Abstract
Purpose
Patients undergoing treatment for cancer often report problems with their cognitive function, which is an essential component of health-related quality of life. Pursuant to this, a two-arm randomized, placebo-controlled, double-blind, phase III clinical trial was conducted to evaluate Ginkgo biloba (EGB 761) for the prevention of chemotherapy-related cognitive dysfunction in patients with breast cancer.
Methods
Previously chemotherapy naïve women about to receive adjuvant chemotherapy for breast cancer were randomized to receive 60 mg of EGB 761 or a matching placebo twice daily. The study agent was to begin before their second cycle of chemotherapy and to be taken throughout chemotherapy and 1 month beyond completion. The primary measure for cognitive function was the High Sensitivity Cognitive Screen (HSCS), with a secondary measure being the Trail Making Tests (TMT) A and B. Subjective assessment of cognitive function was evaluated by the cognitive subscale of the Perceived Health Scale (PHS) and the Profile of Mood States (POMS). Data were collected at baseline and at intervals throughout and after chemotherapy, up to 24 months after completion of adjuvant treatment. The primary statistical analysis included normalized area under the curve (AUC) comparisons of the HSCS, between the arms. Secondary analyses included evaluation of the other measures of cognition as well as correlational analyses between self-report and cognitive testing.
Results
One hundred and sixty-six women provided evaluable data. There were no significant differences in AUC up to 12 months on the HSCS between arms at the end of chemotherapy or at any other time point after adjuvant treatment. There were also no significant differences in TMT A or B at any data point. Perceived cognitive functions, as measured by the PHS and confusion/bewilderment subscale of the POMS, were not different between arms at the end of chemotherapy. There was also little correlation between self-reported cognition and cognitive testing. No differences were observed in toxicities per Common Terminology Criteria for Adverse Events (CTCAE) assessment between Ginkgo biloba and placebo throughout the study; however, after chemotherapy, the placebo group reported worse nausea (p = .05).
Conclusion
This study did not provide any support for the notion that Ginkgo biloba, at a dose of 60 mg twice a day, can help prevent cognitive changes from chemotherapy. These analyses do provide data to further support the low associations between patients’ self-report of cognition and cognitive performance, based on more formal testing.
Keywords: Ginkgo biloba, Chemotherapy-related cognitive dysfunction, Adjuvant treatment, Breast cancer, Dietary supplements
Introduction
Cognitive impairment is a frequent topic of concern in the cancer community, where it has colloquially been termed “chemo-fog” or “chemo-brain”[1]. Patients frequently describe a change in cognitive function as a mental slowing or an inability to multitask to the same degree as they could before their cancer diagnosis. This perceived impairment of brain function can profoundly affect psychological well-being and the ability to perform usual activities of daily living [2]. Several published articles report that these changes are relatively subtle, compared to major head trauma or Alzheimer disease, but can continue to have long-term effects for many months to years after cancer treatment is completed [3–9]. Results of a multicenter study of 595 cancer patients noted that 82 % of patients self-reported problems with their memory and concentration during chemotherapy [10].
Changes in cognitive function may be confused with, or confounded by, other problems commonly associated with cancer and its treatment, such as depression, anxiety, pain medications, and fatigue caused by sleep deprivation [11–13]. Although the phenomenon is not completely understood, cognitive deficits in patients with cancer are assuming greater significance as the breast cancer survival rate continues to improve. Advances in basic sciences, imaging, and clinical sciences are beginning to unravel pathophysiologic mechanisms which may underlie this problem [12]. Pharmacologic treatment options for cognitive deficits in cancer survivors have been borrowed from diverse diseases including attention-deficit/hyperactivity disorder and neurodegenerative diseases and, to date, have shown minimal to no effect [14–16]. It has been hypothesized that therapies being evaluated for dementia and Alzheimer’s disease (AD), such as Ginkgo biloba, may have some potential for patients who experience cognitive impairment due to chemotherapy [17, 18].
