Abstract
Aims
To determine whether an insulin algorithm could be used in a similar manner in the setting of diabetes and stress hyperglycemia following cessation of intravenous (IV) insulin after cardiac surgery.
Materials and Methods
Subjects who were clinically stable, requiring ≥ 1 unit/hr of IV insulin 48 hours after surgery, were randomized to once daily detemir at 50, 65, or 80% of IV insulin requirements and received aspart according to carbohydrate intake. Diabetes was defined as any history of diabetes or preoperative HbA1c 6.5%.
Results
The AM glucose in patients with diabetes was 143 mg/dl (N=61) vs. 124 mg/dl in those with stress hyperglycemia (N=21, p=0.05) on day 1 and 127 vs. 110 mg/dl over 72 hours (p=0.01). This was unaffected by adjustment for initial dosing group. At 72 hours, 56% of patients with stress hyperglycemia reached AM (80–130 mg/dl) and 87% reached overall (80–180 mg/dl) glucose targets, compared to 90 and 100% of patients with stress hyperglycemia, , respectively. There was no difference in hypoglycemia in patients with stress hyperglycemia or diabetes. The percentage of patients with diabetes receiving insulin was 46% on admission and 77% at discharge, compared to 0% and 42% of patients with stress hyperglycemia.
Conclusions
Following cardiac surgery, patients with stress hyperglycemia may be converted from IV insulin to detemir with a 50% conversion factor, while patients with diabetes may require a higher conversion factor. Stress hyperglycemia may be prolonged; the intensity and duration of insulin therapy required for optimal outcomes warrants further examination.
INTRODUCTION
In the cardiac surgery population, a large proportion of patients require intravenous (IV) insulin, even where no previous diagnosis of diabetes exists. In one study, 36–49% of patients without previously diagnosed diabetes required an insulin infusion [1]. However, the duration and extent of metabolic derangements is less well known. Many such patients have evidence of pre-diabetes or metabolic syndrome [2–4]. Since perioperative hyperglycemia is associated with worse outcomes regardless of diabetes status [5–6], strategies for management must be evaluated in all patients.
Glycemic control using IV insulin infusions is known to improve morbidity and mortality following cardiac surgery [7], and insulin infusions are considered routine in this setting [8]. However, there are little data guiding providers on subsequent care post-infusion. One approach is to administer long-acting basal insulin at a dose of 80% of the predicted 24 hour requirements in patients who are receiving minimal exogenous carbohydrate (CHO) exposure based on the previous 6–8 hours of insulin dosing [8–10]. However, the magnitude and duration of insulin resistance may change rapidly as the stress of the surgery resolves [11]. Therefore, an identical approach for patients with diabetes or “stress hyperglycemia” may not be suitable. The current investigation is a subgroup analysis of a prospective randomized study designed to determine the effectiveness of an algorithm that contains one of 3 conversion factors for the initial dose of detemir, as well as flexible prandial and supplemental insulin aspart in cardiac surgical patients who are being transitioning off of an IV insulin infusion. The objective of this analysis is to determine whether the management of patients with persistent IV insulin requirements following cardiac surgery differs among patients with stress hyperglycemia or diabetes.
MATERIALS AND METHODS
Patients were eligible for study enrollment if they were requiring at least 1 unit/hour of IV insulin 48 hours following cardiac surgery and were no longer requiring vasopressors or mechanical ventilation. Patients receiving glucocorticoids or enteral or parenteral nutrition were excluded. Other exclusion criteria included pregnancy, end-stage kidney or liver disease, or inability to give consent in English. The protocol was approved by the study institution's Institutional Review Board, and all patients signed informed consent to participate.
Procedures
Details of the study procedures were published previously [12]. Briefly, all patients undergoing cardiac surgery who develop hyperglycemia (two consecutive glucose measurements 150 mg/dl perioperatively) at the study institution were placed on a standardized, hospital-wide, nursing-run insulin infusion algorithm [13] that was continued for a minimum of 48 hours postoperatively. For the initial dose of detemir, patients were randomly assigned to one of 3 groups, calculated based upon 50%, 65%, or 80% of projected basal IV insulin requirements. The projected total daily basal need was calculated from the average infusion rate in the previous 8 hours (from 9 AM to 5 PM) multiplied by 3. If the insulin infusion was unstable (change greater than 2 unit per hour) in the preceding 6 hours prior to discontinuation, the dose was calculated based upon the 24 hour insulin requirement. Patients received their first dose of detemir at 6 PM, once daily, and the infusion was continued for four additional hours.
