Abstract
INTRODUCTION
Airway fluid glutathione (GSH) reactivity with inhaled vapors of diisocyanate, a common occupational allergen, is postulated to be a key step in exposure-induced asthma pathogenesis.
METHODS
A mixed (vapor/liquid) phase exposure system was used to model the in vivo reactivity of inhaled HDI vapor with GSH in the airway fluid. HDI-GSH reaction products, and their capacity to transfer HDI to human albumin, were characterized through mass spectrometry and serologic assays, using HDI-specific polyclonal rabbit serum.
RESULTS
HDI vapor exposure of 10 mM GSH solutions resulted in primarily S-linked, bis(GSH)-HDI reaction products. In contrast, lower GSH concentrations (100 μM) resulted in mainly mono(GSH)-HDI conjugates, with varying degrees of HDI hydrolysis, dimerization and/or intra-molecular cyclization, depending upon the presence/absence of H2PO4-/HPO42- and Na+/Cl- ions. The ion composition and GSH concentration of the fluid phase, during HDI vapor exposure, strongly influenced the transfer of HDI from GSH to albumin, as did the pH and duration of the carbamoylating reaction. When carbamoylation was performed overnight at pH 7, twenty-five of albumin's lysines were identified as potential sites of conjugation with partially hydrolyzed HDI. When carbamoylation was performed at pH 9, more rapid (within 3 hours) and extensive modification was observed, including additional lysine sites, intra-molecular cross-linkage with HDI, and novel HDI-GSH conjugation.
CONCLUSIONS
The data define potential mechanisms by which the levels of GSH, H2PO4-4/HPO42-, and/or other ions (e.g. H+/OH-, Na+, Cl-) affect the reactivity of HDI vapor with self-molecules in solution (e.g. airway fluid), and thus, might influence the clinical response to HDI respiratory tract exposure.
Keywords: hexamethylene diisocyanate (HDI), vapor, aliphatic, albumin, carbamoylation, glutathione (GSH), glutathione, diisocyanate, HDI, GSH
INTRODUCTION
Isocyanate (N=C=O) chemicals used to make polyurethane are a well-recognized cause of occupational asthma (Bernstein 1999; Redlich and Karol 2002; Tarlo and Liss 2002). Two classes of isocyanates, aromatic and aliphatic, possess different chemical properties and are typically used for distinct applications (http://www.cdc.gov/niosh/topics/isocyanates/; Klees and Ott 1999; Ulrich 1996). Aromatic isocyanates, such as toluene diisocyanate (TDI) † and methylene diphenyl diisocyanate (MDI) are excellent for foam production, elastomers and durable coatings (e.g. truck-bed liners), but are sensitive to photo-oxidation (Allport et al. 2003; Davis and Sims 1983; Ulrich 1996). Aliphatic isocyanates, such as HDI, possess better UV light resistance, and are typically used for exterior coatings, such as protective finishes on automobiles and aircrafts (Davis and Sims 1983; http://www.alipa.org/; Ulrich 1996).
The pathogenic mechanisms through which isocyanates cause asthma remain uncertain but, are believed to depend upon N=C=O reactivity with “self” molecules at exposure sites (Day et al. 1996; Day et al. 1997; Lange et al. 1999, Lantz et al. 2001, Wisnewski and Jones 2010). The fluid lining the human lower respiratory tract contains a relatively high level (>100 μM) of an essential tri-peptide, GSH, postulated to be a primary reactant for inhaled isocyanates (Cantin et al. 1987; Day et al. 1997, Lange et al. 1999, Lantz et al. 2001). Airway fluid GSH levels are normally maintained within a limited range, by complex genetically-defined homeostatic feedback mechanisms (Day et al. 2004; Rahman and MacNee 2000). However, substantial individual variability may occur secondary to environmental exposures (e.g. smoking, infection), or genetic mutations/polymorphisms, and has been suggested to influence the pathogenesis of several different diseases (Day 2005, 2009; Pacht et al. 1991; Rahman and MacNee 1999, 2002; Roum et al. 1999; Smith et al. 1993; van der Vliet et al. 1999). In animal studies, inhalation of isocyanate vapors acutely alters airway fluid GSH levels, while GSH depletion exacerbates MDI's respiratory tract toxicity (Pauluhn 2000a, b). Among occupationally exposed workers, the development of isocyanate asthma, and rate of chemical excretion have been associated with genetic polymorphisms in GSH-dependent enzymes (Broberg et al. 2010; Littorin et al. 2008; Mapp et al. 2002; Piirila et al. 2001; Wikman et al. 2002).
In vitro studies have begun to dissect possible mechanisms through which isocyanate reactivity with GSH might influence immunologic and/or other biological responses to exposure. For the aromatic diisocyanate, TDI, it has been demonstrated that chemical vapors readily cross a liquid phase barrier to react with GSH, and cause acute depletion of intracellular GSH in human airway epithelial cells (Lantz et al. 2001; Wisnewski et al. 2011). Furthermore, thiol-linked vapor TDI-GSH reaction products are capable of carbamoylating (transferring TDI to) peptide/protein molecules, including human albumin, the major “carrier” protein for TDI in vivo, and essential element of allergenic (IgE) recognition by the human immune system (Day et al. 1997; Lange et al. 1999; Wass and Belin 1989; Wisnewski et al. 2011).
The reactivity of GSH with HDI, and other aliphatic isocyanates used in polyurethane manufacturing, remains less clear and may differ from aromatic isocyanates, given inherent differences in their chemical reactivity. The hydrolysis of aliphatic isocyanates is much slower than aromatic isocyanates, which may provide greater opportunity to react with GSH in solution (Brown et al. 1987). Thiol-linked (e.g. GSH) reaction products with aliphatic isocyanates are likely more stable than reaction products with aromatic isocyanates, based on prior studies with cysteine-methyl-ester (Chipinda et al. 2006). In short-term (1hr) vapor exposure studies in vitro, GSH has been reported to prevent HDI protein conjugation, rather than mediate transcarbamoylation as observed with TDI (Day et al. 1997; Wisnewski et al. 2011; Wisnewski et al. 2005). A better understanding of the GSH reactivity with HDI vapor should provide insight into the basic mechanisms underlying the biological effects of HDI exposure.