Ginkgo biloba is from a tree species of the family Gink-goaceae, originally from China, which is purported to be over 200 million years old [19]. The leaves are divided into two lobes, hence the name biloba. The principal components in ginkgo extract are flavonoids (flavone glycosides) and terpenoids (terpene lactones–ginkgolides and bilobalides) [19, 20]. Ginkgo is thought to work through a variety of mechanisms: antioxidant activity (free radical scavenger), increasing cerebral blood flow, improving glucose utilization, and stimulating choline uptake in the hippocampus [21, 22].
Based on early and positive evaluations in dementia and AD, providing some proof of principle for the preservation of cognitive function [17, 18] and a proposed mechanism of action related to antioxidant activity and neuroprotective properties [22], this current study was developed to evaluate Ginkgo biloba for the prevention of cognitive decline associated with adjuvant treatment for breast cancer.
Methods
The North Central Treatment Group (NCCTG) conducted a two-arm randomized, placebo-controlled, double-blind, phase III trial evaluating 60 mg of Ginkgo biloba (GB) versus a matching placebo (P) twice a day, for the prevention of chemotherapy-related cognitive dysfunction. Eligible women were newly diagnosed with breast cancer and were to receive adjuvant treatment. They had to be chemotherapy naïve and were required to begin study treatment before their second cycle of adjuvant chemotherapy. Women were excluded for use of Ginkgo biloba in the past 6 months, antithrombotic therapy, or significant comorbidities, such as arterial vascular disease or diabetes. Randomization, using dynamic allocation, was accomplished via computer and participants were stratified by type of chemotherapy, age, menopausal status at the start of treatment, and lymph node involvement. All patients participating in this trial signed a consent form as per the United States federal guidelines and the trial received approval from local institutional review boards.
Objective cognitive functioning was evaluated by research personnel using the High Sensitivity Cognitive Screen (HSCS). The HSCS is an instrument that was developed to be a briefer way to evaluate cognitive function compared to other standard neuropsychological batteries and also to be more sensitive than the brief measures already validated, such as the Mini-Mental State Examination [23, 24]. It has been used in a population with breast cancer and is considered to be relatively convenient to use in an outpatient, community setting. The HSCS assesses various aspects of cognitive functioning, including memory, language, visual–motor/spatial, attention/concentration, and self-regulation and planning. Inter-rater reliability for the instrument ranges from .86 to .99 for the various sections [23]. When compared with neuropsychological testing, the HSCS had a true positive rate of 94 %, a true-negative rate of 92 %, a false-positive rate of 8 %, and a false-negative rate of 6 % [23]. In addition, the Trail Making Tests (TMT), A and B, were also used to evaluate objective cognitive functioning [25].
At the time this study was developed (2000), there were no published reliable nor validated cancer-specific subjective measures available which assessed cognitive function in patients undergoing treatment. Subjective measures of cognition used in this study included the Profile of Mood States (POMS) (confusion/bewilderment subscale), and the cognitive subscale of the Perceived Health Scale (PHS). The PHS is a questionnaire developed by Dr. Brenda Lyons (Indiana University) to evaluate various aspects of health and functioning in a general aging population, which includes a cognitive subscale [26]. Questions are phrased so that participants are rating elements of their cognition compared to what they perceive as their normal functioning. The response set is categorical and ranges from 1 —“extremely less than normal” to 7—“extremely better than normal”. It has been highly correlated with the SF-36 and has been used in a population of breast and prostate cancer patients (N = 99), demonstrating good reliability [27].
Data were collected at the following time points: baseline (before first or second cycle of chemotherapy), during chemotherapy, at the first visit postchemotherapy (generally 1 month after chemotherapy and commensurate with the completion of the study agent), and at 6, 12, 18, and 24 months after chemotherapy. Potential side effects were evaluated at each data point by provider toxicity grading as per the NCI CTCAE-version 2, and by a self-report questionnaire using a numeric analogue scale, having participants rate side effects on a 0 (none) to 10 (worst possible) point scale.
The intervention consisted of capsules containing Ginkgo biloba 60 mg taken twice a day vs. an identical placebo that was provided by Schwabe Pharmaceuticals, Germany, IND # 63,522. The study drug was to begin prior to the secondcycle of chemotherapy and was taken throughout adjuvant treatment and 1 month beyond the completion of chemotherapy.