During the study, patients received subcutaneous aspart at 1 unit/10 grams of carbohydrates as soon as a diet was ordered, even while receiving IV insulin therapy. Patients with very large basal insulin requirements (70 units) received 1 unit/5 grams of carbohydrate intake. Overlap with rapid acting insulin was intended to prevent large fluctuations in drip requirements associated with eating, thus allowing more precise calculation of basal insulin needs. If the patient received carbohydrates that were not otherwise covered by aspart, the total daily basal insulin requirement was adjusted downward by 1 unit per 10 gm prior to calculation of the final subcutaneous insulin dose. Patients received a supplemental correction factor of 1 unit of aspart per 25 mg/dl increment of glucose above 150 mg/dl, once off the insulin drip. The aspart was continued throughout the first 72 hours of the study without adjustment, at which point adjustment in prandial insulin was allowed.
Subsequent doses of detemir were titrated upwards or downwards as needed according to a fasting glucose target of 80–130 mg/dL. The detemir was reduced 20% for any glucose <80 mg/dl. On day 1, the dose was increased 5, 10, 15, or 20% for AM glucose greater than 150, 180, 200, or 250 mg/dl respectively. On days 2 and 3, the dose was increased 5, 10, 15, or 20% for a fasting glucose greater than 130, 150, 200, or 250 mg/dl respectively. The patient could be reverted to the IV insulin infusion for persistent hyperglycemia 200 mg/dl. Following study completion (at 72 hours or discharge if earlier than 72 hours), adjustments in therapy and regimen at discharge were implemented at the discretion of the inpatient diabetes consult team.
HbA1c is drawn preoperatively as part of a standardized order set. Glucose was monitored at a minimum of before meals and at bedtime using the Accuchek Inform® glucometer.
Statistical Analysis
Outcomes of interest included AM glucose and mean glucose, % of patients meeting AM glucose target 80–130 mg/dl or mean glucose target 80–180 mg/dl, and hypoglycemia (defined as any blood glucose <65 mg/dl). Glucose may be converted to SI units (mmol/l) by multiplying by 0.0555. Glycemic variability was calculated using coefficient of variation (CV), mean distance travelled (mean daily range), and absolute daily risk range (ADRR) [14].
The diagnosis of diabetes was defined as a history of diabetes or glucose-lowering medication at admission, or HbA1c 6.5%. Differences between groups were determined with the ANOVA or X2 test as appropriate. Values were reported as mean +/− SD unless otherwise stated. P-values less than 0.05 were considered statistically significant. Stepwise nominal logistic regression was performed using dosage group and either diabetes or stress hyperglycemia diagnosis or HbA1c as independent variables in order to determine relationships with the following dependent variables: requirement for repeated detemir dose titration and insulin therapy at discharge. Analyses were performed using JMP 8.0 software.
RESULTS
Seventy per cent of patients had a diagnosis of diabetes at admission, whereas one additional patient was found to have diabetes when classified by an HbA1c 6.5% (table 1). The remaining patients (26%) were classified as having stress hyperglycemia. Other baseline characteristics are shown in table 1. The initial dose of detemir and initial 24 hour total insulin dose were not statistically different between those with diabetes or stress hyperglycemia (table 2).
Table 1.
