Abstract
Both type 1 diabetes mellitus and end stage renal disease are associated with increased fracture risk, likely due to metabolic abnormalities that reduce bone strength. Simultaneous pancreas-kidney transplantation is a treatment of choice for patients with both disorders, yet the effects of simultaneous pancreas-kidney versus kidney transplantation alone on post-transplantation fracture risk are unknown. From the United States Renal Data System we identified 11, 145 adults with type 1 diabetes undergoing transplantation of whom 4,933 had a simultaneous pancreas-kidney while 6, 212 had a kidney alone transplant between 2000 and 2006. Post-transplantation fractures resulting in hospitalization were identified from discharge codes. Time to first fracture was modeled and propensity score adjustment was used to balance covariates between groups. Fractures occurred in significantly fewer (4.7%) of pancreas-kidney compared to kidney-alone transplant (5.9%) cohorts. After gender stratification and adjustment for fracture covariates, pancreas-kidney transplantation was associated with a significant 31% reduction in fracture risk in men (hazard risk 0.69). Older age, white race, prior dialysis and pre transplantation fracture were also associated with increased fracture risk. Prospective studies are needed to determine the gender-specific mechanisms by which pancreas-kidney transplantation reduces fracture risk in men.
Keywords: pancreas-kidney transplantation, fracture, kidney, diabetes, renal, gender differences, USRDS
Introduction
Fractures are common after kidney transplantation. In comparison to the general population, fracture risk is more than 3-fold higher 1–4 and in comparison to patients on hemodialysis, hip fracture risk is more than 30% higher 1. Unfortunately, therapeutic agents that have been demonstrated to prevent fracture due to either post-menopausal or glucocorticoid-induced osteoporosis have not been proven effective in kidney transplant recipients 5. Indeed, while small clinical trials have demonstrated that anti-resorptives, calcium and vitamin D prevent bone loss after kidney transplantation, reduction in fracture risk has not been clearly deomonstrated 5. Although the lack of efficacy of these fracture preventative agents is very likely an artifact of inadequately powered clinical trials, it remains concerning that definitive therapeutic strategies to prevent fractures are lacking for kidney transplant recipients in light of their 60% increased mortality risk after hip fracture in comparison to the general population 6. Therefore, there is urgent need to identify and study novel strategies that lower fracture risk in the more than 170,000 kidney transplant recipients that are living in the United States (U.S.) 7.
Epidemiologic studies have reported that type 1 diabetes mellitus (T1DM) increases fracture risk in patients with 8 and without 9–11 kidney transplantation. Indeed, our group 12, 13 and others 4, 14, 15 have shown that pre-transplantation diabetes more than doubles the risk of fractures after kidney transplantation. The mechanisms by which T1DM increases fracture risk are not completely understood, but may include low bone mineral density 10, 16, 17, low circulating levels of insulin like growth factor1 (IGF-1) 18, decreased bone formation rates 19, elevated glucose levels 20, the development of altered collagen structure due to the accumulation of advanced glycation end products 21–23, micro-vascular complications 9, 24, 25 and an increased risk of falls due to peripheral neuropathy. It is not known whether correction of T1DM by pancreas transplantation decreases fracture risk.
Simultaneous pancreas-kidney transplantation is the treatment of choice for patients with end stage renal disease (ESRD) and T1DM 26. While animal 27 and human 28, 29 data suggest that exogenous insulin administration may correct abnormal bone and mineral metabolism due to T1DM, other data suggest that exogenous insulin may increase fracture risk 24, 28, 30 as a complication of hypoglycemia associated falls.
However, simultaneous pancreas-kidney transplantation provides physiologic-type insulin repletion without the hypoglycemic consequences of exogenous insulin administration 31. Moreover, in patients with T1DM and ESRD, simultaneous pancreas-kidney transplantation provides superior clinical outcomes compared to kidney transplantation alone 32, including normalized levels of insulin and glucose 32, improvements in micro- and macro-vascular complications 33, 34, prevention of diabetic nephropathy 31 and stabilization of diabetic neuropathy 35. Therefore, based on data suggesting that the adverse metabolic and clinical outcomes associated with T1DM are improved by pancreas-kidney transplantation, we hypothesized that in patients with T1DM and ESRD, simultaneous pancreas-kidney transplantation would be associated with lower fracture risk than kidney transplantation alone. To test our hypothesis, we used the United States Renal Data System (USRDS), the largest U.S. kidney and pancreas transplantation database.
Materials and Methods
Patients
The USRDS is the largest registry of kidney transplantation recipients and combines the United Networks for Organ Sharing (UNOS) transplantation registry data with payment data from the Centers for Medicare and Medicaid Services, which is the primary payer for the majority of patients with ESRD 36, 37. We used the USRDS data to estimate the incidence of fractures resulting in hospitalization among patients with T1DM undergoing either simultaneous pancreas-kidney or kidney transplantation alone between January 1, 2000 and December 31, 2006. Diabetes type was determined by the primary patient diagnosis reported on UNOS forms and included in the USRDS database. Patients were excluded from this analysis for: age less than 18 years; transplantation occurring prior to 2000; a history of multiple kidney or other organ transplantations; residence in an institution; being unable to ambulate; and requiring assistance with activities of daily living. We did not exclude patients who received a living donor kidney because in a sensitivity analysis including only patients with a deceased donor kidney the hazard ratio (HR) was materially unchanged from that of entire cohort (HR 0.70; 95% CI 0.55–0.89).