The present study used a mixed (vapor/liquid) phase exposure system to model the biophysics of HDI reactivity in the airway microenvironment, where vapor phase chemical contacts the airway lining fluid, which contains high levels of GSH (Wisnewski et al. 2011; Wisnewski et al. 2004). A variety of physiologically relevant, inter-dependent exposure variables (pH, [GSH], and ion/buffer content of the solution) were evaluated for their potential influence on HDI vapor phase reactivity with GSH, and subsequent carbamoylation of human albumin, the major carrier protein for isocyanates in vivo. The potential biological relevance of the in vitro data is discussed.
EXPERIMENTAL PROCEDURES
HDI Vapor Exposure
Solutions (100 μM-10 mM) of reduced or oxidized glutathione (GSH or GSSG respectively) from Sigma (St. Louis, MO) were prepared in 20 mM phosphate buffered saline (PBS, pH 7.4) from Gibco (Grand Island, NY), 140 mM NaCl, or de-ionized water (Sigma). All GSH solutions and reaction products were 0.2 μm filtered (Millipore; Billerica, MA) to ensure sterility. Solutions were exposed as previously described, to room air or HDI vapor, for 18 h, in 35 × 10 mm Petri dishes obtained through VWR International from Bridgeport, NJ (Wisnewski et al. 2011; Wisnewski et al. 2004). HDI vapors were obtained by passive diffusion from puriss grade HDI (PubChem Substance ID: 24874557, CAS Number: 822-06-0) solution (Sigma-Aldrich), ≥99% purity by gas chromatography, with a refractive index of n20/D 1.453, and a density of 1.047 g/mL at 20°C. HDI vapor concentration was monitored with an Autostep toxic gas monitor (GMD Systems, Pittsburgh, PA, USA), and maintained at 180 +/- 20 ppb, by adjusting the intake air flow rate. The mixed phase exposure conditions were empirically developed in prior studies, based on specific recognition of (albumin) reaction products by serum IgG from HDI exposed workers. Each exposure condition was replicated in four independent experiments to determine biological variability of the experimental results.
LC-MS
Experiments were performed on a Waters (Milford, MA) nanoACQUITY ultra-performance liquid chromatography (UPLC) system. Aliquots (1 μL) of each GSH-HDI mixture were injected and trapped/desalted on a 5 μm SymmetryC18 (180 μm × 20 mm) trapping column with 99.5/0.5 A/B (A:0.1% formic acid; B:0.1% formic acid in acetonitrile) at a flow rate of 15 μL/min for 1 minute. Separation was performed on a 1.7 μm BEH130 C18 (100 μm × 100 mm) analytical column utilizing gradient elution at a flow rate of 400 nL/min and a gradient of 99/1 to 60/40 A/B over 60 min. The eluent from the UPLC system was directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Positive ion nanoelectrospray was performed utilizing 10 μm PicoTip (Waters) emitters held at a potential of +3.5 kV. The cone voltage was held constant at +40 V for all experiments. Dry N2 desolvation gas was supplied to the instrument via a nitrogen generator (NitroFlowLab, Parker Hannifin Corp., Haverhill, MA). [Glu]1-Fibrinopeptide B (100 fmol/μL in 75/25 A/B) was supplied to an orthogonal reference probe and the [M+2H]2+ ion (m/z = 785.84265u) measured as an external calibrant at 30 sec intervals. Ultra-high purity (UHP) argon was used as collision gas. Data were analyzed with MassLynx v. 4.1 (Waters). Samples from four independent experiments were analyzed to assess reproducibility of the experimental findings.
GSH-HDI Mediated Carbamoylation of Human Albumin
Solutions of HDI vapor exposed GSH were co-incubated 1:2 (v/v) with a 5 mg/mL solution of human albumin (Sigma) at 37°C. Initial studies, including those analyzed by ELISA and Western blot (Figure 3A and B), were performed overnight in 0.1 M carbonate, pH 9.0. Subsequent carbamoylation reactions were performed for varying time periods (1 hr to 3 days) in carbonate buffer, or at pH 7.0 using 0.1 M (mono/dibasic) phosphate buffer. Four different experiments were performed in triplicate using GSH-HDI reaction products from four independent experiments.
Figure 3. Carbamoylation of human albumin by GSH-HDI based on immunochemical analyses.
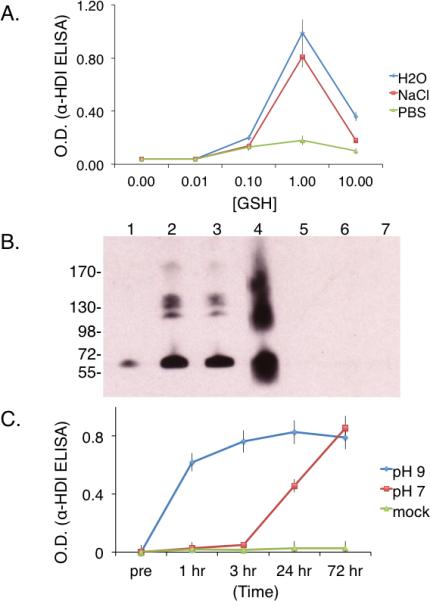
Panel A- HDI vapor exposed solutions containing different concentrations of GSH (X-axis = mmoles/L) in PBS, NaCl, or H20, were tested for their ability to transfer HDI to human albumin, based on HDI-specific polyclonal rabbit serum IgG binding. *p < 0.002 comparing PBS vs. H20 or NaCl when [GSH] is 1 mM. Panel B- Western blot with HDI-specific antiserum of human albumin co-incubated with HDI vapor exposed solutions of 1mM GSH in PBS (lane1), H2O (lane 2), or NaCl (lane 3). For comparison, albumin directly conjugated by HDI vapors (lane 4), or incubated with control (room air exposed) GSH solutions prepared in PBS (lane 5), H2O (lane 6), or NaCl (lane 7). Panel C- Solutions of HDI vapor exposed 1 mM GSH in H2O were tested for their ability to transfer HDI to albumin over time (X-axis) at pH 7.0 or pH 9.0 as labeled. *p < 0.001 comparing pH 7 vs. pH 9 at 1, 3 and 24 hrs. Note: ELISA O.D. values (Y-axis) in Panels A & C reflect “antigenic” HDI transfer (mean and standard error) observed in N=4 independent experiments, while Western blot in Panel B is representative of N=3 independent experiments.