The primary analysis involved compiling each subscale score for the HSCS into area under the curve (AUC) scores for the data points from baseline to the 12-month data point. Each subscale score was compiled into an AUC score for each individual patient. This score was compared between the two arms by standard two-sample independent T tests. A sample size of 100 patients per group provided 80 % power to detect a difference of 40 % times the standard deviation using two-sided alternative hypothesis testing and a 5 % Type I error rate. TMT A and B and subjective measures were analyzed by evaluating changes from baseline. Mixed modeling was used to evaluate the differences between groups for PHS scoring from baseline to the 24-month data point. Changes from baseline were compared between arms for self-reported side effects. Correlational analyses were calculated to evaluate the associations between self-reported cognition and the HSCS and TMT A and B.
Results
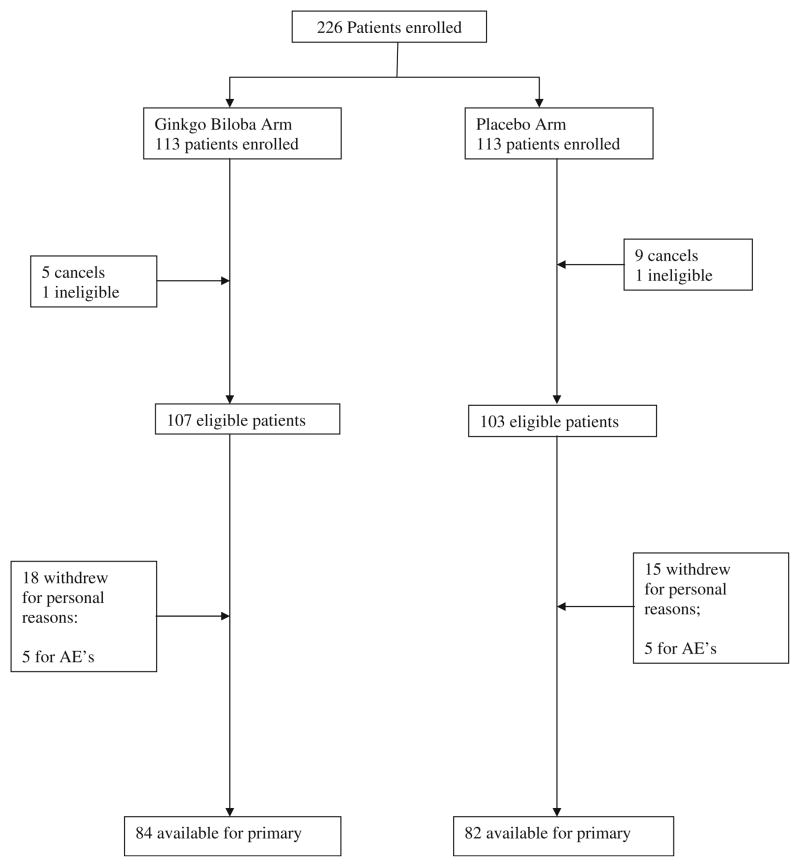
Twenty-three institutions across the US enrolled 226 patients in this study between October 2002 and October 2006. There were 14 cancellations and two ineligible patients removed from the analysis; these participants were randomized but withdrew before beginning any study interventions. Baseline characteristics of the remaining 210 participants are shown in Table 1 and were balanced between the two arms for all eligible patients. One hundred sixty-six patients provided data for the primary analysis. A CONSORT diagram is shown in Fig. 1.
Table 1.