Baseline Characteristics
| Total N=82 | Stress Hyperglycemia N=25 | Diabetes N=61 | P-value | |
|---|---|---|---|---|
| African American | 6 (7.3%) | 1 (4.8%) | 5 (8.2%) | 0.99 |
| White American | 76 (93%) | 20 (95%) | 56 (92%) | |
| Female | 17 (21%) | 1 (4.8%) | 16 (26%) | 0.06 |
| Age (years) | 59 (8.4) | 57 (7.3) | 59 (8.7) | 0.82 |
| BMI (kg/m2) | 33.2 (6.9) | 32 (4.1) | 34 (7.6) | 0.11 |
| Time to extubation (days) | 1.3 (0.78) | 1.2 (0.51) | 1.3 (0.86) | 0.41 |
| Days on pressors | 1.1 (0.80) | 1.1 (0.65) | 1.0 (0.86) | 0.54 |
| Creatinine | 0.99 (0.44) | 1.1 (0.44) | 0.95 (0.44) | 0.17 |
| HbA1c | 7.2 (1.8) | 5.7 (0.33) | 7.7 (1.9) | <0.0001 |
| Insulin (admission) | 28 (34%) | 0 | 28 (46%) | NA |
| Any glucose-lowering drug (admission) | 56 (68%) | 0 | 56 (92%) | NA |
| Indication for surgery | ||||
| Coronary artery bypass surgery | 54 (66%) | 14 (67%) | 40 (66%) | 0.99 |
| Valve | 23 (28%) | 8 (38%) | 15 (35%) | 0.27 |
| Aneurysm/dissection | 5 (6.1%) | 3 (14%) | 2 (3%) | 0.10 |
| Other | 11 (13%) | 3 (14%) | 15 (25%) | 0.38 |
| Coronary artery disease | 61 (77%) | 15 (71%) | 46 (79%) | 0.55 |
| Hypertension | 70 (86%) | 18 (86%) | 52 (87%) | 0.99 |
| Hyperlipidemia | 65 (82%) | 16 (76%) | 49 (84%) | 0.51 |
| Tobacco (current) | 11 (13.6%) | 4 (20%) | 7(11%) | * |
| Peripheral vascular disease | 4 (6.0%) | 0 | 4 (8%) | 0.57 |
| Stroke | 9 (12%) | 0 | 9 (16%) | 0.10 |
Table 2.
Study Data
| DM N=61 | Stress N=21 | P-value | |
|---|---|---|---|
| Detemir 24 hour | 37 (18) | 30 (19) | 0.18 |
| Total Insulin 24 hour | 54 (22) | 44 (19) | 0.06 |
| Revert to drip | 3 (4.9%) | 0 | 0.99 |
| AM glucose | |||
| Mean | 127 (33) | 110 (21) | 0.01 |
| % at Target | 34 (56%) | 19 (90%) | 0.004 |
| Overall glucose | |||
| Mean | 147 (31) | 112 (16) | <0.0001 |
| % at Target | 53 (87%) | 21 (100%) | 0.11 |
| Hypoglycemia | |||
| Day 1 | 1 (1.6%) | 1 (4.8%) | 0.45 |
| Overall | 6 (9.8%) | 3 (14%) | 0.69 |
| Coefficient of Variation* | 0.24 (0.18–0.32) | 0.30 (0.21–0.35) | 0.11 |
| Daily distance travelled* | 84.7 (54–115) | 40 (26–56) | <0.0001 |
| ADRR* | 16 (11) | 5.5 (3.9) | <0.0001 |
| Insulin (discharge) | 47 (77%) | 9 (42%) | 0.006 |
| Any glucose lowering drug (discharge) | 59 (97%) | 17 (81%) | 0.04 |
Data are presented as mean (standard deviation) or number (%) for days 1–3 (72 hours) following cessation of the insulin drip unless otherwise noted. Overall glucose consists of 4 glucose checks per day.
Data presented as median (interquartile range) and analyzed using Wilcoxon Rank Sum. Glucose may be converted to SI units (mmol/l) by multiplying by 0.0555.