Determination of Date of First Fracture
First-time fracture events resulting in hospitalization were determined from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for fracture (ICD-9-CM codes 805.0–829.9) contained within USRDS. Both phalangeal (ICD-9-CM 816.0–816.9; 826.0–826.9) and skull (ICD9-CM 850–854) fractures were excluded. In the event of multiple fractures in the same patient, we considered the first listing of a fracture specific ICD-9-CM code as the fracture event. Both traumatic and fragility fractures were included. An analysis excluding traumatic fractures was not conducted because the severity of trauma associated with fracture was not completely recorded in USRDS. However, similar to low-trauma fractures, high-trauma fractures are associated with low-bone mineral density (BMD) and increased risk of future fracture 38.
Ascertainment of Fracture Covariates
Data on fracture covariates were obtained from the USRDS, selected on the basis of epidemiologic studies that demonstrated their ability to predict fracture risk in the general, chronic kidney disease (CKD) and kidney transplant populations 1, 2, 39–43. These included age at transplantation (years), gender, race (White, Black, Asian and other), body mass index (BMI), human leukocyte antigen (HLA)-matching (0; 1–2; 3–4; and 5–6), a history of and length of pre-transplantation dialysis and a history of prior fracture. BMI was evaluated both as continuous and categorical parameters: underweight (BMI <18.5), normal (BMI between 18.5 and 24.9), overweight (BMI between 25 and 29.9), and obese (BMI of >30). BMI >50 (n = 20) was considered a measurement error and was classified along with those missing BMI measurements (n = 1513 patients). A history of pre-transplantation fracture was determined by the presence of an ICD-9-CM fracture code with a date of service prior to transplant.
Statistical Analysis
All analyses were performed using STATA (version 8.2; StataCorp LP, College Station, TX) and SAS (v9.2, Gary, NC) statistical software. Analyses were designed to: (1) compare characteristics of T1DM patients with either a first kidney or simultaneous pancreas-kidney transplant; (2) quantify fracture risk by transplant type while controlling for fracture covariates; and (3) evaluate time to first fracture controlling for patient and transplant characteristics. Categorical parameters were compared using chi-square tests and continuous parameters were compared using Student's t tests. Time to first fracture was modeled using the Kaplan-Meier method with comparisons made between strata with the log-rank test. Proportional hazard regression was used to quantify fracture risk of simultaneous kidney-pancreas transplantation in comparison to kidney transplantation alone, while adjusting for predefined covariates of fracture determined from univariate analyses. Finally, due to the observational nature of this investigation, multiple fracture covariates were unequally distributed between transplantation groups, potentially favoring a fracture reduction benefit in pancreas-kidney recipients. Therefore, propensity scores were created for the probability of receiving a pancreas-kidney versus kidney transplant 44. Covariates included in the propensity score model included demographic and co-morbidity characteristics. The multivariable proportional hazard regression model included the predictor of interest (simultaneous pancreas-kidney versus kidney alone) adjusted for the propensity score.
Results
Cohort Characteristics
Using the USRDS, 11,145 adults with a primary diagnosis of T1DM were identified who underwent either simultaneous pancreas-kidney (N = 4,933) or kidney (N = 6,212) transplantation between January 1, 2000 and December 31, 2006. Patient survival rates after transplantation with either a pancreas-kidney or a kidney alone were similar at one year (95.1% versus 94.8%, respectively; p-value NS) but superior for pancreas-kidney at both three (90.7% vs. 87.8%; p-value < 0.001) and five years (86.0% vs. 79.6%; p-value < 0.0001). There were small but significant differences between patients transplanted with a pancreas-kidney and a kidney alone (Table 1). Although transplant recipients in both groups were young, patients who received a pancreas-kidney were 5.6 years younger on average than patients who received a kidney alone (p-value < 0.0001). In addition, patients with a simultaneous pancreas-kidney transplant had slightly lower BMI, were more likely to be male or white, had fewer HLA mismatches and although they were slightly more likely to have received dialysis, they were on dialysis for less time. Between-group differences in glucocorticoid use were small and not significant. There was no difference in the prevalence of pre-transplantation fractures.
Table 1.