Patient characteristics
| Ginkgo biloba (N = 107) | Placebo (N = 103) | |
|---|---|---|
| Age | ||
| <50 | 53 (50 %) | 51 (50 %) |
| ≥50 | 54 (50 %) | 52 (50 %) |
| Race | ||
| White | 99 (93 %) | 98 (95 %) |
| Black or African American | 8 (7 %) | 3 (3 %) |
| Native Hawaiian or other Pacific Islander | 0 (0 %) | 1 (1 %) |
| Asian | 0 (0 %) | 1 (1 %) |
| Chemotherapy treatment | ||
| Doxorubicin/cyclophosphamide | 35 (33 %) | 37 (36 %) |
| Doxorubicin/cyclophosphamide/taxane | 56 (52 %) | 54 (52 %) |
| Other anthracycline-based chemo | 6 (6 %) | 0 (0 %) |
| Other nonanthracycline-based chemo | 10 (9 %) | 12 (12 %) |
| Menopausal status | ||
| Premenopausal | 55 (51 %) | 56 (54 %) |
| Postmenopausal | 44 (41 %) | 43 (42 %) |
| Unknown | 8 (8 %) | 4 (4 %) |
| Lymph node involvement | ||
| 0–3 | 93 (87 %) | 89 (86 %) |
| ≥4 | 14 (13 %) | 14 (14 %) |
| Adjuvant TAM planned | ||
| Yes | 55 (51 %) | 56 (54 %) |
| No | 52 (49 %) | 47 (46 %) |
Fig. 1.
Patient Flow diagram
There were no significant differences for the AUC on any of the subscales of the HSCS over 12 months between arms (Table 2). There were also no statistically significant differences between the two arms on either TMT, A or B, over 24 months (Table 3). On TMT A, the change from baseline after chemotherapy was a median of −4 s for both GB and P, (p = 0.80), while on TMT B, it was −6.5 s for both arms (p = 0.44). At 12 and 24 months, change from baseline for TMT A was a median of −5 s (GB) and −2 s for (P) (p = 0.12) and −7 s (GB) and −5 s (P) (p = 0.87), respectively. For TMT B, 12-and 24-month median changes were −7 s (GB) and −3 s (P) (p = 0.10) and −5 s (GB) and −7 s (P) (p = 0.87), respectively.
Table 2.
Area under the curve High Sensitivity Cognitive Screen (HSCS)
| HSCS | Ginkgo (SD) (N = 107) | Placebo (SD) (N = 103) | p value |
|---|---|---|---|
| Memory | 12.2 (9.18) | 12.6 (9.16) | 0.78 |
| Language | 3.9 (6.84) | 2.6 (2.72) | 0.82 |
| Visual–motor | 1.1 (1.29) | 0.7 (0.84) | 0.12 |
| Spatial | 1.4 (0.92) | 1.3 (0.92) | 0.43 |
| Attention/concentration | 2.2 (3.59) | 1.8 (2.16) | 0.96 |
| Self-regulation | 2.4 (2.14) | 2.0 (2.10) | 0.17 |
| Total | 20.7 (19.59) | 18.0 (12.26) | 0.84 |
Table 3.
Median scores (s) for Trail Making Tests A and B (lower scores are better)
| Tests | Treatment | Baseline | Time after starting chemotherapy
|
||||
|---|---|---|---|---|---|---|---|
| 1 month | 6 months | 12 months | 18 months | 24 months | |||
| TMT A | Ginkgo biloba (N = 73) | 30 | 25 | 24 | 23.5 | 25 | 23 |
| Placebo (N = 67) | 28 | 25 | 22 | 25 | 23 | 21 | |
| TMT B | Ginkgo biloba (N = 73) | 48 | 45 | 41 | 40 | 44.5 | 40 |
| Placebo (N = 67) | 50 | 44 | 42 | 45 | 40 | 40 | |
Change from baseline in the confusion/bewilderment subscale of the POMS did not differ between groups at any time point. Likewise, the cognitive subscale of the PHS yielded no significant differences between groups at any data point using repeated measures mixed modeling estimates (data not shown).
According to CTCAE grading, there were no significant differences between groups for any toxicity, including anemia, bruising, insomnia, nausea, vomiting, thrombocytopenia, or overall worst toxicity. Self-reported effects are shown for the time frame over which Ginkgo biloba or placebo was taken (Table 4). There was only one significantly different side effect, nausea, which was worse in the group taking placebo.
Table 4.