Anti-HDI ELISA
Maxisorp® microtiter plates from Nunc (VWR International) were incubated overnight at 4°C with 5 μg/well of human albumin that had been co-incubated with solutions of HDI vapor-exposed GSH. Plates were coated in 0.1 M carbonate buffer, pH 9.5, washed, and “blocked” with 3% (w/v) dry milk, before addition of anti-HDI rabbit serum, diluted 1:200 (v/v). As previously described, the HDI-specific rabbit polyclonal antiserum was raised against HDI–KLH, depleted of KLH binding activity by affinity chromatography, and specifically recognizes HDI when bound to a larger carrier protein, such as human albumin (Wisnewski et al. 2010; Wisnewski et al. 1999; Wisnewski et al. 2008; Wisnewski et al. 2004; Wisnewski et al. 2000). ELISA plates were developed with peroxidase-conjugated anti-rabbit IgG (Pharmingen; San Diego, CA), diluted 1:2000 (v/v), and TMB substrate. Optical density (OD) measurements (absorbance of light at 450 nm, minus absorbance at a reference wavelength), reflecting detection of HDI, were obtained on a Benchmark microtiter plate reader from Bio-Rad. Samples from four independent experiments, described above, were analyzed in triplicate to obtain mean and standard error values. Statistical differences in IgG binding data were calculated using the Wilcoxon rank-sum test.
Anti-HDI Western Blot
Human albumin carbamoylation reactions (1 μL) or control samples from three independent experiments were electrophoresed under reducing conditions (without heating to avoid protein aggregation) on precast 4-15% gradient gels and transferred to nitrocellulose membrane using a trans-blot system from BioRad (Hercules, CA). Nitrocellulose membranes were blocked with 3% dry milk in PBS, and probed with rabbit anti-HDI polyclonal antiserum (anti-KLH depleted), diluted 1:200 as previously described (Wisnewski et al. 2010; Wisnewski et al. 1999; Wisnewski et al. 2008; Wisnewski et al. 2004; Wisnewski et al. 2000). Following incubation with 1:1000 peroxidase-conjugated anti-rabbit IgG (Pharmingen; San Diego, CA), blots were developed with enhanced luminescence reagent from Thermo Fisher Scientific (Rochester, NY).
Quantitative Measurements of Hexamethylene Diamine (HDA)
Samples were treated with equal volume of 3M H2SO4 at 100°C for 16 hours, and then neutralized with 2.5 volumes of saturated sodium hydroxide, vortexed and cooled in an ice bath to cool for 10 minutes. Samples were extracted 2X with dichloromethane (DCM), evaporated at 40°C under N2, to 1 mL. Five hundred μL of 0.5% H2SO4 was then added to the extract, of which two-hundred fifty μL was finally used for derivatization. An equal volume of saturated borate buffer (pH 8.5) and 450 μL of acetonitrile were added and vortexed for 1 minute before adding 50 μL of 15 mg/mL fluorescamine in acetonitrile. Samples were separated on a 250 × 4.6 mm, 5μm particle size Discovery C18 column run using a Shimadzu Prominence HPLC system (Columbia, MD, USA) consisting of an online vacuum degasser (model DGU-20A5), a quaternary pump (model LC-20AT), autosampler (model SIL-10AD-VP), and fluorescence detector (model RF-10AXL). HDA-fluorescamine derivatives were eluted from the column into a fluorometer at 1 mL/min using a linear solvent gradient of 0 to 30% ACN/water. Analytes were excited at 410nm and emission was measured at 510nm. The concentration of HDA in albumin samples carbamoylated by GSH-HDI was based on calibration standards containing 10-1600 ng/mL HDA in 1.65 mg/ml albumin solution in PBS, which were acid hydrolyzed at the same time. Analysis of samples from three independent experiments is provided in supplemental material (on-line Fig. E6).
MS/MS analysis of GSH-HDI reaction products
For FT-ICR MS analysis, total GSH-HDI reaction products were desalted using a C18 ZipTip, eluted into 60% acetonitrile/ 0.1% formic acid and infused, via Triversa NanoMate, into a Bruker 9.4T FT-ICR MS (BrukerDaltonics; Billerica, MA), as previously described (Stone et al. 2007; Wisnewski et al. 2011).
Preparative Reverse Phase HPLC Purification of GSH-HDI Reaction Products
GSH-HDI reaction products from three independent experiments were fractionated by the Yale Keck Center on a Hewlett-Packard 1090 HPLC system equipped with an Isco Model 2150 Peak Separator and a 1 mm × 25 cm Vydac C-18 (5 μm particle size, 300 Å pore size) reverse phase column (Williams and Stone 1997). Following equilibration with 98% buffer A (0.06% TFA) and 2% buffer B (0.052% TFA, 80% acetonitrile), mono(GSH)-HDI* and bis(GSH)-HDI reaction products eluted with distinct retention times (~12 and ~20 minutes respectively), when buffer B was increased from 2 to 37% over the course of 1 h. Elution was tracked by A210, and purity was verified by MS/MS. For carbamoylation reactions, fractions containing equimolar amounts of mono(GSH)-HDI* or bis(GSH)-HDI, based on HDA released by acid hydrolysis, were speed-vacuumed to dryness and resuspended with 100 μL of 3.3 mg/mL human albumin in 0.1 M carbonate, pH 9.0 and incubated overnight at 37°C.
MS/MS identification of albumin carbamoylation sites
Samples of albumin carbamoylated by GSH-HDI, were reduced, acetylated, and trypsin digested, prior to LC-MS/MS at the Yale University Keck Center, as previously described (Stone et al. 2007; Williams and Stone 1997). Samples from 3 independent experiments were analyzed on an LTQ Orbitrap Elite mass spectrometer and all MS/MS spectra were searched using the automated Mascot algorithm against the NCBInr database. A 95% confidence level was set within the MASCOT search engine for protein hits based on randomness search. In addition, 2 or more MS/MS spectra must have matched the same protein entry and been derived from trypsin digestion. Peptide scores >20 are likely correct based on past experience, and the higher the score the better the match. In addition to oxidation of methionine and acetylation of cysteine (during workup), the data were further queried for anticipated mass modifications due to carbamoylation by GSH-HDI reaction product(s). Supplemental material Figure E1 provides chemical structures of the predicted Δ mass modifications shown in Table 1.
Table 1.