Characteristics: Patients with Type 1 Diabetes Mellitus Stratified by Transplant Type
| Variable | Kidney Transplantation (n = 6212) | Simultaneous Pancreas-Kidney Transplantation (n = 4933) | P value |
|---|---|---|---|
|
| |||
| Pre-Transplantation Fracture (%) | 2.2 | 1.8 | 0.1 |
|
| |||
| Age at transplantation in years (SD) | 46.0 (10.9) | 40.4 (8.1) | <0.0001 |
|
| |||
| Female Gender (%) | 41 | 39 | 0.02 |
|
| |||
| Race (%) | |||
| White | 79.3 | 83.7 | <0.0001 |
| Black | 14.7 | 12.7 | 0.002 |
| Asian | 1.3 | 0.8 | 0.02 |
| Other | 4.7 | 2.7 | <0.0001 |
|
| |||
| BMI in kg/m2 (SD) | 26.7 (5.6) | 24.8 (5.7) | <0.0001 |
| BMI < 18.5(%) | 1.9 | 2.5 | 0.03 |
| BMI 18.5–24.9 (%) | 34.0 | 47.7 | <0.0001 |
| BMI 25–30 (%) | 29.5 | 28.4 | 0.2 |
| BMI 30–50 (%) | 19.9 | 9.1 | <0.0001 |
| Missing (%) | 14.7 | 12.4 | <0.0001 |
|
| |||
| Mean HLA-A, HLA-B, and HLA-DR mismatches (SD) | 2.4 (1.7) | 1.3 (1.2) | <0.0001 |
| 0 mismatches (%) | 14.2 | 26.8 | <0.0001 |
| 1–2 mismatches (%) | 38.2 | 59.2 | <0.0001 |
| 3–4 mismatches (%) | 32.7 | 11.1 | <0.0001 |
| 5–6 mismatches (%) | 13.8 | 1.8 | <0.0001 |
|
| |||
| Deceased Donor (%) | 48 | 100 | <0.0001 |
|
| |||
| Pre-Transplant Dialysis (%) | 78.4 | 80.9 | 0.001 |
|
| |||
| Years on Pre-Transplant Dialysis (SD) | 2.4 (2.1) | 2.0 (1.7) | <0.0001 |
|
| |||
| Parathyroidectomy (%) | 1.8 | 1.0 | <0.0001 |
|
| |||
| Corticosteroid Maintenance Immunosuppression (%) | 79.6 | 80.0 | 0.6 |
|
| |||
| Year of Transplant - Number (%) | |||
| 2000 | 835 (53.5) | 725 (46.5) | |
| 2001 | 895 (55.6) | 715 (44.4) | |
| 2002 | 941 (57.5) | 696 (42.5) | |
| 2003 | 821 (55.4) | 660 (44.6) | |
| 2004 | 992 (59.0) | 688 (41.0) | |
| 2005 | 894 (55.3) | 723 (44.7) | |
| 2006 | 834 (53.5) | 726 (46.5) | |
Cumulative Incidence and type of hospitalization-associated fractures according to type of transplantation
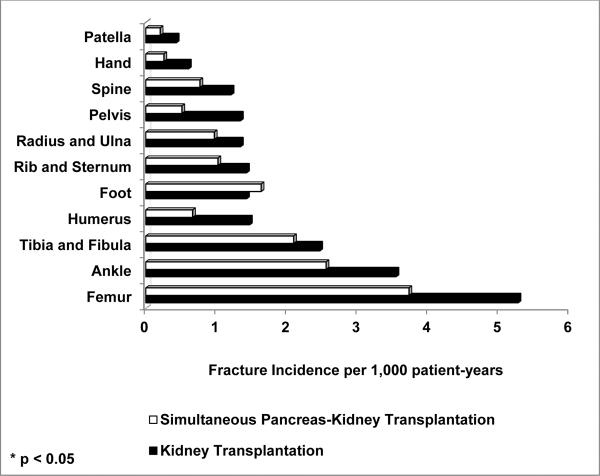
The cumulative incidence of fractures leading to hospitalizations during follow-up was slightly but significantly lower for patients transplanted with a pancreas-kidney than a kidney alone (4.7% vs. 5.9%, respectively, p-value 0.005); the absolute risk reduction was 1.2 percentage points. Out of all fractures, the most common sites were femur (25%), ankle (16%) and tibia/fibula (13%). Fractures of the hip, pelvis and humerus were significantly less common after transplantation with a pancreas-kidney than a kidney alone (Hip: 0.8% versus 1.3%, respectively, p-value 0.01; Pelvis: 0.2% versus 0.5%, respectively, p-value 0.01; and Humerus: 0.3% versus 0.6%, respectively, p-value 0.03).
Risk of fractures leading to hospitalization according to type of transplantation
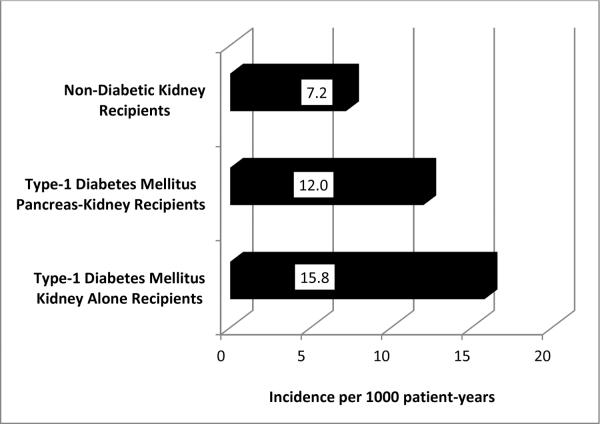
Five-hundred ninety four fractures resulting in hospitalizations were indentified over 42,755 patient-years of follow-up. For patients transplanted with a simultaneous pancreas-kidney and kidney alone, median (interquartile range) follow-up was 3.8 (2.1 – 5.8) and 3.5 (2.0 – 5.4) years, respectively. Incidence rates of fracture per 1000 patient-years for patients who received a pancreas-kidney or kidney alone were 11.7 and 15.7, respectively (Figure 1). Fracture incidence rates adjusted for patient-years of follow-up at all anatomic sites and stratified by type of transplantation are shown in Figure 2.
Figure 1.
Fracture incidence per 1000 patients per year by transplant type
Figure 2.
Fracture incidence per 1000 patients per year at each skeletal site by transplant type
Fracture free survival was determined by the Kaplan-Meier method (Figure 3). At three months after transplantation, fracture incidence rates began to differ between groups; there were fewer fractures among patients with T1DM who received a simultaneous pancreas-kidney, compared to kidney alone. The beneficial effect of transplantation with a pancreas-kidney compared to kidney alone on fracture risk persisted for the duration of follow-up (p-value <0.0003).
Figure 3.
Kaplan-Meier plot of time to fracture resulting in hospitalization, stratified by kidney transplant type.
In univariate hazard regression analysis, pancreas-kidney transplantation, compared to kidney transplantation, was protective against fractures in general (HR 0.74; 95% CI 0.63–0.88) and at the hip (HR 0.59; 95% CI 0.42–0.82), pelvis (HR 0.38; 95% CI 0.19–0.78) and humerus (HR 0.45; 95% CI 0.24–0.85). However, in multivariable analysis, pancreas-kidney transplantation was protective against fractures in general (HR 0.79; 95% CI 0.66–0.96) and at the pelvis (HR 0.46; 95% CI 0.21–1.00). While black race was also protective against fractures (HR 0.76; 95% CI 0.57–0.99), older age (HR 1.02; 95% CI 1.01–1.03), BMI <18.5 kg/m2 (HR 1.91; 95% CI 1.25–2.94) and both pre-transplantation fracture (HR 3.58; 95% CI 2.53–5.07) and dialysis (HR 1.48; 95% CI 1.18–1.86) were associated with increased fracture risk.