Self-reported symptoms or side effects mean change from baseline to 1st postchemo visit (negative numbers indicate worsening symptoms)
| Self-reported side effect/symptoms | GB change from baseline (N = 73) | SD | Placebo change from baseline (N = 67) | SD | P valuea |
|---|---|---|---|---|---|
| Appetite loss | −0.5 | (2.90) | −0.8 | (2.81) | 0.34 |
| Nausea | −0.1 | (2.78) | −0.9 | (2.67) | 0.05 |
| Dizziness | −0.4 | (1.96) | −0.3 | (2.07) | 0.91 |
| Constipation | −0.7 | (2.77) | −0.9 | (2.68) | 0.43 |
| Headache | −0.6 | (2.13) | −1.2 | (2.31) | 0.14 |
| Diarrhea | −0.3 | (1.74) | 0.0 | (2.10) | 0.14 |
| Indigestion | 0 | (2.61) | −0.4 | (2.67) | 0.28 |
| Bruising | −0.4 | (2.33) | −0.9 | (2.42) | 0.35 |
| Overall QOL | −0.4 | (2.08) | −0.6 | (2.40) | 0.71 |
| Thinks memory affect QOL | 0.5 | (2.82) | −0.5 | (3.06) | 0.13 |
Two-sample independent t test
Since there were no differences between groups throughout the study period, data were combined to evaluate the relationship between self-report items and cognitive testing for similar areas of cognition. Correlational analyses demonstrated that there were low to no correlations between areas of cognition measured by the HSCS and similar self-report questions (Table 5). There were also very low associations between TMT A and B and self-reported questions of ability to solve problems, plan ahead, and make decisions (Table 5). The negative correlations in Table 5 indicate that better perceived functioning was associated with a lower number of seconds on TMT A and B; however, these coefficients are very small.
Table 5.
Pearson correlation coefficients conceptually related subjective and objective measures (N = 158)
| Variable | Self-report Stay focused | Self-report Think clearly | Self-report Balance checkbook | |
|---|---|---|---|---|
| HSCS attention concentration at: | 1 month | 0.01 | 0.08 | −0.02 |
| 6 months | −0.06 | 0.00 | −0.02 | |
| 12 months | −0.02 | 0.02 | −0.08 | |
| 18 months | 0.14 | 0.07 | −0.03 | |
| 24 months | −0.06 | −0.07 | −0.10 | |
| Solve problems | Plan ahead | Make decisions | ||
| TMT A | 1 month | −0.01 | 0.05 | −0.05 |
| 6 months | 0.04 | 0.20* | 0.10 | |
| 12 months | −0.09 | 0.03 | −0.01 | |
| 18 months | 0.06 | 0.08 | 0.08 | |
| 24 months | −0.01 | −0.02 | −0.02 | |
| TMT B | 1 month | −0.08 | −0.02 | −0.06 |
| 6 months | 0.09 | 0.28* | 0.21* | |
| 12 months | 0.13 | 0.16 | 0.10 | |
| 18 months | 0.06 | 0.08 | 0.10 | |
| 24 months | 0.00 | −0.05 | 0.00 |
p = .02
Discussion
Despite a promising rationale and some earlier positive results in AD and multi-infarct dementia, this study does not provide any data to support the use of Ginkgo biloba at a dose of 120 mg/day in the prevention of cognitive changes due to treatment with adjuvant chemotherapy for breast cancer. None of the endpoints, either self-report or cognitive testing, revealed differences between groups at any of the data points evaluated. The Ginkgo biloba was well tolerated, with no side effects significantly different than placebo and no signs of bleeding issues. The significant increase in nausea for the placebo group compared to the Ginkgo biloba group is likely a spurious finding due to the number of comparisons conducted. Most of the symptom scores are worse than baseline over time as all of these participants were receiving chemotherapy.
These results are in contrast to four old studies in populations of patients with AD or multi-infarct dementia [18], but are in agreement with more recent studies published on the use of Ginkgo biloba as a prevention strategy in age-related dementia in populations of healthy individuals without any known dementia processes [28–30]. In patients diagnosed with AD or dementia, Ginkgo biloba, in doses of 120 or 240 mg/day, resulted in maintenance or small improvements in cognitive function over 6 to 12 months, while participants receiving a placebo declined over the same time [17, 31]. A review of the data evaluating Ginkgo biloba for AD and dementia reported effect sizes of 0.40 over placebo in 424 patients (p<.0001) [18].