Predicted mass modifications due to carbamoylation with HDI via GSH
| Δ Mass | Chemical formula | Description |
|---|---|---|
| 142.11 | C7H14N2O | HDI*, *2nd NCO hydrolyzed |
| 168.09 | C8H12N2O2 | HDI, cross-linked |
| 532.19 | C20H32N6O9S | HDI-GSH (2nd NCO conjugated to γ-glu of GSH, acetylation of GSH's thiol) |
| 674.30 | C27H46N8O10S | HDI*-HDI-GSH (2 HDI molecules linked via 1 hydrolyzed NCO, conjugated to GSH's γ-glu, acetylation of GSH's thiol) |
RESULTS
HDI-GSH Reaction Products Resulting from Mixed Phase Exposure
Reaction products of HDI vapor with reduced glutathione (GSH) in fluid phase, were analyzed by LC-MS methods. Initial experiments were performed with solutions of GSH at two different concentrations, 10 mM and 100 μM, to mimic approximate human intra- and extra-cellular (e.g. airway fluid) concentrations. When the aqueous phase contained 10 mM GSH, HDI vapor exposure consistently resulted in a single major product, with an LC-column retention time around 20 minutes, and an m/z of 783.3 (Figure 1). Further MS/MS analysis (see supplemental materials, Fig. E2) identified the product as bis(GSH)-HDI, in which HDI's 2 NCO groups were conjugated via thiocarbamate linkages to GSH's cysteine side chains (rather than via carbamide linkages to the primary amine of γ-glutamate) as shown in Figure 2A. Formation of bis(GSH)-HDI was similarly observed when 10 mM GSH was dissolved in PBS, 140 mM NaCl, or deionized H2O (Figure 1A-C).
Figure 1. LC-MS analysis of GSH-HDI reaction products formed under different conditions.
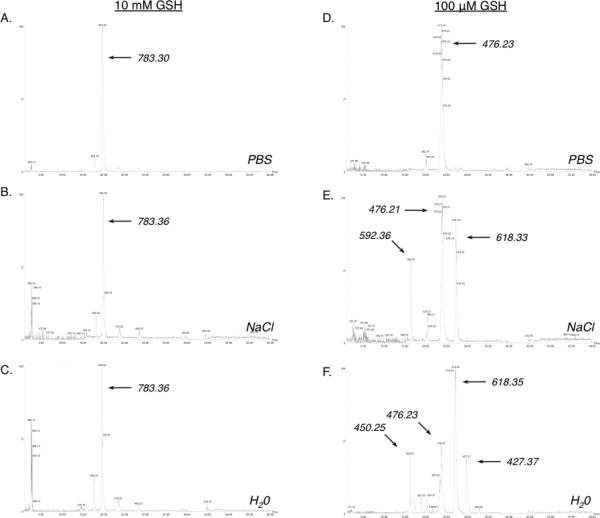
LC-MS base peak intensity chromatograms of the reaction products formed in the presence of 10 mM (left) or 100 μM GSH, dissolved in PBS, or H2O as labeled. The m/z of the major products are highlighted. Data shown are representative of N=4 independent experiments.
Figure 2. Chemical structures of GSH-HDI reaction products.
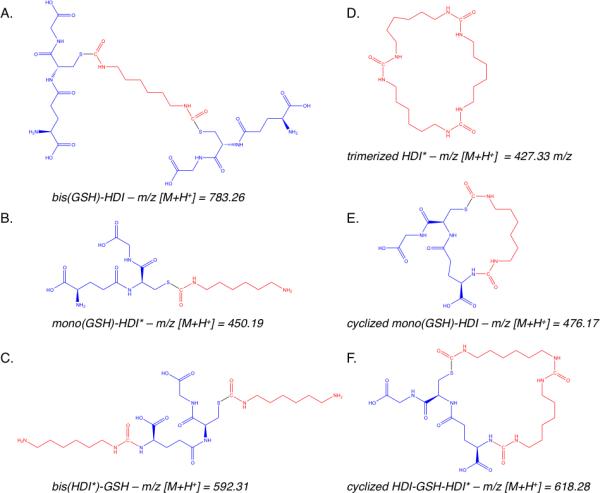
The chemical structures of the major reaction products of HDI vapor with fluid phase GSH are based on the exact mass and MS/MS data included in the Supplemental Materials.
When HDI vapor exposed solutions contained 100-fold lower concentrations of GSH (e.g. 100 μM), LC-MS analysis revealed different reaction products, which further varied depending upon the ionic composition of the fluid phase (Fig. 1D-F). In PBS, a single major product, with a m/z of 476.2 was observed. In saline solution (140 mM NaCl), the 476.2 m/z product was similarly present; however, two other major products were also observed, with m/z of 618.3 and 592.3. In deionized H2O, four major GSH-HDI reaction products were observed; the 618.3 m/z and 476.23 m/z products described above, as well as unique products with m/z of 450.2 and 427.4. The structures of the 100 μM GSH reaction products with HDI vapor were further characterized by MS/MS (see supplemental materials Figs. E3-E5), which confirmed molecular assignments based on the exact mass (Fig. 2). The data identify four different GSH-HDI reaction products, each containing a single GSH molecule, in contrast to the major bis(GSH) product formed at higher (10 mM) GSH concentrations. Furthermore, lower (100 μM) GSH concentration resulted in more reaction with GSH's “amino” terminus, intramolecular cyclization, and/or reaction products with two HDI molecules. Together, the data describe the formation of markedly different fluid phase reaction products, despite identical HDI vapor exposure, depending upon the GSH concentration and ionic composition.
Carbamoylation of human albumin by GSH-HDI reaction products
The potential influence of the fluid phase starting composition (GSH concentration, buffer/ion content) on the subsequent carbamoylation capacity of different HDI vapor exposed solutions was initially investigated through ELISAs and Western blots with HDI-specific polyclonal rabbit serum. Under the conditions tested, HDI vapor exposed-solutions possessed the greatest capacity to “antigenically” transfer HDI to human albumin, when GSH was present at 1 mM (Fig. 3A). However, the relationship between starting GSH concentration and carbamoylating capacity appeared to be non-linear, and significantly (p < 0.002) influenced by the presence of H2PO4-/HPO42- ions (Fig. 3A and B). Further studies suggested that the pH level during the albumin carbamoylation reaction (after HDI vapor exposure) also had substantial affects on HDI transfer kinetics. As shown in Fig. 3C, “antigenic” HDI transfer occurred rapidly (within 1 hr), and reached near maximal levels within 3 hours at pH 9. In contrast, modification occurred significantly (p < 0.001) slower at pH 7, requiring 3 days to reach maximal levels.