Gender specific associations between fracture risk and simultaneous pancreas-kidney transplantation
Female gender is an important risk factor for fracture after kidney transplantation4, 12–14. A test of interaction between gender and pancreas-kidney transplantation was near-significant (HR 1.39; 95% CI 0.98–1.96, p-value 0.06). Therefore, we evaluated gender-specific effects on post-transplantation fracture (Table 2). In men with T1DM, simultaneous pancreas-kidney transplantation was associated with a 31% reduction in overall fracture risk (HR 0.69; 95% CI 0.53–0.88). Higher BMI (>25 kg/m2) was also associated with reduced fracture risk (BMI 25–30 kg/m2 HR 0.75; 95% CI 0.58–0.97 and BMI 30–50 HR 0.62; 95% CI 0.43–0.90). For pelvic fractures in men, pancreas-kidney transplantation was associated with a 91% risk reduction (HR 0.09; 95% CI 0.01–0.71). In women, simultaneous pancreas-kidney transplantation was not associated with decreased fracture risk. To evaluate the potential influence of menopausal status on fracture risk we stratified the female population by age 50 years; there was no fracture prevention benefit to pancreas-kidney transplantation in either younger or older women. Black race was protective against fractures in women (HR 0.52; 95% CI 0.33–0.81). Older age, lower BMI (<18.5 kg/m2) and both pre-transplantation fracture and dialysis were all independent predictors of increased fracture risk in both men and women.
Table 2.
Multivariable Hazard Ratios: Patients with Type 1 Diabetes who received either Kidney or Simultaneous Pancreas-Kidney Transplantation
| Men (N=6690) | Women (N=4455) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |||
| Simultaneous Pancreas-Kidney Transplantation | 0.69 | 0.53 | 0.88 | 0.003 | 0.95 | 0.71 | 1.26 | 0.7 |
|
| ||||||||
| Pre-KTx Fracture | 4.13 | 2.64 | 6.45 | <0.0001 | 2.62 | 1.48 | 4.63 | 0.0009 |
|
| ||||||||
| Age at transplantation (per year over 18) | 1.01 | 1.00 | 1.03 | 0.02 | 1.03 | 1.02 | 1.05 | <0.0001 |
|
| ||||||||
| Race | - | |||||||
| White | Reference | Reference | ||||||
| Black | 0.94 | 0.67 | 1.33 | 0.9 | 0.52 | 0.33 | 0.81 | 0.004 |
| Asian | 0.52 | 0.13 | 2.08 | 0.5 | - | - | - | - |
| Other | 0.96 | 0.51 | 1.83 | 0.9 | 0.91 | 0.42 | 1.96 | 0.8 |
|
| ||||||||
| BMI in kg/m2 | - | |||||||
| BMI < 18.5 | 2.08 | 1.12 | 3.86 | 0.02 | 1.79 | 0.98 | 3.27 | 0.06 |
| BMI 18.5–24.9 | Reference | Reference | ||||||
| BMI 25–30 | 0.75 | 0.58 | 0.97 | 0.03 | 1.09 | 0.79 | 1.49 | 0.6 |
| BMI > 30–50 | 0.62 | 0.43 | 0.90 | 0.01 | 1.02 | 0.70 | 1.48 | 0.9 |
| Missing | 0.79 | 0.56 | 1.11 | 0.2 | 1.20 | 0.82 | 1.76 | 0.3 |
|
| ||||||||
| Mean HLA-A, HLA-B, and HLA-DR mismatches (SD) | - | |||||||
| 0 mismatches | Reference | Reference | ||||||
| 1–2 mismatches | 0.98 | 0.73 | 1.32 | 0.9 | 1.17 | 0.83 | 1.64 | 0.4 |
| 3–4 mismatches | 1.13 | 0.82 | 1.57 | 0.5 | 0.93 | 0.62 | 1.40 | 0.7 |
| 5–6 mismatches | 1.09 | 0.71 | 1.68 | 0.7 | 1.00 | 0.61 | 1.64 | 1.0 |
|
| ||||||||
| Pre-Transplant Dialysis | 1.39 | 1.02 | 1.88 | 0.04 | 1.64 | 1.17 | 2.31 | 0.005 |
|
| ||||||||
| Parathvroidectomy | - | |||||||
| Pre- or Post-Transplant | 0.32 | 0.04 | 2.26 | 0.6 | 1.33 | 0.88 | 3.03 | 0.5 |
|
| ||||||||
| Corticosteroid Maintenance Immunosuppression | 1.08 | 0.76 | 1.54 | 0.7 | 0.81 | 0.56 | 1.17 | 0.3 |
|
| ||||||||
| Year | - | |||||||
| 2000 | Reference | Reference | ||||||
| 2001 | 1.39 | 0.99 | 1.96 | 0.06 | 1.24 | 0.85 | 1.81 | 0.3 |
| 2002 | 0.93 | 0.63 | 1.38 | 0.7 | 1.09 | 0.72 | 1.65 | 0.7 |
| 2003 | 1.28 | 0.87 | 1.90 | 0.2 | 1.00 | 0.63 | 1.58 | 1.0 |
| 2004 | 1.15 | 0.75 | 1.77 | 0.5 | 0.87 | 0.53 | 1.43 | 0.6 |
| 2005 | 1.12 | 0.69 | 1.83 | 0.6 | 0.82 | 0.46 | 1.45 | 0.5 |
| 2006 | 1.60 | 0.93 | 2.76 | 0.09 | 1.00 | 0.51 | 1.93 | 1.0 |
Propensity score adjustment of hazard regression models
Imbalances in the distribution of fracture risk factors between transplantation groups may have biased results in favor of pancreas-kidney recipients. Therefore, we used propensity score models to confirm the validity of our findings in both the whole and gender stratified cohorts. In the whole cohort, there was a 21% reduction in all fractures (HR 0.79; 95% CI 0.65–0.95) and a 58% reduction in pelvic fractures (HR 0.42; 98% CI 0.19–0.94). In men, there was a 33% reduction in all fractures (HR 0.67; 95% CI 0.52–0.86) and a 92% reduction in pelvic fractures (HR 0.08; 98% CI 0.01–0.63). There was no reduction in hip fracture rates in the whole cohort, but in men there was a trend towards a 39% reduction in risk (HR 0.61; 95% CI 0.38–1.00, p-value 0.054). Similar to models unadjusted for propensity score, there was no association between fracture and transplant type in women.