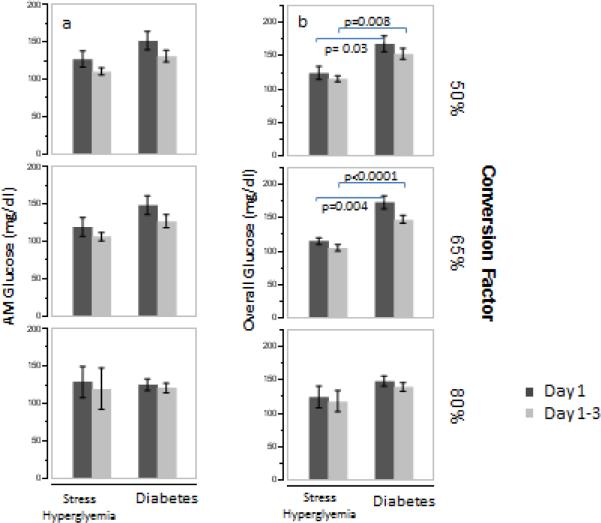
Patients with diabetes had higher 3-day mean morning glucose than those with stress hyperglycemia (127 +/− 33 mg/dl versus 110 +/− 21 mg/dl, p=0.01, table 2) and had a trend for higher day 1 morning glucose (143 +/− 50 versus 124 +/− 34 mg/dl, p=0.05, figure 1) following cessation of the insulin infusion. The overall 3-day and daily mean glucose was also significantly higher in patients with diabetes compared to those with stress hyperglycemia (p<0.0001 for all comparisons). The findings were essentially unchanged after adjusting for dosage group. Within each treatment group, 3-day morning glucose was similar in patients with diabetes and stress hyperglycemia (figure 2). However, overall 3-day mean glucose was significantly higher in the 50% and 65% dosing group among patients with diabetes compared to those with stress hyperglycemia.
Figure 1.
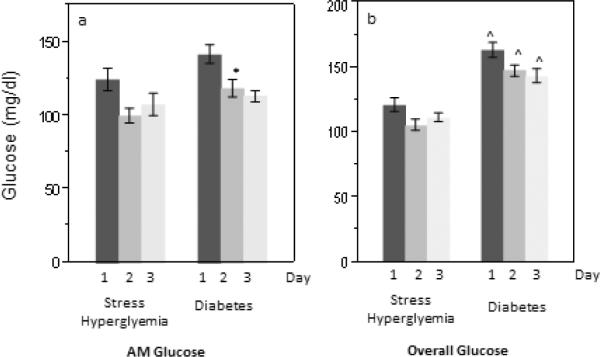
Mean daily morning (a) and overall (based on 4 glucose checks per day, b) glucose, by diabetes or stress hyperglycemia status. Error bars represent 1 standard error. Glucose may be converted to SI units (mmol/l) by multiplying by 0.0555. *p-value 0.02 and ^p-value <0.0001 between patients with stress hyperglycemia and diabetes.
Figure 2.
Mean morning (a) and overall (4 glucose measurements per day, b) glucose, by initial dosing group. Error bars represent 1 standard error. Glucose may be converted to SI units (mmol/l) by multiplying by 0.0555.
Patients with diabetes less frequently achieved the 3-day mean morning glucose target (80–130 mg/dl) compared to patients with stress hyperglycemia (p<0.004, table 2) but the percentage of patients reaching the overall (4-point profile) glucose target 80–180 mg/dl at 72 hours was similar (p=0.11).
Among patients with AM hyperglycemia on day 1, 24% of cases with diabetes and 5% of patients with stress hyperglycemia required repeated detemir dose adjustments (p=0.10). A logistic regression model containing dosing group and either diabetes status or HbA1c revealed that neither dosing group nor diabetes status (p=0.09) was a significant predictor of persistent hyperglycemia, but HbA1c was a significant predictor (p=0.004).
There was no difference in the frequency of hypoglycemia among patients with diabetes or stress hyperglycemia (10 versus 14% respectively, p=0.69, table 2). However, patients with diabetes had greater glycemic variability than those with stress hyperglycemia, demonstrated by the mean daily distance traveled (p<0.0001), but CV did not differ (p=0.11). Patients with diabetes had a greater ADRR compared to patients with stress hyperglycemia (p<0.0001). Neither hypoglycemia nor measures of glycemic variability were affected substantially by the initial insulin dosing group.
Patients with stress hyperglycemia were frequently discharged on glucose-lowering therapy, although the number was fewer than those with diabetes (77 versus 42% discharged on insulin respectively, p=0.006, 97 versus 81% discharged on any glucose-lowering therapy, p=0.04). In pooled analysis of both diabetes groups, patients in the higher dose groups were more likely to be discharged on insulin therapy (54, 76, and 76% in the 50, 65, and 80% groups respectively, p=0.04). However, a nominal logistic regression model containing dosing group and diabetes or stress hyperglycemia status revealed that only diabetes was a significant predictor of discharge on insulin therapy (p=0.0074).