In contrast, a study evaluating a dose of 240 mg/day of Ginkgo biloba versus placebo in over 3,000 men and women aged 75 or over with mild or no cognitive impairment, reported no differences in the rate of dementia or AD or the rate of progression to dementia, or in the changes on various tests of cognitive function over a follow up period of 6 years [29]. Other studies that have evaluated preventive effects [28] or enhancement effects [30] in healthy populations without defined risk factors or signs of any cognitive impairment have also not demonstrated efficacy for either 240 mg or 120 mg/day, respectively.
The current study evaluated Ginkgo biloba for the prevention of cognitive impairment due to chemotherapy, targeting a patient population experiencing a reported insult to cognition. Therefore, these negative findings support either the lack of efficacy for Ginkgo biloba related to cognitive preservation or the fact that chemotherapy-induced changes are not physiologically similar to processes at work in AD or multi-infarct dementia.
In this study, all scores on the cognitive tests improved from baseline to first chemotherapy follow up and then stabilized. Due to repetitive testing, patients improved on these measures from the initial testing at baseline to the end of chemotherapy, likely due to practice effects. Alternate versions of neuropsychological testing are generally used to avoid this issue, but alternate versions of the HSCS were not available when this study was developed.
Multiple studies have reported no significant association between objective cognitive impairment after chemotherapy and patients’ self-report of their cognitive function [32–34]. A study by Vardy et al. compared three different cognitive assessments (the HSCS, the FACT-COG and computer-based programs, the Headminder and CogHealth) in a group of 31 survivors, mostly with a history of breast cancer, who had completed chemotherapy. Each assessment provided different rates of cognitive impairment and none of the more objective measures were correlated with the FACT-COG [34]. This current study’s findings coincide with what has been reported previously. In fact, the correlational analysis in this study compared items from self-report and more objective tests that were purportedly evaluating similar aspects of cognitive function. This was done in order to more accurately compare these measures. Despite these efforts, correlation coefficients remained extremely low throughout the 2 years of follow up. Some of the proposed reasons for the discrepancy between subjective and objective measures include that some studies indicate subjective cognitive changes that tend to be correlated more with emotional scales than with objective test results [35, 36]. Additionally, patients report that the negative changes in cognitive function they experience tend to be subtle and these may not be sensitive to tease out during neuropsychological testing [32, 36, 37]. For example, imaging studies show differences in brain scans between women who have had chemotherapy for breast cancer compared to those who did not, despite similar performance on neuropsychological tests [38, 39].
Cancer therapy-related cognitive decline, along with fatigue, anxiety, and depression are some of the most common symptoms negatively impacting health-related quality of life [11, 40]. During the past decade, studies have been performed to better define this treatment-related cognitive change and to study its prevalence. Unfortunately, the precise etiology and definitive risk factors remain unknown with this unsettling phenomenon.
Limitations of this study include weaknesses of some of the measures. This study was developed before well-validated and accepted self-report measures of cognition, such as the FACT-COG were published. Second, the HSCS has been shown to have a large practice effect, owing likely to the fact that alternate versions have not been developed [34]. Finally, it was challenging to educate and consent patients about this trial before receiving their first cycle of chemotherapy due to all of the educational and logistical needs encountered when a patient learns of the need for such treatment. Hence, patients were allowed on study after their first cycle of chemotherapy but before they were exposed to their secondcycle. This happened equally between arms and is, therefore, not thought to have impacted the outcomes of this trial (p = .22).
The strengths of this study include the fact that it is a rigorously designed, randomized placebo, controlled trial that utilized several measures, both objective and subjective, to capture cognition. In addition, the study was longitudinal, collecting data for a full 24 months after adjuvant therapy.