Discussion
These results are the first to show that fracture rates in patients with T1DM are lower after transplantation with a simultaneous pancreas-kidney compared to kidney alone. In men with T1DM and ESRD, the benefit of simultaneous pancreas-kidney transplantation was independent of other risk factors for fracture and was apparent within three months of transplantation. Over five years, the incidence of fracture was 31% lower in men but not significantly different in women. We also noted that transplantation with a simultaneous pancreas-kidney was associated with a reduction in pelvic fractures and a trend towards a reduction in hip fractures compared to a kidney alone. In light of the 60% increased mortality risk6 and the substantially increased health economic costs45, 46 that are associated with fractures after kidney transplantation, simultaneous pancreas-kidney transplantation confers a clinically important fracture prevention benefit to this group of young men. These results are even more striking considering that no single treatment has been proven to be effective for reducing fracture risk after kidney transplantation.
We reported that the incidence of fractures resulting in hospitalizations was 12.0 and 15.8 per 1000 patient-years for patients transplanted with either a pancreas-kidney or kidney alone. This advantageous effect of pancreas-kidney transplantation contrasts with previously published data. Ramsey-Goldman et al3 noted higher percentages of men, women and postmenopausal women with fractures among those who received a pancreas-kidney compared to a kidney alone. A retrospective cohort study by Chiu et al47 demonstrated a significantly increased risk of fracture after simultaneous pancreas-kidney transplantation compared with kidney transplantation. Likewise, Vautour et al4 found that pancreas-kidney transplantation was associated with increased fracture risk. It is important to note that the control groups in all these studies were not well matched to the pancreas-kidney groups. In particular, patients were not matched on either T1DM status, which is itself an extremely important risk factor for fracture12, 13 or race47, which in the case of blacks confers a fracture prevention benefit. In fact, ours is the only study with sufficient power both to match patients with and without pancreas transplantation based on T1DM status and to perform analyses that were adjusted for multiple confounders of fracture risk. Therefore, our comparisons more clearly reflect the effects of pancreas transplantation on fracture risk.
Our results also suggest that the fracture reduction benefits of simultaneous pancreas-kidney transplantation apply to men but not women. Gender specific differences in rates of bone loss after kidney transplantation have been reported48–54. Male gender was noted to be a significant risk factor for low bone mineral density after kidney transplantation51. Other studies also suggest that female gender was protective against bone loss at the lumbar spine49, 50 and femoral neck52, 53 after kidney transplantation. Indeed, higher levels of circulating estrogen correlated with less severe bone loss at the lumbar spine49 and with histologic markers of bone structure and osteoblast function51. In our USRDS analysis, 75% of the women who received a pancreas-kidney from January 1, 2000 to December 31, 2006 were younger than 50 years of age and the majority of these women were likely premenopausal. Therefore, it is possible that the skeletal effects of pancreas-kidney transplantation in women were attenuated because they were estrogen replete. However, in gender- and age-stratified statistical analyses, we did not find any fracture prevention benefit in older women. Furthermore, these findings may have been influenced by our inability to ascertain and quantify the effects of medications that have active bone affects, such as oral contraceptives, hormone replacement therapy, vitamin D and calcium supplements and bisphosphonates, all of which are more commonly used by women. Further investigations are needed to explore the microstructural and biochemical mechanisms of fracture that underlie these gender differences.
T1DM is an important risk factor for fracture both in the general population9, 28 and in patients with chronic kidney disease (CKD) both before9 and after8 kidney transplantation. In this study we report an overall fracture incidence rate of 14.0 per 1000 patient-years, which is markedly higher than the rate of fractures in kidney transplant recipients without diabetes (7.2 per 1000 patient-years). Furthermore, we reported previously that kidney transplant recipients with diabetes, compared to non-diabetics, had a more than 2-fold increased risk of fractures requiring hospitalization, regardless of age, gender, pre-transplantation dialysis and immunosuppression regimen13. Mechanisms of fracture due to T1DM are not fully understood. A recent study of patients with T1DM, without either renal disease or micro-vascular complications, suggested there was no difference in bone mass, microstructure or remodeling in comparison to healthy controls55. However, in other cohorts, T1DM was associated with low BMD10, 16, 17. This may have been due to low levels of circulating IGF-118, decreased bone formation rates19, elevated blood glucose levels20, abnormal mineralization due to altered collagen structure from advanced glycation end products21, 22, poor bone blood flow from micro-vascular complications9, 24, 25, endothelial cell dysfunction56 or peripheral neuropathy57. In addition, hypoglycemic events due to exogenous administration of insulin have been implicated as a cause of falls which in turn, may result in fracture28, 30. It is also important to note that mechanisms of increased fracture risk in patients with co-incident T1DM and CKD have not been fully explored. CKD is an important diabetic complication that is independently associated with an increased risk of fracture. Compared to the general population, CKD is associated with a 2- to 4- fold increased risk of fracture1, 2, 40, 58–60 and compared to patients with T1DM alone, co-incident T1DM and CKD has been reported to increase fracture risk 40%9 to 42-fold17. Studies are needed to clarify mechanisms of fracture in patients with co-incident T1DM and CKD in order to develop effective fracture prevention strategies that can be used in conjunction with pancreas-kidney transplantation in men and to provide fracture prevention therapies to women in whom pancreas transplantation may offer no fracture reduction benefit at all.