Quantitation of the amount of HDI transferred to human albumin by GSH-HDI was subsequently evaluated by measuring the amount of hexamethylene diamine (HDA) released from selected samples upon acid hydrolysis (e.g. mol hydrolyzed HDA/mol albumin). The maximum amount of HDI/albumin (e.g. substitution ratio) mediated by GSH transfer was 0.47 (± 0.18), while albumin directly exposed to HDI vapor contained 1.99 (± 0.4) mol HDI/ mol albumin. Overall, the levels of HDA released by acid hydrolysis correlated well with anti-HDI ELISA absorbance measurements of individual samples (r2 = 0.91) (supplemental Fig E6, and data not shown).
Evidence for mono(GSH)-HDI* as the major carbamoylating reaction product of HDI vapor with GSH
Additional comparative LC-MS was performed to characterize HDI exposed GSH solutions with potent vs. weak carbamoylating capacity, as defined immunochemically. As shown (Fig 4), mono(GSH)-HDI* is uniquely present in HDI vapor exposed solutions with potent carbamoylating capacity (1 mM GSH in deionized H2O), and consistently absent from HDI exposed solutions (1mM GSH in PBS) with weak carbamoylating activity. Further (anti-HDI) Western blot analysis (Fig. 5), with different HPLC purified GSH-HDI products, similarly implicate mono (GSH)-HDI as the major carbamoylating species formed upon HDI vapor exposure of fluid phase GSH.
Figure 4. Effect of buffer on GSH-HDI products formed at 1 mM GSH.
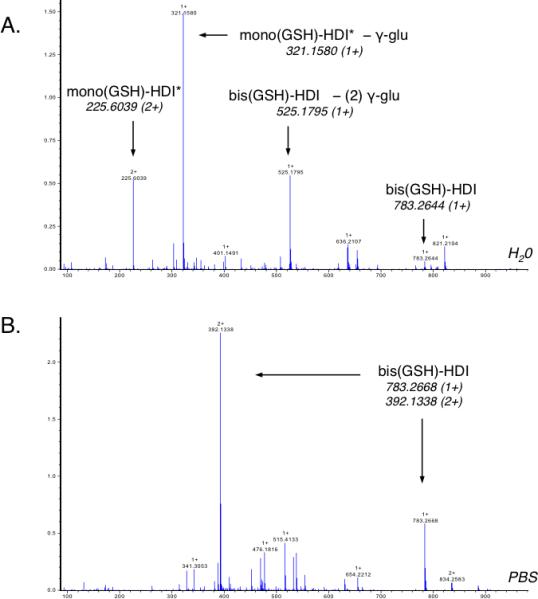
FT-ICR MS analysis of GSH-HDI reaction products formed in (HDI vapor exposed) solutions of 1 mM GSH, prepared in H2O (A) or PBS (B). The major species were identified based on exact mass and MS/MS (see supplemental materials, Figs E2 and E3). Data shown are representative of N=3 independent experiments.
Figure 5. Immunochemical identification of mono(GSH)-HDI as the major carbamoylating reaction product of GSH with vapor phase HDI.
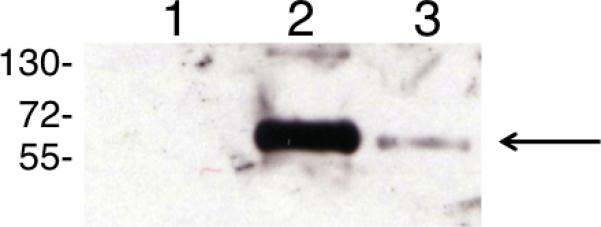
HDI-specific Western blot was performed on albumin that had been co-incubated overnight at pH 9, with reverse phase HPLC purified GSH (lane 1), or equimolar amounts of mono(GSH)-HDI* (lane 2), or bis(GSH)-HDI (lanes 3), based on quantitation of HDA released by acid hydrolysis. Data shown are representative of N=3 independent experiments.
Loci of albumin carbamoylation by GSH-HDI
The loci of albumin modification by HDI vapor exposed-GSH reaction products were assessed by LC-MS/MS. Data from three independent experiments consistently identify GSH-mediated HDI conjugation of up to twenty-five of albumin's lysines (Fig. 6, and supplemental materials, Table E2) when carbamoylation was performed overnight at pH 7, with the total reaction products of 1mM GSH in deionized H2O (which exhibited maximal “antigenic” HDI transfer). Ion fragmentation patterns upon CID further suggested hydrolysis of the unbound NCO group of HDI, to a free amine (see supplemental materials Fig. E1a and Table E1) (Hettick et al. 2011; Wisnewski et al. 2004). The potential sites of albumin modification by GSH-HDI were numerous compared with those directly conjugated by HDI vapors in previous reports, and overlapped with, but were qualitatively distinct from those conjugated by GSH-TDI, or direct exposure to TDI or MDI (Hettick and Siegel 2012; Hettick et al. 2011; Wisnewski et al. 2010; Wisnewski et al. 2011; Wisnewski et al. 2004). Among the sites carbamoylated by GSH-HDI were all (four) of albumin's di-lysine site motifs, and Lys351, recently suggested to be most susceptible to direct TDI vapor conjugation (Hettick et al. 2011). It should be noted that although the number of potential HDI conjugation sites (via GSH-HDI) was numerous, the absolute amount of conjugation/albumin molecule was relatively low, based on HDA analysis as described above, suggesting conjugation to different lysine groups in different albumin molecules.
Figure 6. Loci of HDI conjugation to human albumin, resulting from carbamoylation by GSH-HDI.

Sites of carbamoylation at pH 7 and/or pH 9, identified by MS/MS, are highlighted. K -conjugated w/ HDI* (*=2nd NCO hydrolyzed) resulting from pH 7 carbamoylation reaction overnight. Additional loci of conjugation resulting from carbamoylation reaction at pH 9 for 3 hr are also highlighted, including K (no underline)-conjugated w/ HDI* (*=2nd NCO hydrolyzed), K (double underline)-conjugated w/ HDI-GSH (GSH bound via γ-glu), or K̵ (underline and strikethrough)-peptide internally conjugated with HDI.