DISCUSSION
The main results of the study, published previously, indicated that the initial dose of detemir has little impact on overall glycemia by as early as 24 hours following cessation of the insulin infusion, highlighting the greater relative importance of titration and bolus insulin compared to the initial basal insulin dose on overall glycemia [12]. In the current analysis, patients with stress hyperglycemia were uniformly well-controlled on the low dose (50%) conversion factor, whereas patients with diabetes were more likely to experience hyperglycemia. The findings are in agreement with a retrospective study that used a 50% conversion factor in which the majority of patients did not have known diabetes [15]. The 50% conversion dose is lower than previous recommendations for transitioning patients off of insulin infusions [8–10].
In the previously published study, hypoglycemia occurred only in the 65 and 80% groups [12]. This would lend further support to the use of a 50% conversion factor in patients with stress hyperglycemia, although hypoglycemia did not differ by diabetes status. The current analysis also suggests that patients with diabetes had more glycemic variability, as evidenced by mean daily distance travelled, and greater ADRR, which is a strong predictor of hypoglycemia [14], compared to patients with stress hyperglycemia. Both measures provide a snapshot of glucose fluctuation that may hinder safe insulin titration. Special efforts to control sources of variability, such as more meticulous adjustments in prandial insulin coverage, may be required, particularly in those patients with long-standing diabetes [19].
The initial published results suggested that more aggressive dose titration may be indicated for subjects with hyperglycemia on the morning following cessation of the insulin infusion [12]. The current analysis adds to this finding by demonstrating a role for HbA1c as a significant predictor of the need for more aggressive dose titration than was performed in the study.
Expert guidelines recommend treatment of stress hyperglycemia, whether or not the patient is classified as having diabetes, but do not discuss the unique potential ramifications of stress hyperglycemia on the transition to outpatient care [8]. The current analysis illustrates the protracted course of hyperglycemia in patients with stress hyperglycemia following cardiac surgery. Similarly, patients with diabetes also had intensification of their treatment regimens at discharge, suggesting the presence of superimposed stress hyperglycemia. The extent and duration of intensified treatment following cardiac surgery among patients with stress hyperglycemia (or diabetes) are not well known. However, providers should be aware that discharge potentially leaves such patients vulnerable to hypoglycemia since hyperglycemia and stress-induced insulin resistance are likely to lessen over the ensuing weeks [16]. Close follow-up and a specific plan for pre-emptive adjustment in therapy are crucial.
Post-cardiac surgery, patients frequently have a predictable surge in inflammatory and stress responses that result in hyperglycemia [17]. Such patients often have underlying abnormalities in glucose metabolism [2–4]. Although the HbA1c may be helpful in some cases, the sensitivity is limited for detecting diabetes [18]. Therefore, it is possible that some of the patients classified as having “stress” hyperglycemia in this study in fact had diabetes. At the very least, a persistent insulin requirement following cardiac surgery may be regarded as an important risk factor for the subsequent development of clinically recognized diabetes.
The study is limited by the post-hoc nature and small sample size. Treatment allocation at discharge was not randomized, and no post-discharge follow-up data were collected. However, assessments were adjusted for initial dosing group, and the differences between diabetes and stress hyperglycemia were in some cases very large. Despite the limitations, the findings in this study are important for directing further research, given that little prospective data in this population of patients is otherwise available.
In conclusion, transitioning patients with stress hyperglycemia from insulin infusions may require a lower conversion factor compared to patients without diabetes following cardiac surgery. Furthermore, stress hyperglycemia may be prolonged following cardiac surgery, underscoring the need for further study to optimize management.
ACKNOWLEDGEMENTS
This study was supported by an investigator-initiated grant from Novo Nordisk and by NIH grant number 1K23DK080891-02. The authors wish to thank the Ohio State University Clinical Research Center (supported by Award Number UL1RR025755 from the National Center for Research Resources) for assistance with data collection and analysis and Rita Burris, study coordinator.