Although we found that pancreas-kidney transplantation conferred a fracture reduction benefit compared to kidney transplantation alone, fractures continued to occur even after dual organ transplantation. Potential mechanisms of increased skeletal fragility after simultaneous pancreas-kidney transplantation have been reported in several small investigations61–63. Both pre- and post- transplantation characteristics of the skeleton had important implications for future fracture risk. Before transplantation, up to 58% and 23% of patients with T1DM and ESRD had osteoporosis at the femoral neck and lumbar spine, respectively61, 62. In patients with kidney transplantation, glucocorticoid use is an important risk factor for fracture13. During the first six months after transplantation, reported rates of bone loss were 6.9% and 6% at the femoral neck and spine, respectively, possibly due to the high doses of glucocorticoids used during that time period. After glucocorticoid doses were lowered, bone density did not recover and remained significantly below pre-transplantation levels61. The pattern of bone loss after pancreas-kidney transplantation was predominantly cortical63, which may explain the strong association between pancreas-kidney transplantation and peripheral fractures47, 61, 62, 64.
Unfortunately, no study has evaluated whether the rates and patterns of bone loss after pancreas-kidney transplantation are due to effects of either dual organ transplantation or T1DM. Simultaneous pancreas-kidney transplantation results in the correction of pre-transplantation metabolic derangements. This includes normalized regulation of serum glucose, phosphate and calcium, restored secretion of calcitriol, reversal of pre-transplantation hyperparathyroidism and improvement in micro-vascular disease50, 65. We hypothesize that the reversal of these derangements after simultaneous pancreas-kidney transplantation may account for some of the beneficial effects on fracture risk.
Finally, an uneven distribution of fracture risk factors between transplant recipients may have biased the unadjusted risk estimates of fracture in favor of the pancreas-kidney group. For example, recipients of a kidney alone had a longer mean duration of pre-transplantation dialysis, were commonly older and female and had a greater prevalence of HLA mismatches and pre-transplantation parathyroidectomy. On the other hand, other risk factors for fracture were more common in recipients of pancreas-kidney transplantation; all recipients of a pancreas-kidney received a deceased donor transplant and they were more likely to be white, of lower BMI and to have received pre-transplantation dialysis. Although, these data suggest recipients of a pancreas-kidney may have been healthier than those of a kidney alone, we a priori excluded debilitated patients, including those with an inability to ambulate, requiring assistance and residing in a facility. These imbalances in the distribution of fracture risk factors between transplantation groups are an important limitation of this study and were addressed in two separate analyses. First, all multivariable analyses were adjusted for known independent predictors of post-transplantation fracture. Second, a propensity score approach was used as a statistical method to balance inequalities in covariate structure44. The results of both analytical methods were consistent with each other, indicating a fracture prevention benefit of pancreas-kidney transplantation in men. Therefore, we believe these adjustments limited bias in fracture risk estimates.
This study also has other limitations. It was not a randomized clinical trial and is subject to the limitations of observational research using registry and claims based data. However, the reduced fracture risk after transplantation with a pancreas-kidney compared to a kidney alone was significant both after adjustment for other risk factors for fracture and after the use of propensity scores. If this were a randomized clinical trial, an absolute fracture risk reduction of 1.8 percentage points in men would equate with the prevention of one incident fracture requiring hospitalization for every 56 male patients receiving a simultaneous pancreas-kidney. Therefore, in the 3670 male patients in this study who received a kidney alone, 65 fractures would have been prevented if a pancreas had also been transplanted. As fractures were assessed by ICD9 hospital codes, we were limited to fractures resulting in hospitalization. This almost certainly led to under-detection of smaller peripheral fractures and morphometric vertebral fractures; however, our analysis did identify those fractures that were the most clinically significant. The USRDS does not contain information on either bone mineral density or medication usage after hospital discharge. However, the role of low bone mineral density as a risk factor for fracture in patients with ESRD has not been fully elucidated. Regarding medication usage, we were unable to control for the impact of chronic glucocorticoid and calcineurin inhibitor use on fracture rates. However, multivariable models and propensity scores included surrogate markers of glucocorticoid use, including hospital discharge with a glucocorticoid and a history of graft rejection. Finally, we were unable to differentiate simultaneous pancreas-kidney from simultaneous cadaver pancreas living-donor kidney transplantation and determine if there are differences in fracture risk between these two transplantation approaches.
In conclusion, simultaneous pancreas-kidney transplantation compared to kidney transplantation alone was associated with a 31% reduction in overall fracture risk along with a significant reduction in pelvic fracture risk and a trend towards reduced hip fracture risk in men with T1DM and ESRD. Long-term prospective mechanistic studies are needed to elucidate the effects of T1DM and gender on fracture risk after pancreas-kidney and kidney transplantation so that effective fracture prevention strategies can be developed and implemented.
Acknowledgements
This work was supported by grants from the Doris Duke Charitable Foundation (L.E.N. and S.P.I.), the National Institutes of Health K24 AR052665 (E.S.) and K23 DK080139 (T.L.N.) and a Columbia University Herbert Irving Scholars Award (T.L.N.)
This work was supported by a grant from the Bette Midler Foundation to fund Melanie Foley.
We would like to thank Melanie Foley for her administrative assistance.
Footnotes
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Disclosures The authors of this manuscript have no conflicts of interest to disclose.