Conclusion
Based on the results of this study, Ginkgo biloba, 120 mg/day, does not present a pharmacologic treatment option for women facing treatment for breast cancer, with regard to preserving or enhancing cognitive function. Research to better characterize the physiology and risk factors that lead to cognitive dysfunction is needed in order to identify therapeutic biologic targets for treatment and prevention. As there remain no known effective pharmacologic strategies to prevent cognitive changes, this area of research remains of critical importance.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported, in part, by Public Health Service grants CA-25224, CA-37404, CA-63849, CA-35195, CA-35431, CA-35269, CA-35101, CA-63848, CA-37417, CA-35415, CA-35448, CA-35103, CA-35119, CA-35103, and CA-35272. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional participating institutions include: Siouxland Hematology-Oncology Associates, Sioux City, IA 51105, USA (Donald Wender, M.D.); Toledo Community Hospital Oncology Program (Paul L. Schaefer, M.D.); Medical College of Georgia, Augusta, GA 30912, USA (Anand P. Jillella M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309, USA (Robert J. Behrens, M.D.); Rapid City Regional Hospital, Inc., Rapid City, SD 57701, USA (Richard Charles Tenglin, M.D.); Columbus CCOP, Columbus, OH 53215, USA (J. Philip Kuebler, M.D., Ph.D.); Mayo Clinic Florida, Jacksonville, FL 32224, USA (Kurt A. Jaeckle, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106, USA (Philip J. Stella, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122, USA (Preston D. Steen, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822,USA (Albert M. Bernath, Jr, M.D.); Upstate Carolina CCOP, Spartanburg, SC 29303, USA (James D. Bearden, III, M.D.); Montana Cancer Consortium CCOP, Billings, MT 59101, USA (Benjamin T. Marchello, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105, USA (Miroslaw Muzurczak, M.D.); Lehigh Valley Hospital, Allentown, PA 18103, USA (Suresh Nair, M.D.); Ochsner CCOP, New Orleans, LA 70121, USA (Jyotsna Fuloria, M.D.); Colorado Cancer Research Program, Denver, CO 80224, USA (Eduardo R. Pajon, Jr., M.D.).
Footnotes
Conflict of interest None of the authors have any conflict of interest. Schwabe did supply Ginkgo biloba and placebo for this study, but none of the authors have any financial relationship with them. Our biostatisticians had full control of all primary data throughout data collection and analysis and still have these data under their control.
Contributor Information
Debra L. Barton, Email: barton.debra@mayo.edu, Mayo Clinic Rochester, Rochester, MN 55905, USA
Kelli Burger, Mayo Clinic Rochester, Rochester, MN 55905, USA.
Paul J. Novotny, Mayo Clinic Rochester, Rochester, MN 55905, USA
Tom R. Fitch, Mayo Clinic Arizona, Scottsdale, AZ 85259-5404, USA
Sadhna Kohli, Mayo Clinic Rochester, Rochester, MN 55905, USA.
Gamini Soori, Missouri Valley Cancer Consortium, Omaha, NE 68106, USA.
Mary Beth Wilwerding, Missouri Valley Cancer Consortium, Omaha, NE 68106, USA.
