies that have demonstrated an association between some, but not all 4q25 risk loci on clinical outcome. 12, 13 This is likely due to differences in the degree of linkage disequilibrium between 4q25 marker polymorphisms and the underlying causative polymorphism that can occur due to differences in patient sampling between studies, or more broadly between the degree of genetic admixture in different continental populations.
4q25 and Mechanisms for AF
Our study sought to examine the 4q25 AF risk locus identified by GWA studies.8–10 The minor allele frequency for the SNPs examined range from 13% (rs10033464) to 25% (rs2200733) and therefore provide a significant exposure in the overall population. The 4q25 locus exists in a non-protein coding (intronic) chromosomal segment and the exact mechanism for its association with AF development remains unproven. However, it is suspected to act through modulation of the nearest gene, PITX2, which is known to code for development of the PV myocardial sleeve- a critical source of AF triggers.18 The role of ectopic potentials originating from the PV myocardium is well recognized and the success of AF ablation is based on preventing these ectopic potentials from entering the LA and triggering AF.19 It has been shown that approximately 80% of recurrent AF results from electrical reconnection of the PV myocardium with the body of the LA.20, 21 It is possible that differences exist in the PV myocardium between 4q25 risk allele carriers and WT patients at baseline, or in response to RF injury and scar formation that could account for the differences in the AT/AF recurrence rates after catheter-ablation.
Study Limitations
This study was limited by the variability in the ablation procedure and monitoring protocol that resulted from the extended duration of the study period. To partially adjust for this variability, performing physician and year of ablation were included as covariates in our multivariable model. Year of ablation was statistically associated with earlier time to recurrence from 2009 to 2011, which may in-part reflect more rigorous post-ablation monitoring that coincided with the full adoption of HRS/EFRA/ECAS expert consensus standards first published in mid-2007. The study was not sufficiently powered to test for the presence of an interaction between genotype and performing physician. Additionally, follow-up data was captured at the time of clinically scheduled return visits, and 28.8% of patients underwent data censoring due to being within the 18-month window from date of ablation, or being lost-to-follow-up. Due to data-censoring the absolute time to recurrence of AT/AF should be deemphasized. However, the primary goal of this study was to establish the relative contribution of 4q25 genotype on time to AF recurrence, and given risk allele status is a fixed, concealed determinant it is not expected that variability in follow-up provided a source of bias. Second, recurrence of AF after ablation is a complex phenomenon with many potential contributors. We selected 11 covariates for our final multivariable model that we felt best adjusted for factors affecting time to recurrence with the least redundancy amongst individual covariates. However, different or additional covariates could have been selected to improve the model. Finally, as common to all prognostic study designs, the mechanism by which AF risk loci affect recurrence of AF after catheter ablation was not evaluated. The findings of this study should serve to prioritize the rs2200733 SNP for further studies to better understand the mechanism of its association with variability in clinical response to ablation.
Conclusion
The 4q25 AF risk polymorphism rs2200733 predicts impaired clinical response to catheter ablation in multivariable analysis among a highly generalizable population of patients undergoing de-novo and repeat AF ablation. Overall, our findings demonstrate that a 4q25 AF risk allele may hold promise as an objectively measured patient characteristic that can used as a clinical tool for selecting patients for AF ablation.
Acknowledgments
Financial Support: American Heart Association Established Investigator (0940116N) and Clinical Research Program (11CRP7420009) Awards, and National Institutes of Health grants U19 HL65962, HL092217 and UL1 RR024975.
Acknowledgements: None
Abbreviations
- AF
atrial fibrillation
- GWA
genome wide association
- SNP
single nucleotide polymorphism
- PITX2
paired-like homeodomain transcription factor 2
- AAD
antiarrhythmic drug
- DCCV
direct current cardioversion
- BMI
body mass index
- LA
left atrial
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- TTE
transthoracic echocardiogram
- PV
pulmonary vein
- RF
radiofrequency
- CFAE
complex fractionated atrial electrograms
- AT/AF
atrial tachycardia, atrial flutter, or atrial fibrillation
- WT
wildtype
Footnotes
Conflicts of Interest: None
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004 Aug 31;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005 Nov;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie MD, Rowan S, Kucera G, et al. Chromosome 4q25 variants are genetic modifiers of rare ion channel mutations associated with familial atrial fibrillation. J Am Coll Cardiol. 2012 Sep 25;60:1173–1181. doi: 10.1016/j.jacc.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans W, Swann P. Lone auricular fibrillation. Brit Heart J. 1954 Apr;16:189–194. doi: 10.1136/hrt.16.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. J Am Med Assoc. 1985 Dec 27;254:3449–3453. [PubMed] [Google Scholar]
- 6.Levy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists Circulation. 1999 Jun 15;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 7.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007 Jun 19;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 8.Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007 Jul 19;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 9.Gudbjartsson DF, Holm H, Gretarsdottir S, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nature Genetics. 2009 Aug;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010 Mar;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdine RD, Schier AF. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 2000 Apr 1;14:763–776. [PubMed] [Google Scholar]
- 12.Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012 Aug 7;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010 Feb 23;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007 Jun;4:743–749. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9:632–696. e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Alderborn A, Kristofferson A, Hammerling U. Determination of single-nucleotide polymorphisms by real-time pyrophosphate DNA sequencing. Genome Res. 2000 Aug;10:1249–1258. doi: 10.1101/gr.10.8.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. P Natl Acad Sci USA. 2010 May 25;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998 Sep 3;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 20.Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003 Sep 30;108:1599–1604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005 Jan 18;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]