Bibliography
- 1.Ball AM, Gillen DL, Sherrard D, et al. Risk of Hip Fracture Among Dialysis and Renal Transplant Recipients. JAMA: The Journal of the American Medical Association. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 2.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney International. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey-Goldman R, Dunn JE, Dunlop DD, et al. Increased Risk of Fracture in Patients Receiving Solid Organ Transplants. Journal of Bone and Mineral Research. 1999;14:456–463. doi: 10.1359/jbmr.1999.14.3.456. [DOI] [PubMed] [Google Scholar]
- 4.Vautour LM, Melton LJ, 3rd, Clarke BL, et al. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int. 2004;15:160–167. doi: 10.1007/s00198-003-1532-y. [DOI] [PubMed] [Google Scholar]
- 5.Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2007:CD005015. doi: 10.1002/14651858.CD005015.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Abbott KC, Oglesby RJ, Hypolite IO, et al. Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol. 2001;11:450–457. doi: 10.1016/s1047-2797(01)00226-5. [DOI] [PubMed] [Google Scholar]
- 7.System USRD . USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. [Google Scholar]
- 8.Nisbeth U, Lindh E, Ljunghall S, et al. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation. 1999;67:1218–1222. doi: 10.1097/00007890-199905150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcified tissue international. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 10.Janghorbani M, Van Dam RM, Willett WC, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg AH, Johnell O, Nilsson PM, et al. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17:1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 12.Nikkel LE, Hollenbeak CS, Fox EJ, et al. Risk of fractures after renal transplantation in the United States. Transplantation. 2009;87:1846–1851. doi: 10.1097/TP.0b013e3181a6bbda. [DOI] [PubMed] [Google Scholar]
- 13.Nikkel LE, Mohan S, Zhang A, et al. Reduced Fracture Risk With Early Corticosteroid Withdrawal After Kidney Transplant. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Shaughnessy EA, Dahl DC, Smith CL, et al. Risk factors for fractures in kidney transplantation. Transplantation. 2002;74:362–366. doi: 10.1097/00007890-200208150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Akaberi S, Simonsen O, Lindergard B, et al. Can DXA predict fractures in renal transplant patients? Am J Transplant. 2008;8:2647–2651. doi: 10.1111/j.1600-6143.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 16.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 17.Miao J, Brismar K, Nyren O, et al. Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care. 2005;28:2850–2855. doi: 10.2337/diacare.28.12.2850. [DOI] [PubMed] [Google Scholar]
- 18.Moyer-Mileur LJ, Slater H, Jordan KC, et al. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. 2008;23:1884–1891. doi: 10.1359/jbmr.080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhaeghe J, Suiker AM, Nyomba BL, et al. Bone mineral homeostasis in spontaneously diabetic BB rats. II. Impaired bone turnover and decreased osteocalcin synthesis. Endocrinology. 1989;124:573–582. doi: 10.1210/endo-124-2-573. [DOI] [PubMed] [Google Scholar]
- 20.Gopalakrishnan V, Vignesh RC, Arunakaran J, et al. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84:93–101. doi: 10.1139/o05-163. [DOI] [PubMed] [Google Scholar]
- 21.Hein G, Weiss C, Lehmann G, et al. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Ann Rheum Dis. 2006;65:101–104. doi: 10.1136/ard.2004.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito M, Fujii K, Soshi S, et al. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986–995. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 23.Katayama Y, Akatsu T, Yamamoto M, et al. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996;11:931–937. doi: 10.1002/jbmr.5650110709. [DOI] [PubMed] [Google Scholar]
- 24.Ivers RQ, Cumming RG, Mitchell P, et al. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 25.Vogt MT, Cauley JA, Kuller LH, et al. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–289. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP, Davis C, Larsen J, et al. Pancreas transplantation for patients with type 1 diabetes. Diabetes Care. 2003;26(Suppl 1):S120. doi: 10.2337/diacare.26.2007.s120. [DOI] [PubMed] [Google Scholar]
- 27.Hough S, Avioli LV, Bergfeld MA, et al. Correction of abnormal bone and mineral metabolism in chronic streptozotocin-induced diabetes mellitus in the rat by insulin therapy. Endocrinology. 1981;108:2228–2234. doi: 10.1210/endo-108-6-2228. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 29.Campos Pastor MM, Lopez-Ibarra PJ, Escobar-Jimenez F, et al. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporos Int. 2000;11:455–459. doi: 10.1007/s001980070114. [DOI] [PubMed] [Google Scholar]
- 30.Nabarro JD. Compression fractures of the dorsal spine in hypoglycaemic fits in diabetes. Br Med J (Clin Res Ed) 1985;291:1320. doi: 10.1136/bmj.291.6505.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland DE, Gores PF, Farney AC, et al. Evolution of kidney, pancreas, and islet transplantation for patients with diabetes at the University of Minnesota. Am J Surg. 1993;166:456–491. doi: 10.1016/s0002-9610(05)81142-0. [DOI] [PubMed] [Google Scholar]
- 32.Sudan D, Sudan R, Stratta R. Long-term outcome of simultaneous kidney-pancreas transplantation: analysis of 61 patients with more than 5 years follow-up. Transplantation. 2000;69:550–555. doi: 10.1097/00007890-200002270-00015. [DOI] [PubMed] [Google Scholar]
- 33.Becker BN, Odorico JS, Becker YT, et al. Simultaneous pancreas-kidney and pancreas transplantation. Journal of the American Society of Nephrology : JASN. 2001;12:2517–2527. doi: 10.1681/ASN.V12112517. [DOI] [PubMed] [Google Scholar]