Jeff A. Sloan, Mayo Clinic Rochester, Rochester, MN 55905, USA
Lisa A. Kottschade, Mayo Clinic Rochester, Rochester, MN 55905, USA
Kendrith M. Rowland, Jr., Carle Cancer Center CCOP, Urbana, IL 61801, USA
Shaker R. Dakhil, Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882, USA
Daniel A. Nikcevich, Duluth CCOP, Duluth, MN 55805, USA
Charles L. Loprinzi, Mayo Clinic Rochester, Rochester, MN 55905, USA
References
- 1.Huria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Investig. 2007;25:373–7. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 2.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2009 doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 3.Wieneke M, Dienst E. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology. 1995;4:61–66. [Google Scholar]
- 4.Brezden CB, Phillips KA, Abdolell M, et al. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 5.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–69. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson RJ, Ahles TA. Low neuropsychologic performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep. 2003;3:215–22. doi: 10.1007/s11910-003-0081-2. [DOI] [PubMed] [Google Scholar]
- 7.Kreukels BP, van Dam FS, Ridderinkhof KR, et al. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer. 2008;8:80–7. doi: 10.3816/CBC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 8.Schagen SB, van Dam FS, Muller MJ, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–50. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–8. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 10.Kohli S, Griggs J, Roscoe JA, et al. Self-reported cognitive impairment in cancer patients. J Oncol Pract. 2007 doi: 10.1200/JOP.0722001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janz NK, Mujahid M, Chung LK, et al. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16:1348–61. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 13.Mehnert A, Scherwath A, Schirmer L, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns. 2007;66:108–18. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–83. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care. 2007;5:273–80. doi: 10.1017/s1478951507000442. [DOI] [PubMed] [Google Scholar]
- 16.Kohli S, Fisher SG, Tra Y, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–16. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 18.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409–15. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 19.Natural Standard: Natural Standard Database
- 20.Schultz VHR, Tyler V. Rational physotherapy: a physicians guide to herbal medicine, natural standard. Springer; New York: 1998. pp. 38–49. [Google Scholar]
- 21.Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012;46:180–9. doi: 10.1016/j.nbd.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64:465–72. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 23.Faust D, Fogel BS. The development and initial validation of a sensitive bedside cognitive screening test. J Nerv Ment Dis. 1989;177:25–31. doi: 10.1097/00005053-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Nelson A, Fogel BS, Faust D. Bedside cognitive screening instruments. A critical assessment. J Nerv Ment Dis. 1986;174:73–83. doi: 10.1097/00005053-198602000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Army Individual Test Battery. Manual of direction and scoring. Washington, DC: War Department Adjutant General’s Office; 1944. [Google Scholar]
- 26.Fakouri C, Lyon B. Perceived health and life satisfaction among older adults: the effects of worry and personal variables. J Gerontol Nurs. 2005;31:17–24. doi: 10.3928/0098-9134-20051001-06. [DOI] [PubMed] [Google Scholar]
- 27.Barton D. A model to predict negative health outcomes during cancer treatment 2002 [Google Scholar]
- 28.Dodge HH, Zitzelberger T, Oken BS, et al. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70:1809–17. doi: 10.1212/01.wnl.0000303814.13509.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snitz BE, O’Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–70. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon PR, Adams F, Silver A, et al. Ginkgo for memory enhancement: a randomized controlled trial. JAMA. 2002;288:835–40. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 31.Kanowski S, Herrmann WM, Stephan K, et al. Proof of efficacy of the Ginkgo biloba special extract EGb 761 in out-patients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry. 1996;29:47–56. doi: 10.1055/s-2007-979544. [DOI] [PubMed] [Google Scholar]
- 32.Donovan KA, Small BJ, Andrykowski MA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499–507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannock IF, Ahles TA, Ganz PA, et al. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–9. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 34.Vardy J, Wong K, Yi QL, et al. Assessing cognitive function in cancer patients. Support Care Cancer. 2006;14:1111–8. doi: 10.1007/s00520-006-0037-6. [DOI] [PubMed] [Google Scholar]
- 35.Kibiger G, Kirsh KL, Wall JR, et al. My mind is as clear as it used to be: a pilot study illustrating the difficulties of employing a single-item subjective screen to detect cognitive impairment in outpatients with cancer. J Pain Symptom Manage. 2003;26:705–15. doi: 10.1016/s0885-3924(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 36.Cull A, Hay C, Love SB, et al. What do cancer patients mean when they complain of concentration and memory problems? Br J Cancer. 1996;74:1674–9. doi: 10.1038/bjc.1996.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poppelreuter M, Weis J, Kulz AK, et al. Cognitive dysfunction and subjective complaints of cancer patients. a cross-sectional study in a cancer rehabilitation centre. Eur J Cancer. 2004;40:43–9. doi: 10.1016/j.ejca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson RJ, McDonald BC, Saykin AJ, et al. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–70. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819–28. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psychooncology. 2010 doi: 10.1002/pon.1837. [DOI] [PubMed] [Google Scholar]