Consistent with the immunochemical assessment of HDI transfer, LC-MS/MS analysis demonstrated more extensive and rapid albumin carbamoylation by GSH-HDI at pH 9 vs. pH 7. When albumin carbamoylation was performed for three hours at pH 9.0, an additional eight lysines were modified by HDI, in which the unbound NCO was hydrolyzed as described above (Fig. 6 and supplemental materials Table E2). Also observed were internal cross-linking of an individual peptide with HDI (see supplemental materials Fig 1b), and a previously undescribed carbamoylation product apparently resulting from reaction of albumin's K389 with intramolecularly cyclized GSH-HDI, as shown (Fig. 7 and supplemental material Figs. E1c, E7, E8 and Table E2). Together, the mass spectrometry data identify specific loci of structural modification, and describe novel HDI-mediated cross-linking of GSH to human albumin.
Figure 7. Novel type of albumin conjugation by HDI-GSH.
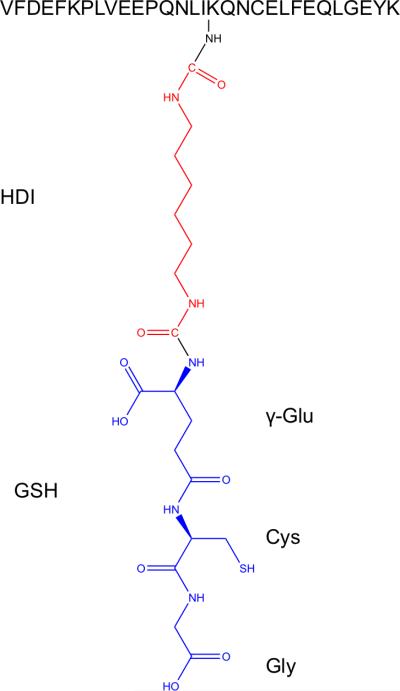
Proposed structure of novel HDI-GSH conjugation to albumin based on MS/MS data, where one NCO of the HDI is linked to K389 side chain, and the 2nd NCO is linked to the “N-terminus” of GSH (e.g. γ-glutamate side chain). Structure is based on identical MS/MS data from N=3 independent experiments.
DISCUSSION
This report demonstrates the ability of the aliphatic diisocyanate, HDI, to react with glutathione across a vapor/liquid phase boundary, and describes unique reaction products formed in the presence of physiologic GSH concentrations. Notably, different reaction products formed under identical exposure conditions, depending upon the GSH concentration and the ionic composition of the fluid phase. Among the different vapor HDI-GSH reaction products, mono(GSH)-HDI* exhibited particularly potent carbamoylating activity, based on immunochemical analyses, and was a major product present in HDI-vapor exposed GSH solutions proven to transfer HDI to albumin by LC-MS/MS. Together, the data support a hypothetical mechanism by which GSH in the airway fluid may mediate HDI vapor uptake from the respiratory tract, as reversible reaction products. The data further suggest that individual differences in airway fluid composition could modify this process.
The present study extends recent investigations modeling mixed (vapor/liquid) phase reactivity between GSH and asthma–causing diisocyanate chemicals used for commercial polyurethane production. The formation of bis(GSH)-diisocyanate conjugates, as observed here upon HDI vapor exposure of 10 mM GSH, is analogous to that previously reported with the aromatic diisocyanate, TDI (Day et al. 1997; Wisnewski et al. 2011). However, the mono(GSH)-HDI reaction products observed at lower GSH concentrations are distinct and include partially hydrolyzed HDI dimers and/or unique cyclized structures. Importantly, mono rather than bis(GSH)-HDI conjugates were the major reaction products resulting from HDI vapor exposure of 100 μM GSH, the approximate concentration of the lower airway fluid in vivo in humans.
The influence of the fluid phase ionic composition and [GSH] on the formation of GSH-HDI reaction products highlights the potential complexity of HDI reactivity in vivo upon inhalational exposure. Inorganic ions such as H2PO4-/HPO42-, which affect pH levels, also alter reactivity of GSH's thiol toward NCO, and may participate in hydrolysis of NCO and solvolysis of GSH-NCO thiocarbamates (Ng et al. 2004). Inter-individual differences in airway fluid GSH levels, due to genetic and/or other environmental exposures (e.g. smoking), might further result in different HDI reaction products (Day et al. 2004; Duan et al. 1993; Rahman and MacNee 1999). Thus, variability in airway fluid GSH and ion composition might alter the clinical response to HDI vapor exposure, and could help explain individual differences in HDI asthma susceptibility.
The carbamoylating activity of the presently described (aliphatic) HDI-GSH conjugates differs from that of (aromatic) TDI-GSH conjugates (Day et al. 1997; Wisnewski et al. 2011). While bis(GSH)-TDI is more potent than mono(GSH)-TDI* at transferring TDI to albumin, the opposite appears to be true for the corresponding GSH-HDI conjugates, at least based on immunochemical (anti-HDI IgG) analysis. Perhaps more importantly, the kinetics of GSH-HDI mediated albumin carbamoylation, at neutral pH, are substantially delayed compared with those of GSH-TDI. Such differences may reflect inherent differences in stability of thiocarbamate linkages between aromatic vs. aliphatic isocyanate (e.g. TDI vs. HDI) as mentioned earlier, and may be especially relevant to “detoxification” and elimination in vivo (Chipinda et al. 2006). As HDI is excreted rapidly (within hours) from exposed workers, the relative stability of GSH-HDI conjugation (vs. GSH conjugates with aromatic isocyanates) may serve an overall protective, rather than pathogenic role, consistent with previous short-term in vitro HDI vapor studies (Liu et al. 2004; Wisnewski et al. 2005).
Differences between albumin carbamoylated by GSH-HDI, and albumin directly conjugated by HDI, are further suggested by the present data. Under the current experimental conditions, the amount of HDI per albumin molecule, resulting from transfer by GSH-HDI, was four times lower than that resulting from direct HDI vapor exposure. However, while over 25 of albumin's lysines could be modified by GSH-HDI at neutral pH, vapor exposure appears to target a limited number of sites (Wisnewski et al. 2004). By native gel electrophoretic analysis, albumin conjugated by GSH-HDI was virtually unaffected, while the migration of albumin directly conjugated by HDI vapor (and liquid, not shown) was dose-dependently increased, suggesting differences in conformation and/or charge. Such data are consistent with the identification of partially hydrolyzed HDI, vs. cross-linked HDI, as the major modification resulting from carbamoylation via GSH-HDI. Further studies will be needed to better understand the difference(s) between albumin directly reacted with HDI vapor vs. GSH-HDI, including, structural/conformational changes and preferential loci of HDI conjugation in vivo.