- 34.Larsen JL, Colling CW, Ratanasuwan T, et al. Pancreas transplantation improves vascular disease in patients with type 1 diabetes. Diabetes Care. 2004;27:1706–1711. doi: 10.2337/diacare.27.7.1706. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Felber W, Landgraf R, Scheuer R, et al. Diabetic neuropathy 3 years after successful pancreas and kidney transplantation. Diabetes. 1993;42:1482–1486. doi: 10.2337/diab.42.10.1482. [DOI] [PubMed] [Google Scholar]
- 36.Greer JW. End stage renal disease and Medicare. Health Care Financ Rev. 2003;24:1–5. [PMC free article] [PubMed] [Google Scholar]
- 37.System USRD . USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Google Scholar]
- 38.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. Jama. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 39.Stehman-Breen CO, Sherrard DJ, Alem AM, et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney International. 2000;58:2200–2205. doi: 10.1111/j.1523-1755.2000.00394.x. [DOI] [PubMed] [Google Scholar]
- 40.Nickolas TL, McMahon DJ, Shane E. Relationship between Moderate to Severe Kidney Disease and Hip Fracture in the United States. J AmSocNephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 41.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. AmJ Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 42.Mussolino ME, Looker AC, Madans JH, et al. Risk factors for hip fracture in white men: the NHANES I Epidemiologic Follow-up Study. J Bone MinerRes. 1998;13:918–924. doi: 10.1359/jbmr.1998.13.6.918. [DOI] [PubMed] [Google Scholar]
- 43.Ensrud KE, Lipschutz RC, Cauley JA, et al. Body size and hip fracture risk in older women: a prospective study. Study of Osteoporotic Fractures Research Group. AmJ Med. 1997;103:274–280. doi: 10.1016/s0002-9343(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 44.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. StatMed. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 45.Burge R, wson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone MinerRes. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 46.Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. ClinOrthopRelat Res. 1990:163–166. [PubMed] [Google Scholar]
- 47.Chiu MY, Sprague SM, Bruce DS, et al. Analysis of fracture prevalence in kidney-pancreas allograft recipients. J Am Soc Nephrol. 1998;9:677–683. doi: 10.1681/ASN.V94677. [DOI] [PubMed] [Google Scholar]
- 48.Patel S, Kwan JT, McCloskey E, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Miner Res. 2001;16:1863–1870. doi: 10.1359/jbmr.2001.16.10.1863. [DOI] [PubMed] [Google Scholar]
- 49.Brandenburg VM, Ketteler M, Heussen N, et al. Lumbar bone mineral density in very long-term renal transplant recipients: impact of circulating sex hormones. Osteoporos Int. 2005;16:1611–1620. doi: 10.1007/s00198-005-1884-6. [DOI] [PubMed] [Google Scholar]
- 50.Julian BA, Laskow DA, Dubovsky J, et al. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325:544–550. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 51.Cueto-Manzano AM, Konel S, Hutchison AJ, et al. Bone loss in long-term renal transplantation: histopathology and densitometry analysis. Kidney Int. 1999;55:2021–2029. doi: 10.1046/j.1523-1755.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 52.Horber FF, Casez JP, Steiger U, et al. Changes in bone mass early after kidney transplantation. Journal of Bone and Mineral Research. 1994;9:1–9. doi: 10.1002/jbmr.5650090102. [DOI] [PubMed] [Google Scholar]
- 53.Almond MK, Kwan JT, Evans K, et al. Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron. 1994;66:52–57. doi: 10.1159/000187765. [DOI] [PubMed] [Google Scholar]
- 54.Wolpaw T, Deal CL, Fleming-Brooks S, et al. Factors influencing vertebral bone density after renal transplantation. Transplantation. 1994;58:1186–1189. [PubMed] [Google Scholar]
- 55.Armas LA, Akhter MP, Drincic A, et al. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone. 2012;50:91–96. doi: 10.1016/j.bone.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanada M, Taguchi A, Higashi Y, et al. Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis. 2004;176:387–392. doi: 10.1016/j.atherosclerosis.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Rix M, Andreassen H, Eskildsen P. Impact of peripheral neuropathy on bone density in patients with type 1 diabetes. Diabetes Care. 1999;22:827–831. doi: 10.2337/diacare.22.5.827. [DOI] [PubMed] [Google Scholar]
- 58.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51:38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J AmSocNephrol. 2007;18:282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 60.Kaji H, Yamauchi M, Yamaguchi T, et al. Mild Renal Dysfunction Is a Risk Factor for a Decrease in Bone Mineral Density and Vertebral Fractures in Japanese Postmenopausal Women. The Journal of clinical endocrinology and metabolism. 2010 doi: 10.1210/jc.2010-0099. [DOI] [PubMed] [Google Scholar]
- 61.Smets YF, de Fijter JW, Ringers J, et al. Long-term follow-up study on bone mineral density and fractures after simultaneous pancreas-kidney transplantation. Kidney Int. 2004;66:2070–2076. doi: 10.1111/j.1523-1755.2004.00986.x. [DOI] [PubMed] [Google Scholar]
- 62.Smets YF, van der Pijl JW, de Fijter JW, et al. Low bone mass and high incidence of fractures after successful simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 1998;13:1250–1255. doi: 10.1093/ndt/13.5.1250. [DOI] [PubMed] [Google Scholar]
- 63.Smets YF, van der Pijl JW, Ringers J, et al. Pattern of bone loss after simultaneous pancreas-kidney transplantation: a prospective study. Transplant Proc. 1998;30:326. doi: 10.1016/s0041-1345(97)01290-6. [DOI] [PubMed] [Google Scholar]
- 64.Bruce DS, Newell KA, Josephson MA, et al. Long-term outcome of kidney-pancreas transplant recipients with good graft function at one year. Transplantation. 1996;62:451–456. doi: 10.1097/00007890-199608270-00005. [DOI] [PubMed] [Google Scholar]
- 65.Chiu MY, Sprague SM, Bruce DS, et al. Analysis of fracture prevalence in kidney-pancreas allograft recipients. Journal of the American Society of Nephrology : JASN. 1998;9:677–683. doi: 10.1681/ASN.V94677. [DOI] [PubMed] [Google Scholar]