Authorship details: The study is supported by a grant from Novo Nordisk. Dungan: consultant/advisor for Eli Lilly Schuster: consultand/advisor for Eli Lilly, Novo Nordisk Osei: consultand/advisor for Eli Lilly, Novo Nordisk
Footnotes
There are no other relevant conflicts of interest to report.
Conflict of interest details: Design: Dungan, Osei, Schuster Conduct/data collection: Dungan, Hall Analysis: Dungan Writing manuscript: Dungan, Osei, Schuster
REFERENCES
- 1.Knapik P, Nadziakiewicz P, Urbanska E, Saucha W, Herdynska M, Zembala M. Cardiopulmonary bypass increases postoperative glycemia and insulin consumption after coronary surgery. Ann Thorac Surg. 2009;87:1859–1865. doi: 10.1016/j.athoracsur.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 2.Donatelli F, Cavagna P, Di Dedda G, et al. Correlation between pre-operative metabolic syndrome and persistent blood glucose elevation during cardiac surgery in non-diabetic patients. Acta Anaesthesiol Scand. 2008;52:1103–1110. doi: 10.1111/j.1399-6576.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 3.Arora S, Gordon MB. High incidence of impaired glucose regulation in patients with no known history of diabetes mellitus but with hyperglycemia after undergoing a cardiac surgical procedure. Endocr Pract. 2009;15:425–430. doi: 10.4158/EP08349.ORR1. [DOI] [PubMed] [Google Scholar]
- 4.Tekumit H, Cenal AR, Polat A, Uzun K, Tataroglu C, Akinci E. Diagnostic value of hemoglobin A1c and fasting plasma glucose levels in coronary artery bypass grafting patients with undiagnosed diabetes mellitus. Ann Thorac Surg. 2010;89:1482–1487. doi: 10.1016/j.athoracsur.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30:823–828. doi: 10.2337/dc06-2184. [DOI] [PubMed] [Google Scholar]
- 6.Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112:860–871. doi: 10.1097/ALN.0b013e3181d3d4b4. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(Suppl 2):21–33. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 8.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Endocr. Pract. 2009;15:353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 9.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(Suppl 2):71–80. doi: 10.4158/EP.10.S2.71. [DOI] [PubMed] [Google Scholar]
- 10.Furnary AP, Braithwaite SS. Effects of outcome on in-hospital transition from intravenous insulin infusion to subcutaneous therapy. Am J Cardiol. 2006;15(98):557–564. doi: 10.1016/j.amjcard.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Dungan KM, Braithwaite SS, Preiser J. Stress Hyperglycemia. Lancet. 2009;373:798–807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dungan KM, Hall C, Schuster D, Osei K. Comparison of three algorithms for basal insulin in transitioning stable post-cardiothoracic surgery patients from intravenous to subcutaneous insulin. Endocr Pract. 2011 doi: 10.4158/EP11027.OR. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12(Suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 14.Kovatchev BP, Otto E, Cox D. Evaluation of a New Measure of Blood Glucose Variability in Diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 15.Olansky L, Sam S, Lober C, Yared JP, Hoogwerf B. Cleveland Clinic Cardiovascular Intensive Care Unit Insulin Conversion Protocol. J Diabetes Sci Technol. 2009;3:478–486. doi: 10.1177/193229680900300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braithwaite SS. The transition from insulin infusions to long-term diabetes therapy: the argument for insulin analogs. Semin Thorac Cardiovasc Surg. 2006;18:366–378. doi: 10.1053/j.semtcvs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Albacker T, Carvalho G, Schricker T, Lachapelle K. High-dose insulin therapy attentuates systemic inflammatory response in coronary artery bypass grafting patients. Ann Thorac Surg. 2008;86:20–27. doi: 10.1016/j.athoracsur.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of Glycated Hemoglobin for the Classification and Prediction of Diabetes. Diabetes Care. 2011;34:84–89. doi: 10.2337/dc10-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata GH, Duckworth WC, Shah JH, Wendel CS, Hoffman RM. Sources of glucose variability in insulin-treated type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES) Clin Endocrinology. 2004;60:451–456. doi: 10.1111/j.1365-2265.2004.02001.x. [DOI] [PubMed] [Google Scholar]