Particular strengths and weaknesses of the present study are important considerations in the data interpretation. The immunology-based assessment of HDI conjugation, using polyclonal HDI-specific rabbit serum, facilitated high-throughput analysis of albumin carbamoylation in a cost and time-effective manner. However, while the approach recognizes “antigenic” HDI, it is possible that HDI carbamoylation may alter albumin's structure in a way that affects antiserum binding in an HDI dose-independent manner. Although this possibility cannot be ruled out entirely, quantitation of hydrolyzable HDA and the LC/MS-MS data from studies performed at different pH levels are consistent with anti-HDI serology, and positively identify numerous sites of HDI conjugation. It should be noted that the workup (reduction/acetylation) of protein samples for LC/MS/MS, prevented potential identification of albumin carbamoylation on cys-34, the only free thiol in albumin, which could have occurred by a thiol-exchange mechanism. Similar S-linked HDI-albumin conjugates, if they occur in vivo, are likely to be relatively unstable, susceptible to further thiol-exchange as they are in vitro.
Inherent limitations of the in vitro mixed-phase exposure system, in modeling the airway microenvironment, should be also recognized. In particular, the exposure system lacks many components essential to airway fluid's functional activity (surfactant, protein, alveolar macrophages, etc.). Furthermore, while the system recapitulates a mixed (vapor/liquid) phase exposure, the HDI vapor concentration is difficult to compare with that in vivo, given differences in the volume:surface area, and lack of fluid phase mixing. As a starting point, we relied upon a fixed HDI vapor concentration established in previous studies, and evaluated the potential influence of variability in (airway) fluid composition. Future studies with titrated levels of HDI vapor, and other structural/functional components of the airway lining fluid mentioned above, should better reflect GSH-HDI interactions in vivo under “normal” working conditions. Ultimately however, in vivo studies will be necessary to confirm the biological relevance of the present in vitro findings.
In summary, the ability of HDI to react with glutathione across a vapor/liquid phase, as exists in the airway microenvironment in vivo, was documented using a mixed-phase exposure system. The concentration of GSH, across a physiologic range, and the ionic composition of the fluid phase markedly influenced the reaction, with five distinct reaction products observed depending upon the experimental conditions. Notably, HDI vapor exposure of liquid solutions with GSH concentrations similar to normal human airway fluid (100 μM) resulted in predominately mono(GSH)-HDI conjugates and the capacity to carbamoylate human albumin in a pH-dependent manner. Together, the data support the hypothesis that GSH serves as a primary reaction target for HDI in vivo, with a potentially important role in the clinical response to respiratory tract exposure.
Supplementary Material
Highlights.
HDI vapor reactivity with GSH may be a mechanism for chemical entry into the body.
GSH reaction with HDI may be protective if, conjugates are rapidly excreted.
Variability in airway fluid [GSH] and pH may affect the response to HDI exposure.
ACKNOWLEDGMENT
We would like to acknowledge Drs. Stone, Lam, Ted Voss, Tom Abbott, and Mary LoPresti, from the Yale Keck Center, for their work on the HPLC, MS, and MS/MS. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health.
FUNDING SOURCES. The studies were supported by grants ES016728 and ES018021 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ABBREVIATIONS GSH, reduced glutathione; GSSG, oxidized glutathione; HDI, hexamethylene diisocyanate; TDI, toluene diisocyanate.
SUPPORTING INFORMATION AVAILABLE: MS/MS spectra of isolated GSH-HDI reaction products, chemical structures of protein modifications, correlation between HDA levels and anti-HDI serology data, raw MS/MS data describing unique HDI-GSH modification, and tables of MS/MS peptide data are provided as on-line supplemental materials Figures E1-E8, and Tables E1 and E2.
REFERENCES
- Allport DC, Gilbert DS, Outterside SM. MDI and TDI : safety, health and the environment : a source book and practical guide. Wiley; Chichester: 2003. [Google Scholar]
- Bernstein IL. Asthma in the workplace. 2nd ed. Dekker; New York: 1999. [Google Scholar]
- Broberg KE, Warholm M, Tinnerberg H, Axmon A, Jonsson BA, Sennbro CJ, et al. The GSTP1 Ile105 Val polymorphism modifies the metabolism of toluene di-isocyanate. Pharmacogenet Genomics. 2010;20(2):104–111. doi: 10.1097/FPC.0b013e328334fb84. [DOI] [PubMed] [Google Scholar]
- Brown WE, Green AH, Cedel TE, Cairns J. Biochemistry of protein-isocyanate interactions: a comparison of the effects of aryl vs. alkyl isocyanates. Environ Health Perspect. 1987;72:5–11. doi: 10.1289/ehp.87725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Chipinda I, Stetson SJ, Depree GJ, Simoyi RH, Siegel PD. Kinetics and mechanistic studies of the hydrolysis of diisocyanate-derived bis-thiocarbamates of cysteine methyl ester. Chem Res Toxicol. 2006;19(3):341–350. doi: 10.1021/tx050311t. [DOI] [PubMed] [Google Scholar]
- Davis A, Sims D. Weathering of polymers. London: Applied Science. 1983 [Google Scholar]
- Day BJ. Glutathione: a radical treatment for cystic fibrosis lung disease? Chest. 2005;127(1):12–14. doi: 10.1378/chest.127.1.12. [DOI] [PubMed] [Google Scholar]
- Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77(3):285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect Immun. 2004;72(4):2045–2051. doi: 10.1128/IAI.72.4.2045-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BW, Jin R, Karol MH. In vivo and in vitro reactions of toluene diisocyanate isomers with guinea pig hemoglobin. Chem Res Toxicol. 1996;9(3):568–573. doi: 10.1021/tx9501703. [DOI] [PubMed] [Google Scholar]
- Day BW, Jin R, Basalyga DM, Kramarik JA, Karol MH. Formation, solvolysis, and transcarbamoylation reactions of bis(S-glutathionyl) adducts of 2,4- and 2,6-diisocyanatotoluene. Chemical Research in Toxicology. 1997;10(4):424–431. doi: 10.1021/tx960201+. [DOI] [PubMed] [Google Scholar]
- Duan X, Buckpitt AR, Plopper CG. Variation in antioxidant enzyme activities in anatomic subcompartments within rat and rhesus monkey lung. Toxicol Appl Pharmacol. 1993;123(1):73–82. doi: 10.1006/taap.1993.1223. [DOI] [PubMed] [Google Scholar]
- Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. International Journal of Mass Spectrometry. 2012;309:168–175. [Google Scholar]
- Hettick JM, Siegel PD, Green BJ, Liu J, Wisnewski AV. Vapor conjugation of toluene diisocyanate to specific lysines of human albumin. Anal Biochem. 2011 doi: 10.1016/j.ab.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [August 20, 2012]; http://www.alipa.org/
- [August 20, 2012]; http://www.cdc.gov/niosh/topics/isocyanates/
- Klees JE, Ott MG. Diisocyanates in polyurethane plastics applications. Occup Med. 1999;14(4):759–776. [PubMed] [Google Scholar]
- Lange RW, Day BW, Lemus R, Tyurin VA, Kagan VE, Karol MH. Intracellular S-glutathionyl adducts in murine lung and human bronchoepithelial cells after exposure to diisocyanatotoluene. Chem Res Toxicol. 1999;12(10):931–936. doi: 10.1021/tx990045h. [DOI] [PubMed] [Google Scholar]
- Lantz RC, Lemus R, Lange RW, Karol MH. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol Sci. 2001;60(2):348–355. doi: 10.1093/toxsci/60.2.348. [DOI] [PubMed] [Google Scholar]
- Littorin M, Hou S, Broberg K, Bjork J, Falt S, Abdoulaye G, et al. Influence of polymorphic metabolic enzymes on biotransformation and effects of diphenylmethane diisocyanate. Int Arch Occup Environ Health. 2008;81(4):429–441. doi: 10.1007/s00420-007-0232-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berode M, Stowe MH, Holm CT, Walsh FX, Slade MD, et al. Urinary hexane diamine to assess respiratory exposure to hexamethylene diisocyanate aerosol: a human inhalation study. Int J Occup Environ Health. 2004;10(3):262–271. doi: 10.1179/oeh.2004.10.3.262. [DOI] [PubMed] [Google Scholar]
- Mapp CE, Fryer AA, De Marzo N, Pozzato V, Padoan M, Boschetto P, et al. Glutathione S-transferase GSTP1 is a susceptibility gene for occupational asthma induced by isocyanates. J Allergy Clin Immunol. 2002;109(5):867–872. doi: 10.1067/mai.2002.123234. [DOI] [PubMed] [Google Scholar]
- Ng AW, Bidani A, Heming TA. Innate host defense of the lung: effects of lung-lining fluid pH. Lung. 2004;182(5):297–317. doi: 10.1007/s00408-004-2511-6. [DOI] [PubMed] [Google Scholar]
- Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991;100(5):1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Acute inhalation toxicity of polymeric diphenyl-methane 4,4'-diisocyanate in rats: time course of changes in bronchoalveolar lavage. Arch Toxicol. 2000a;74(4-5):257–269. doi: 10.1007/s002040000114. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Inhalation toxicity of 1,6-hexamethylene diisocyanate homopolymer (HDI-IC) aerosol: results of single inhalation exposure studies. Toxicol Sci. 2000b;58(1):173–181. doi: 10.1093/toxsci/58.1.173. [DOI] [PubMed] [Google Scholar]
- Piirila P, Wikman H, Luukkonen R, Kaaria K, Rosenberg C, Nordman H, et al. Glutathione S-transferase genotypes and allergic responses to diisocyanate exposure. Pharmacogenetics. 2001;11(5):437–445. doi: 10.1097/00008571-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277(6 Pt 1):L1067–1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and adaptive response of glutathione in bronchial epithelial cells. Clin Exp Allergy. 2002;32(4):486–488. doi: 10.1046/j.0954-7894.2002.01368.x. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2(2-3):213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- Roum JH, Borok Z, McElvaney NG, Grimes GJ, Bokser AD, Buhl R, et al. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J Appl Physiol. 1999;87(1):438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147(6 Pt 1):1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- Stone KL, Bjornson RD, Blasko GG, Bruce C, Cofrancesco R, Carriero NJ, et al. Keck Foundation Biotechnology Resource Laboratory, Yale University. Yale J Biol Med. 2007;80(4):195–211. [PMC free article] [PubMed] [Google Scholar]
- Tarlo SM, Liss GM. Diisocyanate-induced asthma: diagnosis, prognosis, and effects of medical surveillance measures. Appl Occup Environ Hyg. 2002;17(12):902–908. doi: 10.1080/10473220290107101. [DOI] [PubMed] [Google Scholar]
- Ulrich H. Chemistry and technology of isocyanates. Wiley; Chichester: 1996. [Google Scholar]
- van der Vliet A, O'Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999;276(2 Pt 1):L289–296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83(1):126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- Wikman H, Piirila P, Rosenberg C, Luukkonen R, Kaaria K, Nordman H, et al. N-Acetyltransferase genotypes as modifiers of diisocyanate exposure-associated asthma risk. Pharmacogenetics. 2002;12(3):227–233. doi: 10.1097/00008571-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Williams KR, Stone KL. Enzymatic cleavage and HPLC peptide mapping of proteins. Mol Biotechnol. 1997;8(2):155–167. doi: 10.1007/BF02752260. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Jones M. Pro/Con debate: Is occupational asthma induced by isocyanates an immunoglobulin E-mediated disease? Clin Exp Allergy. 2010;40(8):1155–1162. doi: 10.1111/j.1365-2222.2010.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400(2):251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem Res Toxicol. 2011;24(10):1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Lemus R, Karol MH, Redlich CA. Isocyanate-conjugated human lung epithelial cell proteins: A link between exposure and asthma? J Allergy Clin Immunol. 1999;104(2 Pt 1):341–347. doi: 10.1016/s0091-6749(99)70377-5. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35(3):352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Liu Q, Liu J, Redlich CA. Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI-albumin conjugates. Clin Exp Allergy. 2008;38(6):957–967. doi: 10.1111/j.1365-2222.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, et al. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113(6):1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, et al. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162(6):2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


